Abstract
Aims:
The impact of plasma osmolality on clinical outcome in acute coronary syndrome (ACS) patients has not been investigated so far.
Methods:
In a retrospective analysis, we included 985 patients with ACS undergoing percutaneous coronary intervention (PCI). Plasma osmolality was calculated using concentrations of sodium, plasma glucose, and blood urea nitrogen at admission. Patients were stratified by quartiles (Q) of admission osmolality, clinical outcome was compared between those groups. The primary endpoints were in-hospital, 30-day, and 1-year mortality.
Results:
Univariate analysis in the Cox proportional-hazards model revealed significantly higher rates of in-hospital death for patients with osmolality in Q4, as compared to patients with osmolality in Q1–3 (HR 5.4, 95% CI 3.3–9.0, p<0.01). After adjustment for confounding baseline variables, osmolality in Q4 was associated with 2.8-fold hazard of in-hospital death (HR 2.75, 95% CI 1.35–5.61, p=0.005). Upon multivariate analysis, admission osmolality in Q4 vs. Q1–3 was associated with higher mortality rates after 30 days (HR 2.53, 95% CI 1.23–5.21, p=0.012) and 1 year (HR 1.73, 95% CI 1.02–2.91, p=0.04). Moreover, we performed landmark analysis in order to exclude critically ill patients, which revealed similar adjusted rates of death beyond 30 days to 1 year (HR 1.21, 95% CI 0.55–2.66, p=0.642).
Conclusions:
Using the 4th quartile of plasma osmolality at admission as a natural cut-off point, osmolality in Q4, as compared to Q1–3, was significantly predictive of short term but not long-term outcome in ACS patients undergoing coronary stenting. Our data suggest osmolality to be an independent, feasible, and cost-effective tool for rapid risk stratification in ACS patients.
Keywords: Acute coronary syndrome, osmolality, risk stratification
Introduction
Risk stratification of patients admitted with acute coronary syndrome (ACS) is essential to provide optimal treatment. Therefore numerous biomarkers and clinical characteristics have been identified in order to improve risk-factor-guided therapy.1–8 The most important clinical characteristics that are strongly associated with increased mortality, both in ACS patients presenting with and without persistent ST-segment elevation, are age, elevated heart rate, low systolic blood pressure, and signs of heart failure.4,9
In clinical practice, serum biomarkers are popular for risk estimation, since those are sensitive and specific for ACS while additionally correlating well with outcome. Thus, cardiac troponin is not only useful for diagnostic purposes but also independently predicts rates of death in a step-wise fashion for each ng/ml increase.2 While B-type natriuretic peptide (BNP) has its primary implications in guiding heart failure treatment,10,11 it is also a relevant marker for the prediction of death, chronic heart failure, and recurrent myocardial infarction in ACS patients.6,12 Given that the routine measurement of BNP is costly, other tools for rapid risk stratification are needed.
Several studies suggested that in patients presenting with acute myocardial infarction, elevated plasma glucose at admission is associated with increased mortality.3,5,8 Those findings were more distinct in patients without known diabetes.5,8 Moreover, the relationship between impaired renal function and worse clinical outcome is well established.1,13 Importantly, elevated blood urea nitrogen (BUN) is highly predictive of mortality, myocardial infarction, and stroke, independently of serum creatinine, estimated glomerular filtration rate (GFR), and other biomarkers.7
Hence, the incorporation of plasma glucose and BUN into a single marker may yield a higher predictive accuracy as well as feasible application in clinical practice among different subgroups of ACS patients.
As plasma glucose, BUN, and sodium are the main components driving plasma osmolality, we sought to assess the impact of admission osmolality on hard clinical endpoints in ACS patients referred for percutaneous coronary intervention (PCI). To the best of our knowledge, this has not been investigated so far.
Methods
The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (EK 10-046-VK_NZ).
Patients
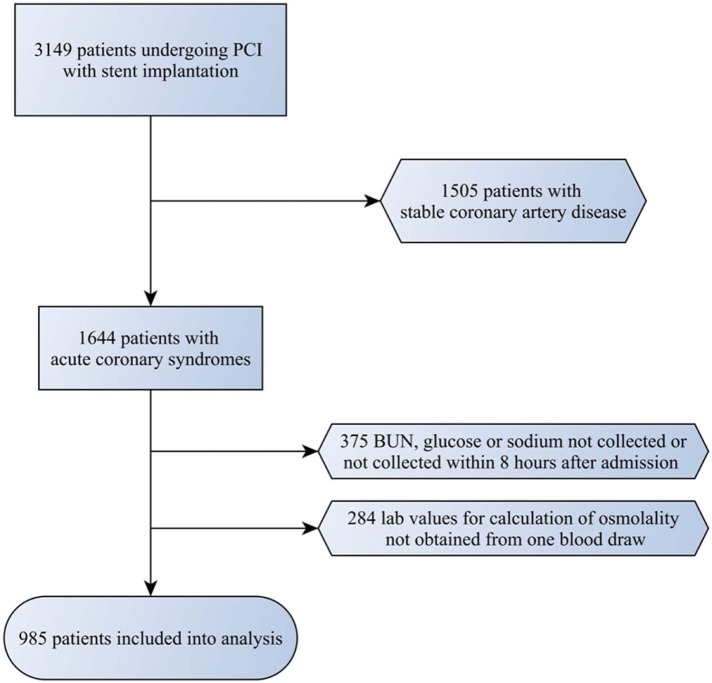
In this post-hoc analysis of a permanent prospective registry, we included 985 patients with ACS, who were referred to our tertiary referral centre for PCI with stent implantation between 2004 and 2011 (Figure 1). We included patients presenting with ST-segment elevation of ≥1 mm in two or more contiguous leads and patients with elevation in troponin I, troponin T or creatine kinase MB levels (CK-MB) above the upper limit of normal and/or ST-segment depression of ≥1 mm. Patients lacking laboratory or electrocardiographic evidence suggestive of myocardial infarction were excluded from this analysis.
Figure 1.
Selection of patients included into the final analysis.
Laboratory results, clinical characteristics, cardiovascular risk factors, comorbidities, coronary morphology, and medication at hospital discharge were registered for all patients. Providing the highest accuracy between multiple calculation methods,14 the following formula was used to assess plasma osmolality at admission: osmolality = 1.86×sodium mmol/l + (glucose mg/dl/18) + (BUN mg/dl/2.8) + 9.
Patients with missing results for sodium, plasma glucose, or BUN within the first 8 hours of admission were excluded, as well as patients where the results were not obtained from the very same blood draw. Iopamidol 300 mg iodine/ml, a nonionic, low osmolal contrast agent, was used in all patients (616 mosmol/kg).
Patients were stratified by quartiles (Q) of osmolality at admission with low osmolality representing the first quartile and high osmolality representing the fourth quartile. All predefined endpoints were compared between those groups.
Endpoints
The primary endpoints were in-hospital, 30-day, and 1-year mortality. Mortality data for all patients were obtained from Statistics Austria. Statistics Austria is an independent and nonprofitmaking federal institution under public law and supports scientific services.
Statistical methods
Descriptive statistics were performed on baseline variables and stratified by quartiles of osmolality. Discrete characteristics are expressed as frequency counts and percentages, differences between groups were determined with the chi-squared test. Continuous characteristics are expressed as medians and quartiles and differences in those variables were examined with the Kruskal–Wallis test throughout all groups. The level of significance used for all tests was a two-sided p-value of ≤0.05.
The Cox proportional-hazards model was chosen for survival analysis. For the inclusion into the model, confounding variables were screened for univariate association with in-hospital, 30-day and 1-year mortality, applying a two-sided p-value ≤0.05. Known correlates of risk and expected confounders (schock, age, estimated GFR, presence of diabetes, clinical presentation (STEMI, NSTEMI), and heart failure) were forced into the model.
Subgroup analysis was performed by stratifying for renal function and the presence of diabetes mellitus. Estimated eGFR was calculated using the Cockcroft–Gault formula. Quartiles of osmolality were calculated for each individual subgroup, survival analysis was then performed using the Cox proportional-hazards model as described above.
Software Package for Social Sciences version 19 (SPSS, Chicago, IL, USA) was used for all statistical calculations.
Results
Registered baseline characteristics included cardiovascular risk factors, comorbidities, coronary morphology, medication at hospital discharge, and laboratory findings and are listed in Table 1.
Table 1.
Patient characteristics, stratified by quartiles of osmolality at admission.
| 1 (n=246) | 2 (n=246) | 3 (n=246) | 4 (n=247) | p-value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age (years, median) | 62 | 61 | 62 | 69 | <0.001 |
| Gender (%) | |||||
| Male | 66.50 | 67.20 | 66.90 | 61.90 | |
| Female | 33.50 | 32.80 | 33.10 | 38.10 | |
| eGFR (ml/min, median) | 93.96 | 95.35 | 84.45 | 62.04 | <0.001 |
| Baseline creatinine (mg/dl, median) | 0.9 | 0.9 | 0.94 | 1.19 | <0.001 |
| BMI (kg/m2, median) | 26.86 | 27.35 | 26.98 | 26.76 | 0.22 |
| Heart rate (bpm, median) | 78 | 75 | 76 | 79.5 | 0.17 |
| SBP (mmHg, median) | 133 | 137 | 135 | 130 | 0.13 |
| CRP (mg/l, median) | 7 | 4.7 | 3.9 | 5.9 | 0.01 |
| Admission troponin I (ng/l, median) | 1.37 | 0.51 | 0.72 | 0.35 | 0.34 |
| Peak troponin I (ng/l, median) | 24.65 | 20.62 | 16.67 | 23.87 | 0.34 |
| Peak CK-MB (U/l, median) | 156 | 148 | 140 | 187.5 | 0.12 |
| Cardiovascular risk factors (%) | |||||
| CAD family history | 22.40 | 17.10 | 17.10 | 13.40 | 0.07 |
| Hypertension | 72.00 | 76.40 | 73.60 | 70.90 | 0.53 |
| Hyperlipidaemia | 72.40 | 80.90 | 76.80 | 72.10 | 0.07 |
| Diabetes | 15.00 | 20.70 | 26.40 | 30.40 | <0.001 |
| Smoking | |||||
| Current | 43.50 | 43.50 | 35.40 | 27.10 | <0.001 |
| Prior | 8.90 | 12.60 | 8.90 | 8.90 | |
| Comorbidities | |||||
| Heart failure (LVEF <45%) | 9.00 | 7.00 | 10.30 | 16.30 | 0.02 |
| Previous MI | 11.40 | 11.80 | 11.80 | 13.40 | 0.91 |
| Previous PCI | 8.10 | 7.30 | 11.80 | 10.50 | 0.29 |
| Previous CABG | 1.60 | 2.80 | 1.60 | 3.20 | 0.52 |
| Atrial fibrillation | 6.90 | 6.50 | 5.70 | 10.50 | 0.18 |
| PAD | 6.90 | 1.20 | 4.90 | 5.70 | 0.02 |
| Prior stroke or TIA | 4.50 | 3.70 | 6.90 | 8.10 | 0.12 |
| History for malignant tumours | 8.10 | 5.70 | 2.40 | 4.00 | 0.03 |
| Clinical presentation | |||||
| NSTEMI | 32.10 | 43.10 | 34.10 | 27.10 | 0.002 |
| STEMI | 67.90 | 56.90 | 65.90 | 72.90 | |
| Vessel disease (stenosis > 50%) | |||||
| 1 vessel | 55.70 | 54.60 | 58.60 | 44.90 | 0.17 |
| 2 vessels | 29.70 | 29.20 | 25.60 | 34.30 | |
| 3 vessels | 14.60 | 16.20 | 15.80 | 20.70 | |
| Stent type | |||||
| DES | 25.80 | 26.80 | 30.20 | 27.20 | 0.79 |
| BMS | 74.20 | 73.20 | 69.80 | 72.80 | |
| Shock | 8.50 | 4.50 | 6.10 | 21.10 | <0.001 |
| Baseline values for osmolality calculation | |||||
| Osmolality (mosmol/kg, median) | 275.53 | 281.5 | 285.37 | 291.77 | <0.001 |
| BUN (mg/dl, median) | 14 | 16 | 17 | 23 | <0.001 |
| Sodium (mmol/l, median) | 136 | 140 | 141 | 143 | <0.001 |
| Glucose (mg/dl, median) | 117 | 119 | 129 | 156 | <0.001 |
| Discharge medication (%) | |||||
| ACEIs | 58.60 | 53.30 | 55.90 | 55.80 | 0.73 |
| ARBs | 8.00 | 14.50 | 7.50 | 9.20 | 0.05 |
| Diuretics | 29.40 | 31.70 | 30.30 | 38.90 | 0.21 |
| Statins | 96.00 | 96.90 | 95.20 | 93.90 | 0.47 |
| Beta-blockers | 85.50 | 84.30 | 86.80 | 83.20 | 0.75 |
| Oral anticoagulation | 1.30 | 2.20 | 3.60 | 3.60 | 0.38 |
Comparisons throughout all groups were performed with the Chi-squared test for discrete characteristics or the Kruskal–Wallis test for continuous characteristics.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BMS, bare metal stent; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CAD, coronary artery disease; CK-MB, creatine kinase-myocardial band; CRP, C-reactive protein; DES, drug-eluted stent; eGFR, estimated glomerular filtration rate (by Cockcroft Gault formula); LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischaemic attack.
From 985 patients presenting with ACS and undergoing PCI plus stent implantation, myocardial infarction with ST-segment elevation was present in 649 (65.9%) patients, while 336 (34.1%) patients presented without persistent ST-segment elevation. Although the distribution of clinical presentation was significantly different between quartiles of osmolality (p=0.002), there was no trend towards increased rates of STEMI with increasing osmolality (p for trend = 0.076).
Age was comparable in Q1–3 (median 62 years, p=0.58); however, patients in Q4 were significantly older (median 69 years, p<001). Likewise, glomerular filtration rate (GFR) was similar in Q1–3 (median 91 ml/min, p=0.33) but was lower in patients with admission osmolality in Q4 (median 62 ml/min, p<0.001).
Further, patients in Q4, as opposed to Q1–3, were more likely to have diabetes (30.4 vs. 20.7%, p=0.002), heart failure (16.3 vs. 8.8%, p=0.003), and to be in cardiogenic shock at any time during hospitalisation (21.1 vs. 6.4%, p<0.001). Moreover, there were significant differences regarding current smoking, peripheral artery disease, and history for malignant tumours between the groups.
Gender and the prevalence of hypertension and hyperlipidaemia were equally distributed. Discharge medication was similar between groups with respect to beta-blockers, angiotensin-converting enzyme inhibitors, diuretics, and statins.
Median osmolality in the entire cohort was 283.4 mosmol/kg (IQR 279.0; 287.9). All three components used for the calculation of osmolality (sodium, glucose, and BUN) increased in a step-wise fashion throughout quartiles of osmolality (p for trend <0.01 for all calculations), as shown in Table 1. Median osmolality in Q1–3 was 281.5 mosmol/kg (range 251.5–287.9 mosomol/kg). Median osmolality in Q4 was 291.8 msomol/kg (range 287.9–368.9 mosmol/kg).
Receiver operating characteristics (ROC) analysis revealed that a cut-off value of 286.22 mosmol/kg would yield the best sensitivity/specificity relation, which was similar to the 75th percentile (287.9 mosmol/ kg).
In STEMI patients, the majority of blood draws (> 90%) were taken at first contact with the patient in the intensive care unit or emergency department. In the minority of the cases, those values were obtained shortly after PCI, but never during the procedure.
In NSTEMI patients, the respective blood draws were taken at first contact in approximately 50% of the cases, but in 80 % before coronary angiography. The remaining results were obtained after angiography, but within 8 hours after admission.
Mortality
Rates of death for all endpoints and multivariate predictors included into the Cox proportional-hazards model are presented in Tables 2 and 3, respectively. Adjusted survival curves for all endpoints are depicted in Figures 2–4.
Table 2.
Rates of death, stratified by quartiles of osmolality at admission in the overall cohort.
| 1 (n=246) | 2 (n=246) | 3 (n=246) | 4 (n=247) | |
|---|---|---|---|---|
| In-hospital mortality | 9 (3.7) | 9 (3.7) | 6 (2.4) | 41 (16.6) |
| 30-day mortality | 7 (2.9) | 9 (3.7) | 6 (2.5) | 38 (15.5) |
| 1-year mortality | 18 (7.3) | 15 (6.1) | 17 (6.9) | 55 (22.3) |
| 30-day to 1-year mortality | 11 (4.7) | 6 (2.6) | 11 (4.7) | 17 (8.2) |
Values are n (%).
Table 3.
Multivariate predictors in the Cox proportional-hazards model.
| HR (95% CI) | p-value | |
|---|---|---|
| In-hospital mortality | ||
| Q4 vs. Q1–Q3 | 2.751 (1.349–5.612) | 0.005 |
| 1-vessel disease | 0.919 | |
| 2-vessel disease | 1.162 (0.512–2.637) | 0.719 |
| 3-vessel disease | 1.01 (0.439–2.323) | 0.981 |
| Smoking | 1.754 (0.841–3.656) | 0.134 |
| STEMI vs. NSTEMI | 1.953 (0.802–4.755) | 0.14 |
| Age | 1.011 (0.974–1.049) | 0.574 |
| eGFR | 0.966 (0.949–0.983) | <0.01 |
| Heart failure | 0.408 (0.167–0.998) | 0.049 |
| Diabetes | 1.722 (0.81–3.663) | 0.158 |
| Shock | 14.429 (6.864–30.334) | <0.01 |
| 30-day mortality | ||
| Q4 vs. Q1–Q3 | 2.531 (1.23–5.205) | 0.012 |
| 1-vessel disease | 0.664 | |
| 2-vessel disease | 1.483 (0.632–3.482) | 0.365 |
| 3-vessel disease | 1.253 (0.531–2.959) | 0.606 |
| Smoking | 1.932 (0.919–4.062) | 0.082 |
| STEMI vs. NSTEMI | 1.827 (0.746–4.478) | 0.187 |
| Age | 1.012 (0.974–1.051) | 0.55 |
| eGFR | 0.962 (0.945–0.98) | <0.01 |
| Heart failure | 0.394 (0.153–1.015) | 0.054 |
| Diabetes | 1.543 (0.715–3.332) | 0.269 |
| Shock | 11.798 (5.606–24.833) | <0.01 |
| 1-year mortality | ||
| Q4 vs. Q1–Q3 | 1.726 (1.024–2.907) | 0.04 |
| 1-vessel disease | 0.81 | |
| 2-vessel disease | 1.013 (0.552–1.856) | 0.968 |
| 3-vessel disease | 1.2 (0.653–2.204) | 0.557 |
| Smoking | 1.146 (0.64–2.053) | 0.646 |
| STEMI vs. NSTEMI | 1.082 (0.613–1.911) | 0.786 |
| Age | 1.035 (1.004–1.066) | 0.026 |
| eGFR | 0.979 (0.966–0.992) | 0.002 |
| Heart failure | 0.673 (0.351–1.292) | 0.234 |
| Diabetes | 1.262 (0.697–2.283) | 0.442 |
| Shock | 12.409 (7.164–21.494) | <0.01 |
| Atrial fibrillation | 0.495 (0.214–1.144) | 0.1 |
| 30-day to 1-year landmark analysis | ||
| Q4 vs. Q1–Q3 | 1.206 (0.547–2.656) | 0.642 |
| Smoking | 0.633 (0.24–1.673) | 0.357 |
| STEMI vs. NSTEMI | 0.767 (0.356–1.656) | 0.5 |
| Age | 1.083 (1.034–1.135) | 0.001 |
| eGFR | 1.007 (0.989–1.026) | 0.438 |
| Heart failure | 1.413 (0.554–3.603) | 0.469 |
| Diabetes | 0.785 (0.313–1.966) | 0.605 |
| Shock | 8.951 (3.85–20.81) | <0.01 |
| Atrial fibrillation | 1.399 (0.496–3.948) | 0.526 |
eGFR, estimated glomerular filtration rate; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Figure 2.
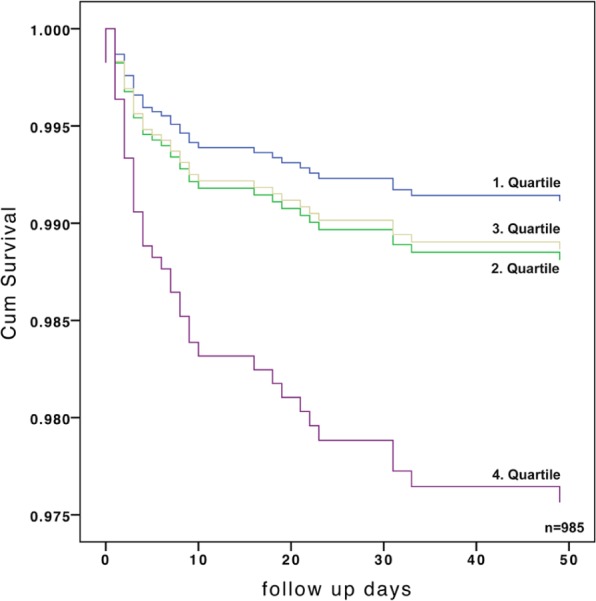
Adjusted in-hospital mortality, stratified by quartiles of admission osmolality.
HR 2.75 (95% CI 1.35–5.61); p=0.005.
Figure 3.
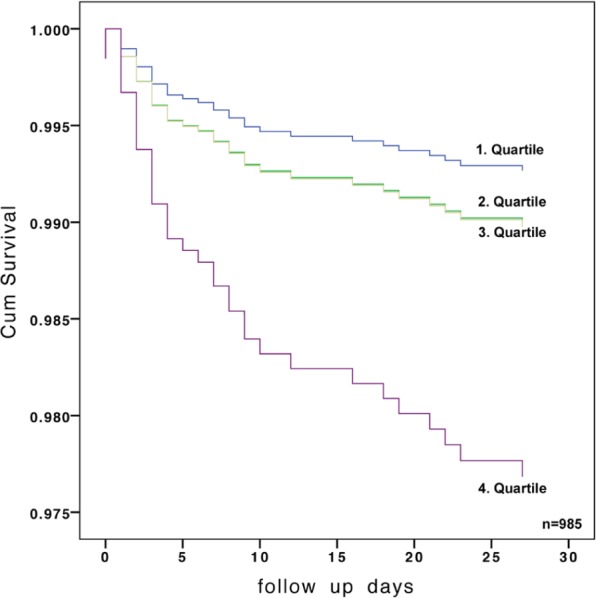
Adjusted 30-day mortality, stratified by quartiles of admission osmolality.
HR 2.53, (95% CI 1.23–5.2); p=0.012.
Figure 4.
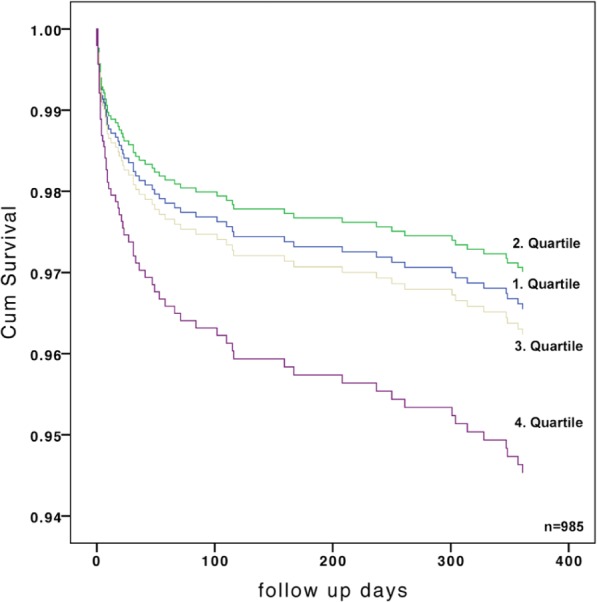
Adjusted 1-year mortality, stratified by quartiles of admission osmolality.
HR 1.73 (95% CI 1.02–2.91); p=0.04 for quartile 4 vs. quartiles 1–3. In order to exclude critically ill patients, landmark analysis from 30 days to 1 year was performed, demonstrating similar rates of death for quartile 4 vs. quartiles 1–3: HR 1.21 (95% CI 0.55–2.66); p=0.642).
Short-term mortality
Since similar rates of death for Q1–3 could be observed (p=0.8), those groups were combined for further analysis. Univariate analysis in the Cox proportional-hazards model revealed significantly higher rates of in-hospital death for patients admitted with osmolality in Q4, as compared to patients with osmolality in Q1–3 (HR 5.4, 95% CI 3.3–9.0, p<0.01). After adjustment for confounding baseline variables this association remained significant. Osmolality in Q4 was associated with a 2.8-fold hazard of in-hospital death (HR 2.75, 95% CI 1.35–5.61, p=0.005). Likewise, patients with admission osmolality in Q4 had significantly higher adjusted 30-day mortality rates, opposed to Q1–3 (HR 2.53, 95% CI 1.23–5.21, p=0.012). When additionally forcing peak troponin I or peak creatine kinase-myocardial band (CK-MB) concentrations into the multivariate model, no changes in significance could be observed (including troponin: HR 2.67, 95% CI 1.26;5.64, p=0.010 for in-hospital mortality and HR 2.41, 95% CI 1.13;5.16, p=0.023 for 30-day mortality; including CK-MB: HR 2.85, 95% CI 1.35;6.05, p=0.006 for inhospital mortality and HR 2.81, 95%CI 1.28;6.17, p=0.010 for 30-day mortality).
One-year mortality
Upon multivariate analysis, admission osmolality in Q4 vs. Q1–3 was associated with higher mortality rates after 1 year of follow up (HR 1.73, 95% CI 1.02–2.91, p=0.04). Results remained significant when including peak CK-MB concentrations into the multivariate model, however, significance was lost after adding peak troponin I levels (including troponin: HR 1.58, 95% CI 0.91;2.75, p=0.102; including CK-MB: HR 2.09, 95% CI 1.18;3.72, p=0.012)
Landmark analysis
In order to exclude critically ill patients, we performed landmark analysis from 30 days to 1 year of follow up, which revealed similar adjusted mortality rates for patients with admission osmolality in Q4 vs. Q1–3 (HR 1.21, 95% CI 0.55–2.66, p=0.642).
Subgroup analysis
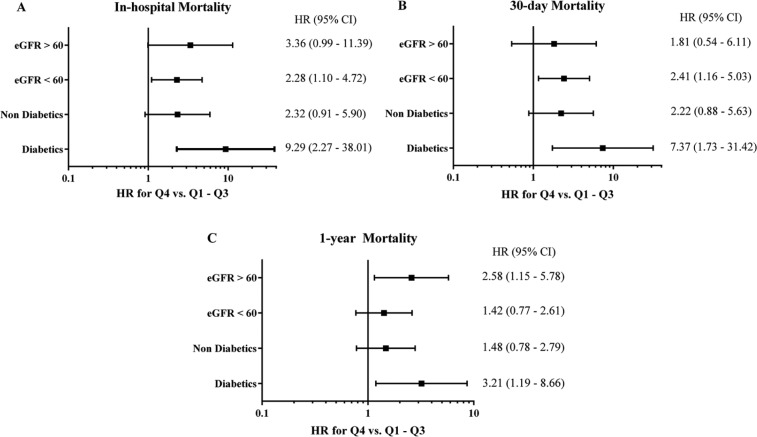
Subgroup analysis for in-hospital, 30-day, and 1-year mortality was performed stratifying for diabetes mellitus and renal function. Outcomes in the Cox proportional-hazards model are presented in Figure 5; multivariate predictors with HRs and CIs can be found in the Appendix (available online). Owing to the lower number of cases and events in the individual subgroups, results did not all remain significant after adjustment. However, there was a trend towards increased rates of mortality in Q4 vs. Q1–3 for all endpoints, irrespective of the presence of diabetes or impaired renal function.
Figure 5.
Adjusted hazard ratios for subgroup analysis of in-hospital (A), 30-day (B), and 1-year (C) rates of all-cause death, stratified by diabetes and estimated glomerular filtration rates.
Adjusted hazard ratios (HR) and confidence intervals calculated by the Cockcroft–Gault formula. Rates of all-cause death are shown for the comparison between admission osmolality in quartile 4 vs. quartiles 1–3. Diabetics, n=228; nondiabetics, 744 eGFR <60 ml/min, 246 eGFR ≥60 ml/min, 664. eGFR, estimated glomerular filtration rate.
Discussion
The main finding of this post-hoc analysis of a permanent prospective registry is the strong association between admission osmolality and all-cause death in ACS patients undergoing PCI. Admission osmolality in the uppermost quartile was highly and independently predictive of in-hospital, 30-day, and 1-year outcome after adjustment for confounders. However, after exclusion of critically ill patients who died within 30 days of follow up, mortality was similar between groups.
Subgroup analysis revealed that the overall trend seems to be independent of the presence of diabetes and renal impairment, although only partly significant owing to lower sample size and event rates in the respective groups.
Interestingly, median osmolality in the uppermost quartile was 292 mosomol/kg, hence below the widely accepted cut-off value of 295 mosomol/kg, and only 28% of patients in Q4 exceeded latter.15 Since we used calculated rather than measured osmolality, a physiological osmolal gap up to 10 momsol/kg has to be considered.16 Nevertheless, we want to emphasise that largely physiological osmolality values, even though close to the upper limit within the normal range, accounted for our results.
The data available on hyperosmolality in acute coronary syndrome is controversial. In animal studies, hyperosmotic pretreatment has been shown to reduce infarct size in the isolated rat heart.17 Consistently, mannitol pretreatment in the ischaemic myocardium of dogs strikingly reduced myocardial necrosis, an effect the authors attributed to the restoration of normal cell volume through hyperosmolality.18 Noteworthy, mannitol is an effective scavenger of the cytotoxic hydroxyl radical.19
On the other hand, hyperosmolality due to hyperglycaemia has been shown to have deleterious effects on survival of ACS patients, particularly in nondiabetics.3,5,8 Likewise, elevated levels of BUN, a major contributor to plasma osmolality, were highly predictive of mortality, recurrent myocardial infarction, and congestive heart failure after 30 days amongst patients with ACS.7,14 Those results were independent of serum creatinine-based estimated GFR, troponin-I, BNP, and C-reactive protein concentrations.7
Bhalla et al. investigated the impact of hyperosmolality in 167 patients admitted for acute stroke (89% ischaemic stroke, 10% intracerebral haemorrhage, 1% unclassified). Mean admission and maximum osmolality, as well as the area under the curve for all measurements were significantly higher amongst patients who died after 3 months of follow up, compared to survivors.20 Osmolality greater than 296 mosmol/kg upon admission therefore resulted in a 2.4-fold increased risk of death.20
The present study is the first to establish the independent relationship between elevated admission osmolality and all-cause death in a cohort of ACS patients undergoing PCI. For the interpretation of our results, the determining components of osmolality and their exclusive impact on mortality have to be kept in mind.
Hyperglycaemia might partly reflect endogenous stress due to high catecholamine state and increased concentrations of circulating factors such as cortisol.8,21 Additionally, in acute myocardial infarction, hyperglycaemia has been associated with increased adipose tissue lipolysis, elevated plasma free fatty acid concentrations, suppression of insulin release, and reduced glucose uptake by the myocardium.8,21 Thus, utilisation of free fatty acids instead of glucose by ischaemic myocardium resulted in impaired regional metabolism along with increased oxygen consumption.8,21
However, conflicting data exist about the relevance of treatment for hyperglycaemia in ACS.8,22–25 Whereas in the DIGAMI trial, tight glycaemic control with insulin markedly improved survival in diabetics after myocardial infarction, the DIGAMI-2 trial failed to replicate those results.24,25 Likewise, in the HI-5 trial, mortality was similar in patients with or without diabetes upon insulin/dextrose infusion after myocardial infarction, compared to conventional treatment.22
In the setting of ACS, the association between renal impairment and worse clinical outcome is well established.1,13,26 Although BUN concentrations themselves, serum creatinine, estimated creatinine clearance and estimated GFR are imperfect measures of renal function, the particular pattern of BUN reabsorption plays a key role for its predictive value.7 Additionally to passive reabsorption of urea in the proximal nephron through solvent drag, in the distal tubule urea reabsorption is closely linked to water reabsorption under the influence of antidiuretic hormone, which in turn is regulated by angiotensin-II.7,27,28 Hence, reduced cardiac output or neurohumoral alterations resulting in renal hypoperfusion are reflected by BUN, irrespective of changes in serum creatinine or GFR, as urea reabsorption is triggered by the sympathetic nervous system and renin–angiotensin–aldosterone system, both established correlates of cardiovascular risk.7,28–30
Whether osmolality has an additive effect on mortality beyond that of the individual components, or the specific treatment for hyperosmolality improves survival, requires further investigations. However, our findings suggest osmolality to be a strong, independent marker for rapid risk stratification in ACS patients.
Limitations
Several limitations have to be considered. Firstly, data from the present study were collected in a single centre and analysed in a retrospective fashion. Significant and important differences were detected between study groups, which we had to statistically adjust for, including heart failure and shock, both previously associated with a disastrous prognosis.31,32 However, it should be mentioned that only 10.8% of patients in our analysis had indeed heart failure, therefore a firm conclusion (with respect to heart failure) out of statistical considerations can not be drawn. This is also represented by the fact that in our population heart failure was not associated with a worse prognosis following statistical adjustment.
Due to high correlations, we did not adjust for the contributors of osmolality included into the formula, therefore we did not identify the sole impact of osmolality, beyond that of the determining components.
In a quarter of all patients, the respective lab values were not obtained immediately upon admission, thus, treatment of patients might have influenced our findings.
Lastly, further investigations are needed to confirm our results. The underlying mechanisms remain elusive.
Conclusion
In conclusion, amongst ACS patients referred for PCI, admission plasma osmolality was highly predictive of in-hospital, 30-day, and 1-year clinical outcome; however, mortality rates were similar beyond 30 days of follow up. To the best of our knowledge, this is the first report on an association between plasma osmolality and all-cause death in ACS patients undergoing PCI. Our data suggest osmolality to be a feasible and cost-effective predictor of death in ACS patients. Whether targeted treatment for hyperosmolality would result in improved survival remains speculative. Nevertheless, our findings imply that incorporation of calculated osmolality on the lab sheet might help to identify patients at particular high risk.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Association for the Promotion of Research in Atherosclerosis, Thrombosis and Vascular Biology and the Ludwig Boltzmann Foundation for Cardiovascular Research.
References
- 1. Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation 2002; 106: 974–980 [DOI] [PubMed] [Google Scholar]
- 2. Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996; 335: 1342–1349 [DOI] [PubMed] [Google Scholar]
- 3. Bellodi G, Manicardi V, Malavasi V, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol 1989; 64: 885–888 [DOI] [PubMed] [Google Scholar]
- 4. Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000; 101: 2557–67 [DOI] [PubMed] [Google Scholar]
- 5. Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–778 [DOI] [PubMed] [Google Scholar]
- 6. de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001; 345: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 7. Kirtane AJ, Leder DM, Waikar SS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol 2005; 45: 1781–1786 [DOI] [PubMed] [Google Scholar]
- 8. Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation 2005; 111: 3078–3086 [DOI] [PubMed] [Google Scholar]
- 9. Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation 1995; 91: 1659–1668 [DOI] [PubMed] [Google Scholar]
- 10. Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000; 355: 1126–1130 [DOI] [PubMed] [Google Scholar]
- 11. Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug–partners in the diagnosis of congestive heart failure. Congest Heart Fail 2004; 10: 3–27 [DOI] [PubMed] [Google Scholar]
- 12. Galvani M, Ferrini D, Ottani F. Natriuretic peptides for risk stratification of patients with acute coronary syndromes. Eur J Heart Fail 2004; 6: 327–333 [DOI] [PubMed] [Google Scholar]
- 13. Gibson CM, Pinto DS, Murphy SA, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol 2003; 42: 1535–1543 [DOI] [PubMed] [Google Scholar]
- 14. Dorwart WV, Chalmers L. Comparison of methods for calculating serum osmolality form chemical concentrations, and the prognostic value of such calculations. Clin Chem 1975; 21: 190–194 [PubMed] [Google Scholar]
- 15. Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology, 12th ed. Philadelphia, PA: Saunders/Elsevier, 2011 [Google Scholar]
- 16. Hoffman RS, Smilkstein MJ, Howland MA, et al. Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol 1993; 31: 81–93 [DOI] [PubMed] [Google Scholar]
- 17. Falck G, Schjott J, Jynge P. Hyperosmotic pretreatment reduces infarct size in the rat heart. Physiol Res 1999; 48: 331–340 [PubMed] [Google Scholar]
- 18. Powell WJ, Jr, DiBona DR, Flores J, et al. The protective effect of hyperosmotic mannitol in myocardial ischemia and necrosis. Circulation 1976; 54: 603–615 [DOI] [PubMed] [Google Scholar]
- 19. Magovern GJ, Jr, Bolling SF, Casale AS, et al. The mechanism of mannitol in reducing ischemic injury: hyperosmolarity or hydroxyl scavenger? Circulation 1984; 70: I91–I95 [PubMed] [Google Scholar]
- 20. Bhalla A, Sankaralingam S, Dundas R, et al. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke 2000; 31: 2043–2048 [DOI] [PubMed] [Google Scholar]
- 21. Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med 2002; 112: 305–311 [DOI] [PubMed] [Google Scholar]
- 22. Cheung NW, Wong VW, McLean M. The Hyperglycemia:Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006; 29: 765–770 [DOI] [PubMed] [Google Scholar]
- 23. Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 1997; 314: 1512–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65 [DOI] [PubMed] [Google Scholar]
- 25. Malmberg K, Ryden L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26: 650–661 [DOI] [PubMed] [Google Scholar]
- 26. Masoudi FA, Plomondon ME, Magid DJ, et al. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J 2004; 147: 623–629 [DOI] [PubMed] [Google Scholar]
- 27. Conte G, Dal Canton A, Terribile M, et al. Renal handling of urea in subjects with persistent azotemia and normal renal function. Kidney Int 1987; 32: 721–727 [DOI] [PubMed] [Google Scholar]
- 28. Usberti M, Federico S, Di Minno G, et al. Effects of angiotensin II on plasma ADH, prostaglandin synthesis, and water excretion in normal humans. Am J Physiol 1985; 248: F254–F259 [DOI] [PubMed] [Google Scholar]
- 29. Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med 2004; 116: 466–473 [DOI] [PubMed] [Google Scholar]
- 30. Dal Canton A, Fuiano G, Conte G, et al. Mechanism of increased plasma urea after diuretic therapy in uraemic patients. Clin Sci 1985; 68: 255–261 [DOI] [PubMed] [Google Scholar]
- 31. Bengtson JR, Kaplan AJ, Pieper KS, et al. Prognosis in cardiogenic shock after acute myocardial infarction in the interventional era. J Am Coll Cardiol 1992; 20: 1482–1489 [DOI] [PubMed] [Google Scholar]
- 32. Rector TS, Cohn JN. Prognosis in congestive heart failure. Annu Rev Med 1994; 45: 341–350 [DOI] [PubMed] [Google Scholar]