Abstract
Aim:
Left ventricular (LV) dysfunction during and after hospitalization for ST-segment elevation myocardial infarction (STEMI) is associated with increased mortality. Whether baseline LV dysfunction impacts STEMI outcomes is not well studied. Furthermore, whether bivalirudin and paclitaxel-eluting stents (PES) are beneficial in patients with LV dysfunction is unknown. We studied the impact of left ventricular ejection fraction (LVEF) on outcomes of patients with STEMI in the HORIZONS-AMI trial.
Methods:
LVEF was determined in 2648 (73.5%) of 3602 enrolled STEMI patients, who were divided into three groups according to LV function: (1) severely impaired (LVEF <40%); (2) moderately impaired (LVEF 40–50%); and (3) normal (LVEF ≥50%).
Results:
Compared to patients with normal LV function, those with severely impaired LVEF had higher 1-year rates of net adverse clinical events (27.1 vs. 14.2%, p<0.0001), major adverse cardiovascular events (20.7 vs. 9.5%, p<0.0001), cardiac death (10.6 vs. 1.2%, p<0.0001), and non-coronary artery bypass graft major bleeding (12.5 vs. 6.6%, p=0.001), differences which persisted after adjustment for baseline characteristics. Among patients with LVEF <40%, treatment with bivalirudin compared to heparin+GPIIb/IIIa inhibitors resulted in reduced 1-year mortality (5.8 vs. 18.3%, p=0.007). Patients with LVEF <40% receiving PES rather than bare metal stents had lower rates of 1-year ischaemia-driven target lesion revascularization (2.9 vs. 12.6%, p=0.02) and reinfarction (4.5 vs. 14.7%, p=0.03).
Conclusions:
Among patients with STEMI undergoing primary percutaneous coronary intervention, adverse events are markedly increased in those with LVEF <40% during the index revascularization procedure. Nevertheless, these high-risk patients experience substantial clinical benefits from bivalirudin and PES.
Keywords: Bivalirudin, drug-eluting stent, left ventricular dysfunction, ST-elevation myocardial infarction
Introduction
Left ventricular (LV) dysfunction is an established correlate of increased short- and long-term mortality after acute myocardial infarction (AMI).1–7 As such, new devices such as coronary stents and pharmacological agents to improve LV function by enhancing microcirculatory reperfusion and decreasing LV remodelling have been developed. These advances have decreased morbidity and mortality in patients with ST-segment elevation myocardial infarction (STEMI).8–11 The prognostic impact of LV dysfunction after the advent and widespread utilization of these new techniques into routine care has not been studied extensively.12–15 Moreover, most prior studies assessed LV function days to months after the index event.1,3,4,14 As primary percutaneous coronary intervention (PCI) has become the preferred treatment for STEMI,16 the ability to directly assess LV ejection fraction (LVEF) by ventriculography at the time of presentation and revascularization allows for early determination of LV function. Only one prior large-scale study performed over a decade ago has examined the prognostic impact of LVEF measured during the primary PCI procedure, whether this practice remains of clinical relevance with contemporary treatments requires re-evaluation.12
The multicentre, prospective, randomized HORIZONS-AMI trial found that usage of bivalirudin rather than heparin with glycoprotein IIb/IIIa inhibitors (GPI) in patients with STEMI undergoing primary PCI reduced the rates of net adverse clinical events (NACE) and major bleeding at 1 year.17 Furthermore, patients randomized to paclitaxel-eluting stents (PES) had lower rates of ischaemia-driven revascularization than those receiving bare metal stents (BMS).18 We examine the prognostic impact of LV function determined during the index revascularization procedure by contrast left ventriculography on 1-year clinical outcomes in the HORIZONS-AMI trial.
Methods
The HORIZONS-AMI study design, major inclusion and exclusion criteria, endpoints, definitions, and results have been previously described in detail.19,20 In brief, 3602 patients with STEMI undergoing primary angioplasty were prospectively randomized in an open-label 1:1 fashion to either heparin plus a GPI (either abciximab or eptifibatide) or to bivalirudin with provisional GPI therapy for predefined thrombotic complications. Dosing regimens were described previously.19 After angiography and contrast left ventriculography, patients were triaged to PCI, coronary artery bypass grafting (CABG), or medical management. The performance of left ventriculography was strongly recommended, but not mandated, during the index procedure. A total of 3006 patients with lesions eligible for stenting were randomized again in a 3:1 fashion to either a PES (TAXUS EXPRESS2, Boston Scientific, Natick, MS, USA) or to an otherwise identical BMS (EXPRESS2, Boston Scientific).
Baseline angiograms were analysed at an independent angiographic core laboratory by technicians blinded to treatment assignments and clinical outcomes. Quantitative angiographic measures including LVEF were performed using the Medis system (Leiden, The Netherlands). Left ventriculograms were excluded from analysis if there was insufficient contrast opacification to visualize LV contours, in the absence of at least three consecutive sinus or supraventricular beats, or if the LV gram was not performed. Clinical follow up was scheduled at 30 days, 6 months, 1 year, and then yearly for 5 years. Primary clinical endpoints were adjudicated by an independent clinical events committee blinded to treatment assignment. The major 30-day primary endpoints were NACE and non-CABG related major bleeding. NACE was defined as a composite of major adverse cardiovascular events (MACE) or non-CABG related major bleeding. MACE (death, reinfarction, stroke, or ischaemic TVR) was the secondary 30-day endpoint. The major 1-year primary endpoint for patients in the stent level of randomization was ischaemia-driven target lesion revascularization (TLR).
Statistical analysis
For the present analysis, patients with baseline left ventricular ejection fraction (LVEF) determined by contrast left ventriculography were divided into those with severely impaired left ventricular function (LVEF <40%), moderately impaired left ventricular function (LVEF 40–50%), and normal left ventricular function (LVEF ≥50%).
Outcomes were assessed according to baseline LVEF in these three groups. Categorical variables are presented as percentages and were compared by either the chi-squared or Fisher’s exact test. Continuous variables are presented as mean±standard deviation (SD) or as median (interquartile range) and were compared by the Kruskal–Wallis or Wilcoxon rank-sum test. Thirty-day and 1-year time-to-event estimates were calculated using Kaplan–Meier methodology and compared with the log-rank test. Multivariable analysis was performed using Cox regression analysis with entry criteria of p=0.20 and stay criteria of p=0.10. Candidate baseline variables included age, gender, body mass index, diabetes, insulin-dependent diabetes, renal insufficiency (creatinine clearance <60 ml/min), smoking, hypertension, hyperlipidaemia, previous MI, previous CABG, prior coronary artery disease, prior angina, history of congestive heart failure, peripheral vascular disease, baseline anaemia, baseline white blood cell count, baseline platelet count, Killip class 2–4 (vs. class 1), LVEF (<40 vs. ≥50%; 40–50 vs. ≥50%), symptom to first balloon time, left anterior descending artery disease, treatment type (PCI, deferred PCI, CABG, medical management), and randomization arm (bivalirudin vs. heparin+GPI). All statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics and procedural outcomes
Of the 3602 randomized patients, 2648 (73.5%) patients had LVEF determined during the index procedure by contrast left ventriculography, in whom LV function was severely impaired in 219 (8.3%), moderately impaired in 396 (15.0%), and normal in 2033 (76.8%). Baseline characteristics according to LV function are detailed in Table 1. Patients with diminished LV function had greater rates of prior MI, prior PCI, and Killip class 2 than patients with normal LV function. Patients with severely impaired LV function were older, more likely women, and had higher rates of peripheral vascular disease and presentation in Killip class 3 or 4. Patients with moderately impaired LV function had a longer interval from symptom onset to hospital presentation than patients with normal LV function.
Table 1.
Baseline characteristics according to index procedure left ventricular function.
| Left ventricular ejection fraction |
p-value |
||||||
|---|---|---|---|---|---|---|---|
| A: <40% (n=219) | B: 40–50% (n=396) | C: ≥50% (n=2033) | All groups | A vs. B | A vs. C | B vs. C | |
| Age (years) | 61.05 (54.90–71.83) | 59.65 (53.18–70.13) | 59.34 (51.79–68.95) | 0.03 | 0.14 | 0.01 | 0.25 |
| ≥65 years | 40.2 (88/219) | 37.6 (149/396) | 34.5 (701/2033) | 0.15 | 0.53 | 0.09 | 0.23 |
| Gender | |||||||
| Male | 67.6 (148/219) | 81.3 (322/396) | 76.9 (1564/2033) | 0.0006 | 0.0001 | 0.002 | 0.06 |
| Female | 32.4 (71/219) | 18.7 (74/396) | 23.1 (469/2033) | 0.0006 | 0.0001 | 0.002 | 0.06 |
| Body mass index (kg/m2) | 26.51 (24.21–29.48) | 27.17 (24.49–30.42) | 27.04 (24.61–29.84) | 0.15 | 0.08 | 0.06 | 0.77 |
| Hypertension | 54.6 (119/218) | 53.5 (212/396) | 52.8 (1073/2033) | 0.86 | 0.80 | 0.61 | 0.78 |
| Hyperlipidaemia | 41.7 (91/218) | 39.4 (156/396) | 41.8 (849/2033) | 0.68 | 0.57 | 1.00 | 0.38 |
| Current smoker | 48.4 (105/217) | 50.6 (200/395) | 46.4 (940/2026) | 0.28 | 0.60 | 0.58 | 0.12 |
| Diabetes mellitus | 19.7 (43/218) | 15.2 (60/396) | 15.6 (317/2033) | 0.26 | 0.15 | 0.11 | 0.82 |
| Insulin requiring | 6.9 (15/218) | 3.8 (15/396) | 4.4 (89/2033) | 0.18 | 0.09 | 0.09 | 0.60 |
| Peripheral vascular disease | 7.8 (17/218) | 1.8 (7/396) | 4.1 (84/2033) | 0.001 | 0.0002 | 0.01 | 0.02 |
| History of renal insufficiencya | 3.2 (7/218) | 2.5 (10/396) | 2.1 (42/2033) | 0.50 | 0.62 | 0.27 | 0.56 |
| Current dialysis | 0.5 (1/218) | 0.3 (1/396) | 0.1 (2/2033) | 0.37 | 0.67 | 0.17 | 0.42 |
| Prior MI | 16.1 (35/218) | 13.1 (52/396) | 9.3 (189/2033) | 0.001 | 0.32 | 0.002 | 0.02 |
| Prior PCI | 13.8 (30/218) | 12.4 (49/396) | 8.4 (171/2033) | 0.004 | 0.62 | 0.009 | 0.01 |
| Prior CABG | 2.3 (5/218) | 2.0 (8/396) | 2.5 (51/2033) | 0.84 | 0.82 | 0.85 | 0.56 |
| Family history of premature CAD | 29.4 (64/218) | 34.1 (135/396) | 29.4 (597/2033) | 0.17 | 0.23 | 1.00 | 0.06 |
| CCS angina class | |||||||
| 1 | 5.0 (11/218) | 4.8 (19/396) | 4.5 (92/2033) | 0.9235 | 0.89 | 0.7267 | 0.8121 |
| 2 | 11.9 (26/218) | 11.9 (47/396) | 8.6 (175/2033) | 0.0503 | 0.98 | 0.1025 | 0.0394 |
| 3 | 4.6 (10/218) | 5.1 (20/396) | 3.7 (76/2033) | 0.4286 | 0.80 | 0.5344 | 0.2202 |
| 4 | 2.8 (6/218) | 4.5 (18/396) | 3.2 (66/2033) | 0.3667 | 0.27 | 0.6936 | 0.1956 |
| NYHA class | |||||||
| 1 | 2.3 (5/218) | 0.8 (3/396) | 0.3 (7/2033) | 0.001 | 0.11 | 0.0002 | 0.24 |
| 2 | 3.7 (8/218) | 1.5 (6/396) | 0.7 (15/2033) | 0.0003 | 0.09 | <0.0001 | 0.13 |
| 3 | 2.8 (6/218) | 0.5 (2/396) | 0.2 (5/2033) | <0.0001 | 0.02 | <0.0001 | 0.38 |
| 4 | 0.0 (0/218) | 0.3 (1/396) | 0.0 (1/2033) | 0.37 | 0.46 | 0.74 | 0.20 |
| Killip class | |||||||
| 1 | 82.1 (179/218) | 88.4 (350/396) | 94.3 (1914/2030) | <0.0001 | 0.03 | <0.0001 | <0.0001 |
| 2 | 12.8 (28/218) | 9.8 (39/396) | 4.8 (98/2030) | <0.0001 | 0.25 | <0.0001 | <0.0001 |
| 3 | 2.8 (6/218) | 1.0 (4/396) | 0.5 (10/2030) | 0.001 | 0.10 | 0.0002 | 0.21 |
| 4 | 2.3 (5/218) | 0.8 (3/396) | 0.4 (8/2030) | 0.003 | 0.11 | 0.0004 | 0.32 |
| Interval from symptom onset to first hospital (h) | 2.46 (1.27–4.17) | 2.50 (1.38–4.08) | 2.08 (1.25–3.87) | 0.04 | 0.60 | 0.24 | 0.02 |
Values are median (interquartile range) or % (n/total).
Renal insufficiency was defined as a creatinine clearance of less than 60 ml/min as calculated at baseline by the Cockcroft–Gault equation.
CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
As shown in Table 2, reduced LV function was associated with left anterior descending infarct artery involvement. Furthermore, patients with impaired LVEF had higher rates of pre-procedural TIMI 0/1 flow and lower rates of post-procedural TIMI 3 flow compared to patients with normal LV function.
Table 2.
Procedural characteristics according to index procedure left ventricular function.
| Left ventricular ejection fraction |
p-value |
||||||
|---|---|---|---|---|---|---|---|
| A: <40% (n=219) | B: 40–50% (n=396) | C: ≥50% (n=2033) | All groups | A vs. B | A vs. C | B vs. C | |
| Any intervention | 99.5 (204/205) | 98.7 (374/379) | 99.4 (1868/1879) | 0.27 | 0.34 | 0.86 | 0.12 |
| Target vessel | |||||||
| LAD | 67.1 (153/228) | 56.1 (230/410) | 33.6 (684/2035) | <0.0001 | 0.007 | <0.0001 | <0.0001 |
| LCX | 12.3 (28/228) | 15.4 (63/410) | 16.9 (343/2035) | 0.18 | 0.29 | 0.08 | 0.46 |
| RCA | 19.3 (44/228) | 27.6 (113/410) | 48.4 (984/2035) | <0.0001 | 0.02 | <0.0001 | <0.0001 |
| PTCA only performed | 3.4 (7/204) | 3.4 (13/378) | 3.6 (67/1866) | 0.98 | 1.00 | 0.91 | 0.88 |
| One or more stents implanted | 95.6 (197/206) | 94.8 (363/383) | 94.5 (1793/1897) | 0.79 | 0.65 | 0.50 | 0.84 |
| Patients with stents implanted | |||||||
| n | 197 | 363 | 1793 | ||||
| Mean±SD | 1.60±0.98 | 1.47±0.73 | 1.51±0.79 | 0.17 | 0.06 | 0.13 | 0.34 |
| Total stent length implanted (mm) | |||||||
| n | 197 | 363 | 1789 | ||||
| Median (interquartile range) | 24.00 (18.00–36.00) | 24.00 (20.00–36.00) | 24.00 (20.00–36.00) | ||||
| No. of vessels treated | 1.06±0.25 | 1.04±0.19 | 1.04±0.21 | 0.33 | 0.15 | 0.18 | 0.64 |
| 1 | 93.6 (190/203) | 96.3 (360/374) | 95.9 (1785/1862) | 0.27 | 0.15 | 0.13 | 0.73 |
| 2 | 6.4 (13/203) | 3.7 (14/374) | 4.0 (74/1862) | 0.23 | 0.15 | 0.10 | 0.83 |
| 3 | 0.0 (0/203) | 0.0 (0/374) | 0.2 (3/1862) | 0.63 | – | 0.57 | 0.44 |
| Multiple vessels treated | 6.4 (13/203) | 3.7 (14/374) | 4.1 (77/1862) | 0.27 | 0.15 | 0.13 | 0.73 |
| No. of lesions treated | 1.15±0.43 | 1.11±0.35 | 1.12±0.38 | 0.56 | 0.28 | 0.36 | 0.65 |
| 1 | 88.2 (179/203) | 89.8 (336/374) | 89.5 (1666/1862) | 0.82 | 0.54 | 0.57 | 0.83 |
| 2 | 8.9 (18/203) | 9.1 (34/374) | 9.0 (168/1862) | 1.00 | 0.93 | 0.94 | 0.97 |
| 3 | 3.0 (6/203) | 1.1 (4/374) | 1.3 (25/1862) | 0.15 | 0.10 | 0.07 | 0.67 |
| 4 | 0.0 (0/203) | 0.0 (0/374) | 0.2 (3/1862) | 0.63 | – | 0.57 | 0.44 |
| Multiple lesions treated | 11.8 (24/203) | 10.2 (38/374) | 10.5 (196/1862) | 0.82 | 0.54 | 0.57 | 0.83 |
| Any side branch lesion treated | 7.3 (15/206) | 8.9 (34/383) | 5.9 (112/1897) | 0.09 | 0.50 | 0.43 | 0.03 |
| No. of vessels with TIMI flow before PCI | |||||||
| 0/1 | 65.5 (146/223) | 64.2 (262/408) | 57.7 (1147/1989) | 0.007 | 0.75 | 0.02 | 0.01 |
| 2 | 17.0 (38/223) | 14.0 (57/408) | 13.6 (271/1989) | 0.38 | 0.30 | 0.16 | 0.85 |
| 3 | 17.5 (39/223) | 21.8 (89/408) | 28.7 (571/1989) | <0.0001 | 0.20 | 0.0004 | 0.005 |
| No. of vessels with TIMI flow after PCI | |||||||
| 0/1 | 1.4 (3/222) | 3.7 (15/408) | 2.2 (43/1988) | 0.11 | 0.09 | 0.42 | 0.07 |
| 2 | 16.7 (37/222) | 14.2 (58/408) | 8.2 (164/1988) | <0.0001 | 0.41 | <0.0001 | 0.0002 |
| 3 | 82.0 (182/222) | 82.1 (335/408) | 89.6 (1781/1988) | <0.0001 | 0.97 | 0.0006 | <0.0001 |
Values are % (n/total) or mean±SD, unless otherwise stated.
CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; LAD, left anterior descending artery; LCX, left circumflex artery; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
Thirty-day and 1-year clinical outcomes
At both 30-days and 1-year, patients with severely impaired LV function had higher rates of NACE, MACE, and non-CABG related major bleeding than the other patient groups (Tables 3 and 4 and Figure 1). Patients with severely impaired LV function also had higher rates of all-cause death, cardiac death, composite death or reinfarction, and stent thrombosis at both time periods (Tables 3 and 4 and Figure 2). Most of the late differences were explained by the increased hazard during the first 30 days, although compared to patients with normal LV function, patients with severely and moderately impaired LV function had increased rates of late (>30 day) all-cause death (4.1 vs. 0.9% and 2.4 vs. 0.9%; p<0.0001 and p=0.01, respectively) and cardiac death (2.6 vs. 0.3% and 1.0 vs. 0.3%; p<0.0001 and p=0.02, respectively). Ischaemic TLR rates were not significantly affected by LV function.
Table 3.
Clinical outcomes at 30-days according to index procedure left ventricular function.
| Left ventricular ejection fraction |
p-value |
||||||
|---|---|---|---|---|---|---|---|
| A: <40% (n=219) | B: 40–50% (n=396) | C: ≥50% (n=2033) | All groups | A vs. B | A vs. C | B vs. C | |
| Net adverse clinical events | 19.6 (43) | 8.6 (34) | 8.8 (178) | <0.0001 | <0.0001 | <0.0001 | 0.87 |
| MACE (death, MI, ischaemic TVR, or stroke) | 12.3 (27) | 3.3 (13) | 3.6 (72) | <0.0001 | <0.0001 | <0.0001 | 0.80 |
| Death | 8.7 (19) | 1.3 (5) | 1.2 (24) | <0.0001 | <0.0001 | <0.0001 | 0.89 |
| Cardiac | 8.2 (18) | 0.8 (3) | 0.9 (19) | <0.0001 | <0.0001 | <0.0001 | 0.73 |
| Non-cardiac | 0.5 (1) | 0.5 (2) | 0.2 (5) | 0.62 | 0.96 | 0.53 | 0.38 |
| Reinfarction | 3.4 (7) | 0.8 (3) | 1.6 (32) | 0.06 | 0.02 | 0.07 | 0.21 |
| Q-wave | 1.4 (3) | 0.3 (1) | 1.2 (24) | 0.23 | 0.09 | 0.78 | 0.09 |
| Non-Q-wave | 2.0 (4) | 0.5 (2) | 0.4 (8) | 0.02 | 0.10 | 0.004 | 0.75 |
| Death or reinfarction | 10.5 (23) | 2.0 (8) | 2.7 (55) | <0.0001 | <0.0001 | <0.0001 | 0.43 |
| TVR, ischaemia driven | 2.8 (6) | 1.3 (5) | 1.8 (36) | 0.39 | 0.17 | 0.294 | 0.47 |
| Stroke | 0.5 (1) | 0.8 (3) | 0.6 (13) | 0.91 | 0.68 | 0.766 | 0.79 |
| TIA | 0.0 (0) | 0.0 (0) | 0.1 (3) | 0.64 | – | 0.578 | 0.44 |
| Bleeding | |||||||
| Major bleeding (non-CABG related) | 12.5 (27) | 6.3 (25) | 6.2 (126) | 0.002 | 0.007 | 0.0004 | 0.97 |
| Intracranial bleeding | 0.0 (0) | 0.0 (0) | 0.0 (0) | – | – | – | – |
| Intraocular bleeding | 0.0 (0) | 0.0 (0) | 0.0 (0) | – | – | – | – |
| Retroperitoneal bleeding | 1.4 (3) | 0.3 (1) | 0.4 (8) | 0.10 | 0.10 | 0.05 | 0.67 |
| Access site haemorrhage | 0.0 (0) | 0.0 (0) | 0.2 (4) | 0.55 | N/A | 0.51 | 0.38 |
| Haematoma ≥5 cm at puncture site | 3.7 (8) | 1.3 (5) | 1.8 (37) | 0.10 | 0.05 | 0.06 | 0.43 |
| Drop in Hb ≥3 g/dl with overt source | 3.2 (7) | 1.5 (6) | 1.8 (37) | 0.29 | 0.16 | 0.15 | 0.67 |
| Drop in Hb ≥4 g/dl without an overt source | 7.5 (16) | 4.1 (16) | 2.8 (56) | 0.0009 | 0.07 | 0.0002 | 0.17 |
| Drop in Hb >5 g/dl without an overt source | 3.3 (7) | 1.5 (6) | 1.3 (27) | 0.09 | 0.15 | 0.03 | 0.77 |
| Reoperation for bleeding | 0.5 (1) | 0.0 (0) | 0.0 (1) | 0.09 | 0.18 | 0.05 | 0.66 |
| Blood product transfusion | 6.0 (13) | 2.3 (9) | 2.7 (54) | 0.01 | 0.02 | 0.005 | 0.65 |
| Major bleeding (including CABG related) | 15.8 (34) | 8.6 (34) | 7.7 (155) | 0.0002 | 0.006 | <0.0001 | 0.56 |
| Stent thrombosis in patients with stent implanted | |||||||
| Definite | 2.5 (5) | 0.8 (3) | 1.8 (33) | 0.27 | 0.10 | 0.48 | 0.17 |
| Probable | 1.6 (3) | 0.3 (1) | 0.2 (3) | 0.003 | 0.08 | 0.0008 | 0.66 |
| Subacute stent thrombosis, definite/probable | 3.6 (7) | 0.8 (3) | 1.3 (23) | 0.02 | 0.02 | 0.01 | 0.47 |
Values are % (n).
CABG, coronary artery bypass graft; Hb, haemoglobin; MACE, major adverse cardiovascular events; TIA, transient ischaemic attack; TVR, target vessel revascularization.
Table 4.
Clinical Outcomes at 1-Year According to Index Procedure Left Ventricular Function.
| Left ventricular ejection fraction |
p-value |
||||||
|---|---|---|---|---|---|---|---|
| A: <40% (n=219) | B: 40–50% (n=396) | C: ≥50% (n=2033) | All groups | A vs. B | A vs. C | B vs. C | |
| Net adverse clinical events | 27.1 (59) | 15.6 (61) | 14.2 (283) | <0.0001 | 0.0004 | <0.0001 | 0.47 |
| MACE (death, MI, ischaemic TVR, or stroke) | 20.7 (45) | 10.8 (42) | 9.5 (187) | <0.0001 | 0.0006 | <0.0001 | 0.38 |
| Death | 12.4 (27) | 3.6 (14) | 2.1 (41) | <0.0001 | <0.0001 | <0.0001 | 0.07 |
| Cardiac | 10.6 (23) | 1.8 (7) | 1.2 (24) | <0.0001 | <0.0001 | <0.0001 | 0.35 |
| Non-cardiac | 2.0 (4) | 1.8 (7) | 0.9 (17) | 0.11 | 0.88 | 0.12 | 0.09 |
| Reinfarction | 6.0 (12) | 3.2 (12) | 3.5 (69) | 0.18 | 0.10 | 0.08 | 0.72 |
| Q-wave | 1.9 (4) | 1.9 (7) | 1.8 (36) | 0.99 | 0.90 | 0.90 | 1.00 |
| Non-Q-wave | 4.6 (9) | 1.3 (5) | 1.8 (35) | 0.02 | 0.02 | 0.009 | 0.52 |
| Death or reinfarction | 16.1 (35) | 6.7 (26) | 5.4 (108) | <0.0001 | 0.0002 | <0.0001 | 0.33 |
| TVR, ischaemia driven | 8.5 (17) | 5.5 (21) | 6.1 (119) | 0.34 | 0.17 | 0.18 | 0.70 |
| Stroke | 1.5 (3) | 1.0 (4) | 0.9 (18) | 0.74 | 0.64 | 0.44 | 0.81 |
| TIA | 0.0 (0) | 0.0 (0) | 0.3 (5) | 0.48 | N/A | 0.47 | 0.32 |
| TLR, ischaemia driven | 4.9 (10) | 4.8 (18) | 5.0 (98) | 0.98 | 0.89 | 1.00 | 0.83 |
| Bleeding | |||||||
| Major bleeding (non-CABG related) | 12.5 (27) | 6.9 (27) | 6.6 (134) | 0.005 | 0.01 | 0.001 | 0.90 |
| Intracranial bleeding | 0.0 (0) | 0.3 (1) | 0.0 (0) | 0.06 | 0.47 | – | 0.02 |
| Intraocular bleeding | 0.0 (0) | 0.0 (0) | 0.0 (0) | – | – | – | – |
| Retroperitoneal bleeding | 1.4 (3) | 0.3 (1) | 0.5 (10) | 0.16 | 0.10 | 0.10 | 0.52 |
| Access site haemorrhage | 0.0 (0) | 0.0 (0) | 0.2 (4) | 0.55 | N/A | 0.51 | 0.38 |
| Haematoma ≥5 cm at puncture site | 3.7 (8) | 1.3 (5) | 1.9 (39) | 0.11 | 0.05 | 0.08 | 0.37 |
| Drop in Hb ≥3 g/dl with overt source | 3.2 (7) | 1.5 (6) | 2.1 (43) | 0.36 | 0.16 | 0.28 | 0.44 |
| Drop in Hb ≥4 g/dl without an overt source | 7.5 (16) | 4.1 (16) | 2.8 (57) | 0.001 | 0.07 | 0.0003 | 0.19 |
| Drop in Hb >5 g/dl without an overt source | 3.3 (7) | 1.5 (6) | 1.3 (27) | 0.09 | 0.15 | 0.03 | 0.77 |
| Reoperation for bleeding | 0.5 (1) | 0.0 (0) | 0.0 (1) | 0.09 | 0.18 | 0.05 | 0.66 |
| Blood product transfusion | 6.0 (13) | 2.8 (11) | 3.0 (60) | 0.04 | 0.04 | 0.01 | 0.84 |
| Major bleeding (including CABG related) | 16.3 (35) | 8.9 (35) | 8.2 (165) | 0.0003 | 0.005 | <0.0001 | 0.68 |
| Stent thrombosis in patients with stent implanted | |||||||
| Definite | 3.6 (7) | 2.2 (8) | 2.7 (48) | 0.61 | 0.32 | 0.43 | 0.62 |
| Probable | 1.6 (3) | 0.6 (2) | 0.2 (3) | 0.005 | 0.22 | 0.0008 | 0.16 |
| Late stent thrombosis, definite or probable | 1.6 (3) | 1.7 (6) | 0.9 (15) | 0.24 | 0.97 | 0.27 | 0.13 |
Values are % (n).
CABG, coronary artery bypass graft; Hb, haemoglobin; MACE, major adverse cardiovascular events; TIA, transient ischaemic attack; TLR, target lesion revascularization; TVR, target vessel revascularization.
Figure 1.
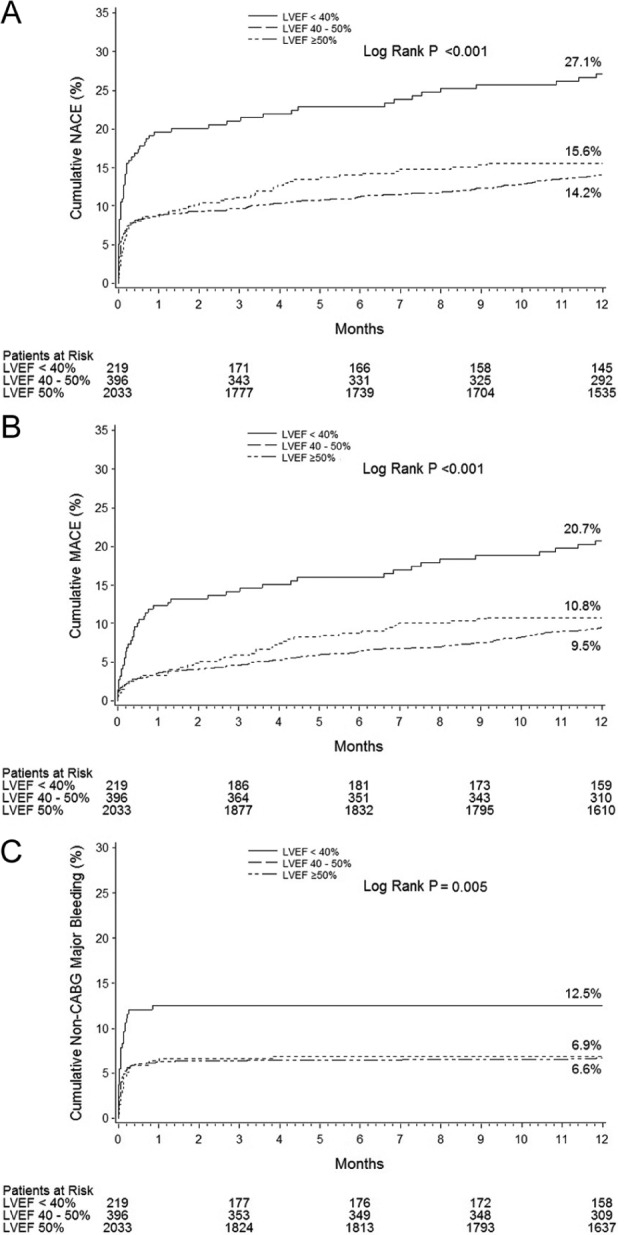
Impact of LV dysfunction on 1-year outcomes: 1-year time-to-event curves according to LVEF for net adverse clinical events (NACE; A), major adverse cardiovascular events (MACE; B), and non-CABG major bleeding (C).
Figure 2.
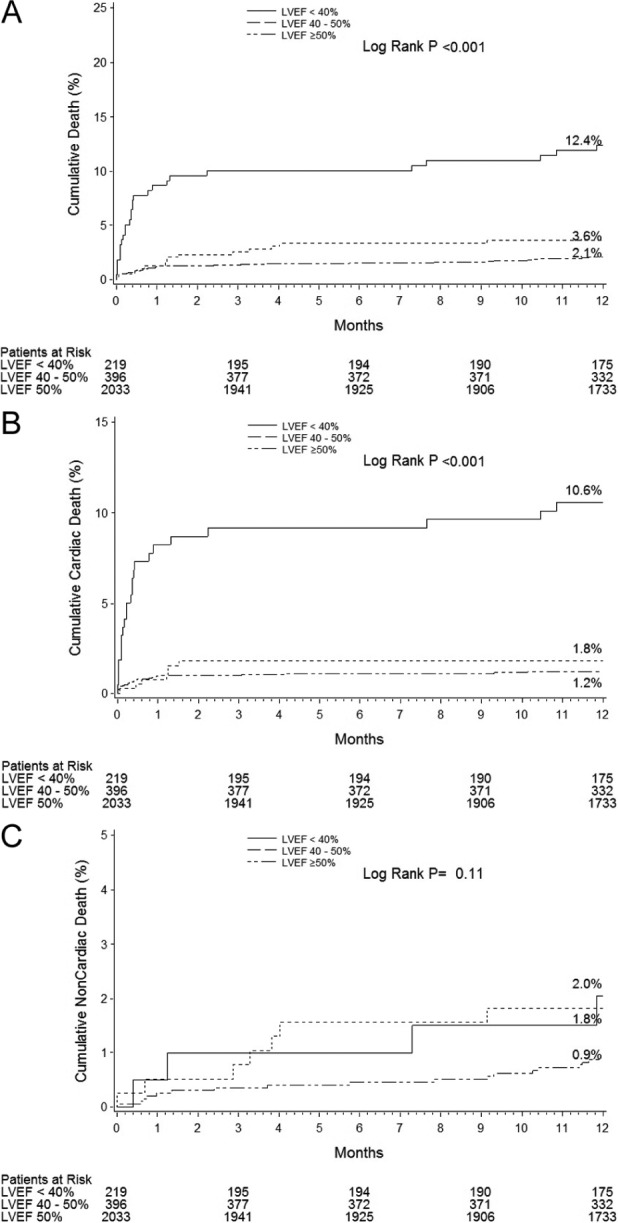
Impact of LV dysfunction on mortality: 1-year time-to-event curves according to LVEF for all-cause death (A), cardiac death (B), and non-cardiac death (C).
After adjustment for baseline differences, LVEF <40% was an independent predictor of 1-year NACE (HR 1.96, 95% CI 1.38–2.78, p<0.001), MACE (HR 2.09, 95% CI 1.49–2.94, p<0.0001), all-cause death (HR 3.54, 95% CI 2.09–5.98, p<0.0001), and non-CABG major bleeding (HR 1.85, 95% CI 1.10–3.11, p=0.02). By multivariable analysis, LVEF 40–50% was not a predictor of poorer outcomes.
Pharmacological randomization in patients with severely reduced LVEF
Of the 219 patients with severely impaired LVEF, 103 (47.0%) were randomized to bivalirudin and 116 (53.0%) were randomized to heparin plus GPI. The groups were well matched except that patients treated with heparin+GPI had slightly higher rates of hypertension (60.9 vs. 47.6%, p=0.049). Although rates of NACE and MACE were similar, patients with LVEF <40% treated with bivalirudin rather heparin+GPI had significantly lower 1-year rates of all-cause death, cardiac death, non-cardiac death and composite death and reinfarction (Table 5). Total major bleeding was also reduced by bivalirudin.
Table 5.
1-year outcomes in patients with LVEF <40% according to pharmacological randomization.
| Bivalirudin (n=103) | UFH+GPI (n=116) | p-value | |
|---|---|---|---|
| Net adverse clinical events | 23.4 (24) | 30.5 (35) | 0.25 |
| MACE (death, MI, ischaemic TVR, or stroke) | 15.6 (16) | 25.3 (29) | 0.09 |
| Death | 5.8 (6) | 18.3 (21) | 0.007 |
| Cardiac | 5.8 (6) | 14.9 (17) | 0.04 |
| Non-cardiac | 0.0 (0) | 4.0 (4) | 0.05 |
| Reinfarction | 3.1 (3) | 8.6 (9) | 0.09 |
| Q-wave | 1.1 (1) | 2.7 (3) | 0.36 |
| Non-Q-wave | 2.1 (2) | 7.0 (7) | 0.10 |
| Death or reinfarction | 8.8 (9) | 22.7 (26) | 0.007 |
| TVR, ischaemia driven | 7.2 (7) | 9.7 (10) | 0.53 |
| Stroke | 2.0 (2) | 1.0 (1) | 0.53 |
| TIA | 0.0 (0) | 0.0 (0) | |
| TLR, ischaemia driven | 4.1 (4) | 5.7 (6) | 0.60 |
| Bleeding | |||
| Major bleeding (non-CABG related) | 8.9 (9) | 15.7 (18) | 0.12 |
| Intracranial bleeding | 0.0 (0) | 0.0 (0) | – |
| Intraocular bleeding | 0.0 (0) | 0.0 (0) | – |
| Retroperitoneal bleeding | 0.0 (0) | 2.6 (3) | 0.10 |
| Access site haemorrhage | 0.0 (0) | 0.0 (0) | – |
| Haematoma ≥5 cm at puncture site | 3.0 (3) | 4.4 (5) | 0.58 |
| Drop in Hb ≥3 g/dl with overt source | 2.0 (2) | 4.4 (5) | 0.32 |
| Drop in Hb ≥4 g/dl without an overt source | 6.0 (6) | 8.9 (10) | 0.43 |
| Drop in Hb >5 g/dl without an overt source | 2.0 (2) | 4.5 (5) | 0.32 |
| Reoperation for bleeding | 0.0 (0) | 0.9 (1) | 0.35 |
| Blood product transfusion | 2.9 (3) | 8.7 (10) | 0.08 |
| Major bleeding (including CABG related) | 10.9 (11) | 21.1 (24) | 0.04 |
| Stent thrombosis in patients with stent implanted | |||
| Definite | 2.1 (2) | 5.1 (5) | 0.27 |
| Probable | 1.0 (1) | 2.2 (2) | 0.56 |
| Late stent thrombosis, definite/probable | 1.1 (1) | 2.2 (2) | 0.54 |
Values are % (n).
CABG, coronary artery bypass graft; Hb, haemoglobin; MACE, major adverse cardiovascular events; TIA, transient ischaemic attack; TLR, target lesion revascularization; TVR, target vessel revascularization.
Stent randomization in patients with severely reduced LVEF
Of 189 stent eligible patients with LVEF <40%, 145 (76.7%) patients were randomized to PES and 44 (23.3%) patients to BMS. Baseline clinical and angiographic characteristics were well matched between the two treatment arms (data not shown). At 1-year, rates of NACE and MACE were similar between the two groups. However, patients with severe LV dysfunction receiving PES rather than BMS had 76% and 66% relative reductions in ischaemia-driven TLR and TVR, respectively (Table 6). PES randomized patients also had a 70% reduction in the 1-year rate of reinfarction. Stent thrombosis rates were similar between patients randomized to PES and BMS.
Table 6.
1-year outcomes in patients with LVEF <40% according to stent randomization.
| TAXUS (n=145) | BMS (n=44) | p-value | |
|---|---|---|---|
| Net adverse clinical events | 25.6 (37) | 36.4 (16) | 0.21 |
| MACE (death, MI, ischaemic TVR, or stroke) | 19.4 (28) | 32.0 (14) | 0.10 |
| Death | 12.5 (18) | 18.3 (8) | 0.37 |
| Cardiac | 10.4 (15) | 18.3 (8) | 0.19 |
| Non-cardiac | 2.3 (3) | 0.0 (0) | 0.35 |
| Reinfarction | 4.5 (6) | 14.7 (6) | 0.03 |
| Q-wave | 2.2 (3) | 2.3 (1) | 0.96 |
| Non-Q-wave | 3.1 (4) | 12.7 (5) | 0.02 |
| Death or reinfarction | 15.9 (23) | 25.1 (11) | 0.20 |
| TVR, ischaemia driven | 6.0 (8) | 17.8 (7) | 0.03 |
| Stroke | 1.6 (2) | 2.3 (1) | 0.65 |
| TIA | 0.0 (0) | 0.0 (0) | |
| TLR, ischaemia driven | 2.9 (4) | 12.6 (5) | 0.02 |
| Bleeding | |||
| Major bleeding (non-CABG related) | 14.8 (21) | 6.8 (3) | 0.19 |
| Intracranial bleeding | 0.0 (0) | 0.0 (0) | – |
| Intraocular bleeding | 0.0 (0) | 0.0 (0) | – |
| Retroperitoneal bleeding | 2.1 (3) | 0.0 (0) | 0.34 |
| Access site haemorrhage | 0.0 (0) | 0.0 (0) | – |
| Haematoma ≥5 cm at puncture site | 2.8 (4) | 6.8 (3) | 0.21 |
| Drop in Hb ≥3 g/dl with overt source | 4.2 (6) | 2.3 (1) | 0.57 |
| Drop in Hb ≥4 g/dl without an overt source | 8.6 (12) | 4.6 (2) | 0.39 |
| Drop in Hb >5 g/dl without an overt source | 3.6 (5) | 4.6 (2) | 0.76 |
| Reoperation for bleeding | 0.7 (1) | 0.0 (0) | 0.58 |
| Blood product transfusion | 7.7 (11) | 4.5 (2) | 0.49 |
| Major bleeding (including CABG related) | 15.5 (22) | 9.5 (4) | 0.30 |
| Stent thrombosis in patients with stent implanted | |||
| Definite | 3.6 (5) | 4.9 (2) | 0.76 |
| Probable | 0.8 (1) | 4.7 (2) | 0.08 |
| Late stent thrombosis, definite/probable | 1.5 (2) | 2.6 (1) | 0.67 |
Values are % (n).
CABG, coronary artery bypass graft; Hb, haemoglobin; MACE, major adverse cardiovascular events; TIA, transient ischaemic attack; TLR, target lesion revascularization; TVR, target vessel revascularization.
Discussion
The present study demonstrates that severe LV dysfunction as assessed by LV angiography during the acute phase of STEMI in patients undergoing a primary PCI management strategy is a powerful independent predictor of poor clinical outcomes. Patients with a LVEF <40% have increased rates of mortality, MACE, major bleeding, and NACE. However, moderate LV dysfunction was not a predictor of poor clinical outcomes. While the major deleterious impact of reduced LVEF on survival is principally seen in the first 30 days after presentation, late mortality after this time point continued to be greater in patients with LV dysfunction. Moreover, patients with severe left ventricular dysfunction had improved survival with bivalirudin monotherapy compared to heparin plus GPI, and reduced rates of reinfarction and recurrent ischaemia necessitating repeat revascularization procedures with PES rather than BMS.
Consistent with prior studies, patients with LV dysfunction in the present study more frequently had co-existent clinical comorbidities and adverse angiographic characteristics.6,12 Many of these, such as advanced age, prior MI, female sex, presentation in Killip class 3 or 4, left anterior descending artery involvement, and an occluded infarct vessel at baseline, have also been associated with increased mortality.2,5,7,14,15,21–25 However, after adjusting for these variables, LVEF measured during index procedure remained a powerful independent determinate of subsequent death, consistent with other studies that measured LVEF during the convalescent phase of STEMI.3–5,12,13,15 In this regard, our results are consistent with a retrospective analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial.12 This trial studied 1620 AMI patients that had baseline LVEF determined by left ventriculography during their primary PCI procedure, and reported that a baseline LVEF <40% compared to ≥40% was associated with decreased 30-day (93.7 vs. 99.1%; p=0.001) and 1-year survival (89.0 vs. 97.2%; p<0.0001).
Both HORIZONS-AMI and CADILLAC studies found that decreased LVEF was not associated with increased rates of reinfarction, revascularization, or stroke. In an earlier era, LV dysfunction had previously been implicated as an independent predictor of 30-day reinfarction after primary balloon angioplasty and BMS from the Primary angioplasty in Acute Myocardial Infarction (PAMI) trials.5 The 30 day reinfarction rate in the present study was 1.6%, somewhat lower than in the PAMI era (2.1%); the extent to which improved pharmacotherapy, devices, and technique may be resulting in greater freedom from reinfarction and a lesser effect of LV dysfunction is unknown. Furthermore, earlier studies suggested that patients with left ventricular dysfunction after myocardial infarction had higher rates of stroke.26–28 This may be related to the increased predisposition for LV thrombus development in patients with areas of LV akinesis or dyskinesis.29,30 In an observational analysis containing 2231 patients with LV dysfunction after an acute myocardial infarction in the Survival and Enlargement (SAVE) trial, a depressed LV ejection fraction was an independent predictor of stroke events during the 5-year follow-up period.28 Unlike earlier reports, our study did not detect any difference in stroke rates between patients with and without LV dysfunction.
Prior studies have shown that a strong association exists between bleeding complications, blood transfusions, and increased mortality in patients with acute coronary syndromes and in those undergoing PCI.31–36 A novel finding of the present study is that patients with severely reduced baseline LVEF had increased rates of non-CABG related major bleeding and blood transfusions, even after adjusting for other comorbidities such as advanced age and female sex which have also been associated with bleeding.31,32,34,37,38 While the causes of increased bleeding in patients with reduced systolic LV function are not known, the increased rate of major haemorrhagic complications and transfusions may be contributing to their poor survival in these patients.
Contrast left ventriculography performed during the index revascularization procedure can safely assess LVEF and provide important information to risk stratify patients, thereby facilitating the selection of therapies to improve the prognosis of these high-risk patients. Consistent with the findings from the overall HORIZONS-AMI trial, when treated with bivalirudin rather than heparin+GPI, patients with LVEF ≤40% had significantly improved survival and reduced bleeding complications. However, the absolute benefit in terms of improved survival in patients treated with bivalirudin rather than heparin+GPI was even greater in those with severely depressed left ventricular dysfunction (12.5% absolute improvement in 1-year survival, or number needed to treat (NNT) to save one life = 8) than in the entire study population (1.3%, NNT = 77).17 Finally, PES rather than BMS implantation led to decreased rates of ischaemia-driven TLR and TVR in patients with severe LV dysfunction, without any safety concerns apparent.
Several study limitations should be considered. This is a post-hoc analysis and is one of many, albeit pre-specified, substudies of the HORIZONS AMI Trial and as such should be considered hypothesis generating, and cannot exclude chance findings due to increased alpha error. However, the impact of LVEF on outcomes after STEMI is consistent with prior data and clinically plausible, despite the modest number of patients with LVEF <40% in the study and the fact that this substudy was not powered to determine the effect of therapies within this group. Given the relatively small subset of patients with severely reduced LV function, an additional sensitivity analysis was performed in which the study population was divided into approximately equal quartile populations. Patients in the lowest LVEF quartile continued to have higher rates of 30-day and 1-year NACE, MACE, and death (data not shown), thus demonstrating the robustness of this analysis despite the small population of patients with a LVEF ≤40%.
The results of this analysis can only be applied to patients who met the inclusion and exclusion criteria of the overall trial. Acute left ventriculography was only available and interpretable in 73.5% of patients; thus, an unknown bias may exist in this subset that might have influenced the results. However, this ascertainment rate is similar to that reported from other observational studies using convalescent LVEF determination,39 and similar to the rates reported in the CADILLAC trial (11%).12 LVEF was assessed using contrast left ventriculography in the acute phase; it is unknown whether LVEF studied using other modalities such as echocardiography, blood pool scintigraphy, or magnetic resonance provides the same prognostic value, and whether the predictive value of LVEF assessment is stronger in the acute or convalescent phases of STEMI. Also, other indicators of LV function, such as wall motion index,4,40 were not examined and might provide incremental information. Finally, left ventricular function was not systematically evaluated at follow-up visits; thus, no conclusions can be drawn regarding the impact of left ventricular function recovery.
These limitations notwithstanding, in the contemporary era of improved drugs and devices for the treatment of STEMI, patients presenting with a reduced LVEF continue to have increased rates of 30-day and 1-year all-cause and cardiac death, MACE, major bleeding, and NACE compared to patients with a normal global LVEF. LVEF determined by contrast left ventriculography during the acute index revascularization procedure can provide important risk stratification information to guide management strategies in STEMI. Furthermore, treatment with bivalirudin rather than heparin+GPI appears to provide substantial improvement in survival in these high-risk patients, and PES may safely be used to reduce recurrent ischaemia and the need for late repeat revascularization procedures.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The HORIZONS-AMI study was sponsored and funded by the Medicines Company (Parsippany, NJ) and Nycomed (Roskilde, Denmark).
References
- 1. Zaret BL, Wackers FJ, Terrin ML, et al. Value of radionuclide rest and exercise left ventricular ejection fraction in assessing survival of patients after thrombolytic therapy for acute myocardial infarction: results of Thrombolysis in Myocardial Infarction (TIMI) phase II study. The TIMI Study Group. J Am Coll Cardiol 1995; 26: 73–79 [DOI] [PubMed] [Google Scholar]
- 2. DeGeare VS, Boura JA, Grines LL, et al. Predictive value of the Killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2001; 87: 1035–1038 [DOI] [PubMed] [Google Scholar]
- 3. Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002; 39: 30–36 [DOI] [PubMed] [Google Scholar]
- 4. Moller JE, Egstrup K, Kober L, et al. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J 2003; 145: 147–153 [DOI] [PubMed] [Google Scholar]
- 5. Kernis SJ, Harjai KJ, Stone GW, et al. The incidence, predictors, and outcomes of early reinfarction after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2003; 42: 1173–1177 [DOI] [PubMed] [Google Scholar]
- 6. Marenzi G, Moltrasio M, Assanelli E, et al. Impact of cardiac and renal dysfunction on inhospital morbidity and mortality of patients with acute myocardial infarction undergoing primary angioplasty. Am Heart J 2007; 153: 755–762 [DOI] [PubMed] [Google Scholar]
- 7. van der Vleuten PA, Rasoul S, Huurnink W, et al. The importance of left ventricular function for long-term outcome after primary percutaneous coronary intervention. BMC Cardiovasc Disord 2008; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zijlstra F, Hoorntje JC, de Boer MJ, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1999; 341: 1413–1419 [DOI] [PubMed] [Google Scholar]
- 9. Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328: 673–679 [DOI] [PubMed] [Google Scholar]
- 10. Zijlstra F, de Boer MJ, Hoorntje JC, et al. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med 1993; 328: 680–684 [DOI] [PubMed] [Google Scholar]
- 11. St John Sutton M, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 1994; 89: 68–75 [DOI] [PubMed] [Google Scholar]
- 12. Halkin A, Stone GW, Dixon SR, et al. Impact and determinants of left ventricular function in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction. Am J Cardiol 2005; 96: 325–331 [DOI] [PubMed] [Google Scholar]
- 13. Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005; 45: 1397–1405 [DOI] [PubMed] [Google Scholar]
- 14. Nienhuis MB, Ottervanger JP, Dambrink JH, et al. Comparative predictive value of infarct location, peak CK, and ejection fraction after primary PCI for ST elevation myocardial infarction. Coron Artery Dis 2009; 20: 9–14 [DOI] [PubMed] [Google Scholar]
- 15. Mehta RH, O’Neill W W, Harjai KJ, et al. Prediction of one-year mortality among 30-day survivors after primary percutaneous coronary interventions. Am J Cardiol 2006; 97: 817–822 [DOI] [PubMed] [Google Scholar]
- 16. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) JACC 2004; 44(3): E1–E211 [DOI] [PubMed] [Google Scholar]
- 17. Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374: 1149–1159 [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med 2009; 360: 1946–1959 [DOI] [PubMed] [Google Scholar]
- 19. Mehran R, Brodie B, Cox DA, et al. The Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial: study design and rationale. Am Heart J 2008; 156: 44–56 [DOI] [PubMed] [Google Scholar]
- 20. Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008; 358: 2218–2230 [DOI] [PubMed] [Google Scholar]
- 21. Guagliumi G, Stone GW, Cox DA, et al. Outcome in elderly patients undergoing primary coronary intervention for acute myocardial infarction: results from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. Circulation 2004; 110: 1598–1604 [DOI] [PubMed] [Google Scholar]
- 22. Henriques JP, Zijlstra F, de Boer MJ, et al. The prognostic importance of heart failure and age in patients treated with primary angioplasty. Eur J Heart Fail 2003; 5: 291–294 [DOI] [PubMed] [Google Scholar]
- 23. Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation 1995; 91: 1659–1668 [DOI] [PubMed] [Google Scholar]
- 24. Kandzari DE, Tcheng JE, Gersh BJ, et al. Relationship between infarct artery location, epicardial flow, and myocardial perfusion after primary percutaneous revascularization in acute myocardial infarction. Am Heart J 2006; 151: 1288–1295 [DOI] [PubMed] [Google Scholar]
- 25. De Luca G, Ernst N, van’t Hof AW, et al. Predictors and clinical implications of early reinfarction after primary angioplasty for ST-segment elevation myocardial infarction. Am Heart J 2006; 151: 1256–1259 [DOI] [PubMed] [Google Scholar]
- 26. Dunkman WB, Johnson GR, Carson PE, et al. Incidence of thromboembolic events in congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation 1993; 87: 533–540 [PubMed] [Google Scholar]
- 27. Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med 1997; 336: 251–257 [DOI] [PubMed] [Google Scholar]
- 28. Lamas GA, Vaughan DE, Pfeffer MA. Left ventricular thrombus formation after first anterior wall acute myocardial infarction. Am J Cardiol 1988; 62: 31–35 [DOI] [PubMed] [Google Scholar]
- 29. Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100: 789–794 [DOI] [PubMed] [Google Scholar]
- 30. Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003; 92: 930–935 [DOI] [PubMed] [Google Scholar]
- 31. Rao SV, O’Grady KO, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 2005; 96: 1200–1206 [DOI] [PubMed] [Google Scholar]
- 32. Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49: 1362–1368 [DOI] [PubMed] [Google Scholar]
- 33. Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol 2007; 100: 1364–1369 [DOI] [PubMed] [Google Scholar]
- 34. Bertrand OF, Larose E, Rodes-Cabau J, et al. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J 2009; 157: 164–169 [DOI] [PubMed] [Google Scholar]
- 35. Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292: 1555–1562 [DOI] [PubMed] [Google Scholar]
- 36. Aronow HD, Steinhubl SR, Brennan DM, et al. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J 2009; 157: 369–374 [DOI] [PubMed] [Google Scholar]
- 37. Iijima R, Ndrepepa G, Mehilli J, et al. Profile of bleeding and ischaemic complications with bivalirudin and unfractionated heparin after percutaneous coronary intervention. Eur Heart J 2009; 30: 290–296 [DOI] [PubMed] [Google Scholar]
- 38. Santolucito PA, Tighe DA, Lessard D, et al. Changing trends in the evaluation of ejection fraction in patients hospitalized with acute myocardial infarction: the Worcester Heart Attack Study. Am Heart J 2008; 155: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galasko GI, Basu S, Lahiri A, et al. A prospective comparison of echocardiographic wall motion score index and radionuclide ejection fraction in predicting outcome following acute myocardial infarction. Heart 2001; 86: 271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]


