Abstract
Objectives
To compare thigh muscle intramuscular fat (intraMF) fractions and area between people with and without knee radiographic osteoarthritis (ROA); and to evaluate the relationships of quadriceps adiposity and area with strength, function and knee MRI lesions.
Methods
Ninety six subjects (ROA: KL >1; n = 30, control: KL = 0,1; n = 66) underwent 3-Tesla MRI of the thigh muscles using chemical shift-based water/fat MR imaging (fat fractions) and the knee (clinical grading). Subjects were assessed for isometric/isokinetic quadriceps/hamstrings strength, function (KOOS, stair climbing test [SCT], and 6-minute walk test [(6MWT]. Thigh muscle intraMF fractions, muscle area and strength, and function were compared between controls and ROA subjects, adjusting for age. Relationships between measures of muscle fat/area with strength, function, KL and lesion scores were assessed using regression and correlational analyses.
Results
The ROA group had worse KOOS scores but SCT and 6MWT were not different. The ROA group had greater quadriceps intraMF fraction but not for other muscles. Quadriceps strength was lower in ROA group but the area was not different. Quadriceps intraMF fraction but not area predicted self-reported disability. Aging, worse KL, and cartilage and meniscus lesions were associated with higher quadriceps intraMF fraction.
Conclusion
Quadriceps intraMF is higher in people with knee OA and is related to symptomatic and structural severity of knee OA, where as the quadriceps area is not. Quadriceps fat fraction from chemical shift-based water/fat MR imaging may have utility as a marker of structural and symptomatic severity of knee OA disease process.
Keywords: quadriceps strength, water/fat imaging, hamstrings, cartilage
INTRODUCTION
Considerable research has focused on the characterization of morphological and compositional changes in cartilage and bone in knee osteoarthritis (OA). [1] However, little attention has been paid to the quantification of adipose tissue at the thigh despite the strong relationship between obesity and knee OA. [2] Greater thigh adiposity is known to be associated with lower strength, worse mobility, and worse lipoprotein profiles in older adults. [3–5] Lower leg lean mass has been shown to be related to a greater risk of incident radiographic knee OA (ROA).[6] Using computed tomography (CT), Conroy et al. found that people with ROA had greater whole body lean and muscle tissue, greater quadriceps cross-sectional area and lower quadriceps specific torque (torque per unit muscle area). [7] Using T1-weighted magnetic resonance (MR) images from the Osteoarthritis Initiative (OAI) datasets, women with ROA were found to have greater intermuscular fat volume; and greater inermuscular fat volume had weak association with lower quadriceps strength and worse physical performance. [8]
CT based techniques for quantifying muscle adiposity require exposure to ionizing radiation and the conventional T1-weighted MR imaging does not allow an accurate determination of intermuscular adipose tissue (IMAT) in localized regions. [9, 10] In T1-weighted images, the IMAT [11] includes the visible fat signal both within the muscles (intramuscular fat) and between the muscles (intermuscular fat). Chemical shift-based water/fat separation methods, including Dixon techniques [12, 13] and the iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) [14], provide a valuable alternative approach for quantification of fatty infiltration. These techniques overcome the limitations of conventional T1-weighted imaging by allowing high spatial resolution for quantification of adipose tissue in localized regions. Chemical shift-based water/fat separation techniques have been used for quantification of fat fractions at the liver [15], with very good agreement with MR spectroscopy. [16] Using this technique, we have earlier found individuals with diabetes to have greater intramuscular fat in the calf muscles when compared with controls, but no difference in intermuscular fat. [17] Quantitative measures of intramuscular fat were observed to correlate very well with established semi-quantitative grading of fatty infiltration at the calf and the shoulder. [18, 19]
In people with knee OA, quadriceps weakness is a ubiquitous clinical finding.[20] Loss of muscle tissue only partly explains the loss of strength in people with OA [7, 20] and fatty infiltration of thigh skeletal muscle is known to affect muscle strength and mobility in the elderly. [3, 4] However, quantitative MR imaging techniques have so far not been used to assess intramuscular fat of quadriceps in people with knee OA. Also, metrics of morphologic and compositional changes in knee cartilage, meniscus and bone with knee OA have not shown strong relationships with patient symptoms and functional outcomes. [21] If quantitative measures of quadriceps adiposity are related to patient symptoms and function, these measures may be used as determinants of the OA disease process. Hence, the aims of this study were (1) to quantify intramuscular fat and area of the quadriceps and other thigh muscles in individuals with and without ROA, and (2) to investigate the relationships between quadriceps fat fractions, muscle area, muscle strength, function and structural severity of knee OA.
PATIENTS AND METHODS
Subjects
Subjects were recruited from the community as a part of a larger study on knee OA. The inclusion criteria for OA patients were age > 35 years, frequent clinical symptoms of OA and radiographic signs of OA [22]. The controls were older than 35 years and without history of diagnosed OA, clinical OA symptoms, previous knee injuries, or signs of OA on radiographs. Standing radiographs using the fixed-flexion protocol [23] using a synaflexor device were obtained for all subjects to determine the Kellgren-Lawrence (KL) grade [24]. The 96 subjects (43 men, 53 women) participated in this cross-sectional study. Of these, 66 were classified as controls (KL=0, 1), and 30 were classified as having ROA (KL score >1). All subjects signed a written informed consent prior to participation in the study and all protocols were approved by a University of California, San Francisco Committee on Human Research.
MRI Acquisition
MRI was performed using a 3-Tesla GE Signa HDx MR Scanner (General Electric, Milwaukee, WI, USA) and an eight-channel transmit-receive knee coil (Invivo, Orlando, FL, USA). For the ROA subjects, the knee with more severe findings on the radiographs was imaged. In controls, the extremity was selected at random. For clinical grading, a high resolution 3-D T2-weighted fast spin echo sequence (Repetition Time [TR]/Echo Time [TE] = 1500/26.69 ms, matrix = 384 × 384, slice thickness = 0.5 mm, echo train length = 32, bandwidth = 37.5 kHz, NEX = 0.5, acquisition time = 10 min 30 sec) was used. For assessment of thigh adiposity and muscle cross-sectional area, the imaging was performed over a volume 14 cm (28 slices) proximal to the superior pole of the patella. Axial 2-D T1-weighted images (TR/TE = 600/5.52 ms, matrix = 384 × 192, slice thickness = 5 mm, echo train length = 7, bandwidth = 93.75 kHz, NEX = 2.0, acquisition time = 1 min 56 sec) were acquired for segmentation of thigh muscles. An investigational version of the chemical shift based water-fat separation method known as IDEAL [14], implemented in a multi-shot multi-echo 3D spoiled-gradient echo (SPGR) acquisition [25] (TR/TE = 11/1.31 ms, acquisition matrix = 180 × 180, slice thickness = 5 mm,, Flip angle = 3, bandwidth = 58.59 kHz, acquisition time = 3 min 00 sec), was used to measure fat content. The separation of water and fat signal was based on the IDEAL algorithm [14] with the multi-peak fat spectrum model and single T2* correction. [15]. In-phase images were calculated by taking the sum of the separated water and fat images. Out-of-phase images were also calculated by taking the absolute value to the difference of the separated water and fat images. Fat fraction images were generated by computing the ratio of the separated fat signal over the sum of the separated water and fat signals.
Semi-quantitative Clinical Grading of knee lesions
A modified-whole-organ magnetic resonance imaging score (mWORMS) University of California, San Francisco (UCSF) classification has been introduced by our research group in which the number of the anatomical compartments is reduced to 6 (patella, trochlea, medial femur, lateral femur, medial tibial, lateral tibia) [26, 27] from 15 in the original score. [28] In the WORMS scoring, higher scores reflect greater severity of the structural feature being reported. This classification system was used to assess severity of cartilage and meniscus and bone marrow lesions (BML), by board certified musculoskeletal radiologists (TML with 22 and LN with 6 years of experience). The radiologists were blinded to subject information and performed separate readings, with a consensus in case of disagreement. For each subject, the scores for all compartments (patella, trochlea, lateral/medial femur/tibia) were added to obtain a total score for each feature – cartilage, meniscus and BML.
Fat Fraction and Lean ACSA quantification
All analyses were performed in a custom written Matlab (Mathworks, Natick, MA, USA) program. Individual muscle regions of interest (ROIs) for quadriceps (vastus medials, vastus lateralis, vastus intermedius, rectus femoris), hamstrings (semimembranosus, semitendinosus, biceps femoris long head, biceps femoris short head), other muscle groups (adductor group, gracilis and sartorius) were manually segmented by a single trained researcher (WL) on the axial T1 weighted images. The volume segmented consisted of a 2 cm section (4 slices) between 10–12 cm proximal to superior pole of the patella. These segmentations were transferred to the fat fraction maps from the axial IDEAL images. For this study, the intramuscular fat fraction, intramuscular fat volume and lean anatomical cross-sectional area (Lean ACSA) variables were calculated for the quadriceps, hamstrings and other muscles compartments as well as for global (all muscles) compartment (Figure 1). The lean ACSA for each muscle was the area of the muscle minus the area of the intramuscular fat. The subcutaneous adipose tissue (SAT) and IMAT regions were segmented using an automatic algorithm published previously. [29] The intermuscular fat compartment consisted of the IMAT region outside of the muscle ROIs within the sub-facial layers (Figure 1). Volumes were calculated for both intermuscular fat and SAT compartments. The intraclass correlation coefficients (ICC) for intra-rater reproducibility was 0.97 (95% confidence intervals of 0.92, 0.99) for quadriceps intramuscular fat and 0.95 (95% confidence intervals of 0.85, 0.98) for quadriceps lean ACSA respectively.
FIGURE 1.
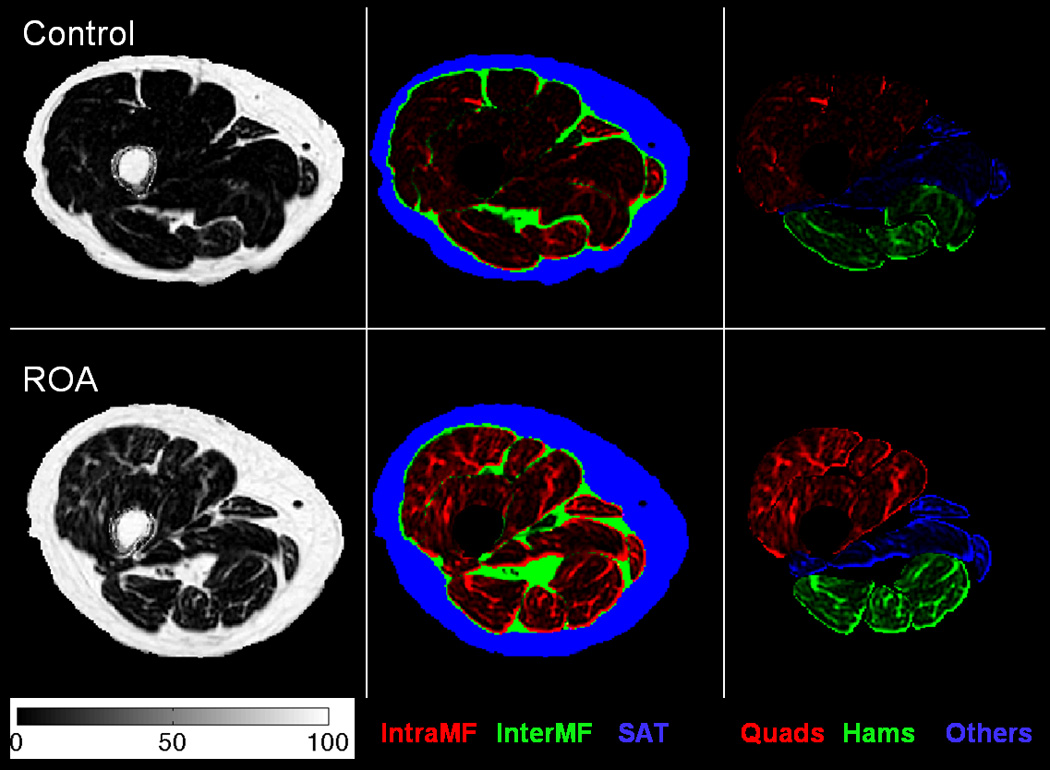
Comparison of fat distribution and fat content at a representative slice between a control subject (first row) and an ROA subject (second row) with the same age, gender and similar total muscle Lean ACSA. The first column shows the fat fraction maps (colorbar units in %). The second column shows color-coded fat fraction maps highlighting the intramuscular fat fraction (red), intermuscular fat fraction (green) and SAT (blue) regions. The third columns shows color-coded fat fraction maps highlighting the quadriceps (red), hamstrings (green) and other muscle (blue) regions. The color-coded maps were weighted by the fat fraction map values.
Strength Testing
Quadriceps and hamstrings strength was measured on an Primus RS instrumented dynamometer (BTE, Hanover, MD, USA) under 2 different conditions – (a) maximal isometric torque at 70 degrees knee flexion, and (b) maximal isokinetic torque at 120 °/sec between 20° – 90° of knee flexion. Three warm-up trials at progressive effort levels preceded the first maximal effort trial for each muscle group. One-minute rest was provided between each repetition. Three trials were acquired for each condition and the trial with the maximum torque was used in the analyses. All torque values were normalized to body mass (Nm/kg).
Function
All subjects completed the Knee injury and Osteoarthritis Outcome Score (KOOS).[30, 31] The KOOS covers 5 separate dimensions: Pain, Symptoms, Activities of Daily Living (ADL), Sport and Recreation Function, and Knee-Related Quality of Life, with higher scores (0–100) representing better function. For this paper the KOOS Pain, Symptoms and ADL subscales were used in the analyses. Physical function was assessed using the - stair climbing test [32] where they were timed with a stopwatch as they ascended and descended a set of 12 stairs (18 cm high); and the 6 minute walk test [33] where they were instructed to cover as much distance as possible during the 6-minute time frame.
Statistics
All analyses were performed in IBM SPSS 20.0 (IBM Corporation, Armonk, NY, USA). Primary analyses were performed with one-way ANOVA to investigate the differences in function, intramuscular fat fractions, lean ACSA and strength between those with and without ROA. All analyses were adjusted for age, gender, and BMI (age and gender only for strength since it was normalized to body mass). Levene’s test for homogeneity of variance was used to ensure homogenous variance in the two groups. Natural log transformations were used in case of non-homogenous variances in the two groups for any variable. Age, gender, BMI, presence of knee OA, quadriceps intramuscular fat fraction and quadriceps lean ACSA were used as predictors of function and quadriceps isometric strength in linear regression analyses. The relationships of quadriceps strength/intramuscular fat fraction/lean ACSA (a) with age and BMI were investigated using Pearson’s correlations, and (b) with KL grade, total cartilage/meniscus/BML mWORMS scores using non-parametric Kendalls τ due to large number of tied ranks.[34] Furthermore, Kendall’s τ is a better estimate of correlation in the population compared to Spearman’s. [34] Exploratory analyses were performed to compare intramuscular fat volumes, intermuscular fat volume and SAT volume between the two groups after adjusting for age, gender, and BMI.
RESULTS
Subject characteristics, function
Age, BMI, gender distribution and functional measures for the 2 groups are shown in Table 1. The ROA group was older (P = 0.001) and had greater BMI (P = 0.032). The proportion of men and women was similar in both groups. The control group had 36 individuals with KL = 0 and 30 individuals with KL = 1. The ROA group had 10, 16, and 4 individuals respectively for KL 2,3 and 4.
Table 1.
Mean and 95% confidence intervals for age, BMI, function, and strength parameters, and the gender distribution for subjects with and without radiographic knee osteoarthritis.
| Control | Osteoarthritis | P | ||
|---|---|---|---|---|
| Age (years) | 50.7 (48.4, 53.1) | 57.7 (54.3, 61.1) | 0.001 | |
| BMI (kg/m2) | 24.1 (23.2, 25.0) | 26.9 (23.5, 30.2) | 0.032 | |
| Gender (M:F) | 28:38 | 15:15 | 0.489* | |
| KOOS | Symptoms | 90.4 (87.7, 93.2) | 81.9 (76.0, 87.8) | 0.014† |
| Pain | 90.6 (87.5, 93.8) | 78.8 (71.4, 86.3) | 0.003† | |
| Activities of Daily Living | 94.7 (92.3, 97.1) | 84.3 (77.5, 91.1) | 0.006† | |
| Stair Climbing Test (sec) | 11.4 (10.9, 12.0) | 12.0 (10.7, 13.3) | 0.865† | |
| 6 Minute Walk Test (m) | 636.7 (613.0, 660.4) | 596.2 (563.3, 630.0) | 0.569† | |
| Quadriceps | Isometric Torque (Nm/kg) | 1.39 (1.29,1.49) | 1.12 (0.99, 1.26) | 0.005# |
| Isokinetic Torque at 120°/sec (Nm/kg) | 0.92 (0.86,0.98) | 0.74 (0.66, 0.83) | 0.001# | |
| Hamstrings | Isometric Torque (Nm/kg) | 0.67 (0.63,0.72) | 0.62 (0.55,0.69) | 0.452# |
| Isokinetic Torque at 120°/sec (Nm/kg) | 0.65 (0.61, 0.70) | 0.60 (0.53, 0.67) | 0.172# | |
P value from the Chi-Square test
- adjusted for age, gender, BMI
adjusted for age and gender.
After adjusting for age, the ROA group had worse self-reported scores on all subscales of KOOS (P < 0.05). The differences in performance based functional tests of stair climbing test (P = 0.865) and six minute walk test (P = 0.569) were not significant.
Thigh muscle strength
After adjusting for age and gender (Table 1), the ROA group had lower quadriceps isometric strength (P = 0.005) and isokinetic strength (P = 0.001) but the differences in hamstring isometric strength (P = 0.452) and isokinetic strength (P = 0.172) were not significant.
Thigh Adiposity and lean ACSA
After adjusting for age, gender, and BMI, the ROA group had greater intramuscular fat fractions for the quadriceps intramuscular fat fraction (P = 0.018) (Figure 2a). The differences in intra MF fractions for the hamstrings (P = 0.490), remaining muscle groups (P = 0.281), and global thigh muscle intramuscular fat (P = 0.102) were not statistically significant (Figure 2a). After adjusting for age, gender, and BMI, the differences in lean ACSA for the quadriceps (P = 0.381), hamstrings (P = 0.905), remaining muscle groups (P = 0.949) and global muscles (P = 0.576) were not statistically significant (Figure 2b).
FIGURE 2.
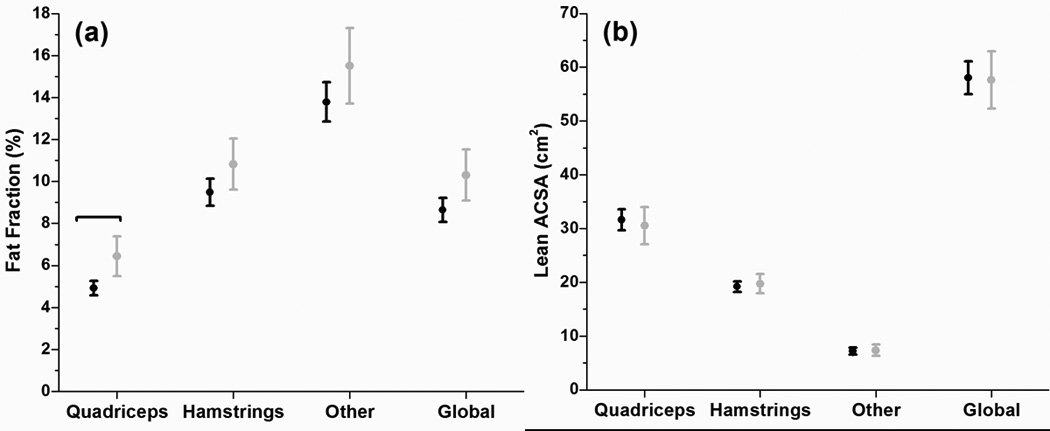
Fat Fractions for control subjects (Black) and OA (Grey) with 95% CI for quadriceps muscle, hamstrings muscle, all remaining muscles and global compartments. (b) Lean Anatomical Cross-sectional area control subjects (Black) and OA (Grey) with 95% CI for quadriceps muscle, hamstrings muscle, all remaining muscles and global compartments. Brackets indicate significant differences at P < 0.05
Exploratory analyses showed that after adjusting for age, gender, and BMI, the differences between the groups were not significant for quadriceps intramuscular fat volume (in cm3) for the quadriceps (Control = 92.8±33.9, ROA = 112.3±45.2, P = 0.198), hamstrings (Control = 81.0±29.5, ROA = 94.7±34.5, P = 0.661), other muscles (Control = 45.0±17.7, ROA = 51.3±19.5, P = 0.622), and global compartments (Control = 218.8±72.0, ROA = 258.2±87.7, P = 0.346). The differences between the groups for intermuscular fat volume (Control = 266.9±92.1, ROA = 293.0±94.4, P = 0.812) and SAT volume (Control = 1547.9±730.6, ROA = 1466.3±668.6, P = 0.564) were not significant after adjusting for age, gender, and BMI.
Relationships
Table 2 has the unstandardized regression coefficients, 95% confidence intervals, standardized regression coefficients, and associated P values from the multiple linear regression analyses. For KOOS Symptoms and ADL scores, the regression models including age, gender, BMI, presence of knee OA, quadriceps intramuscular fat fraction, and quadriceps lean ACSA explained 18.6 % (P = 0.007) and 34.7% (P < 0.001) of the variance in the outcome respectively. Quadriceps intramuscular fat fraction was the only variable that made a significant contribution to the models for both KOOS Symptoms and KOOS ADL (Table 2). For KOOS Pain, the regression model with all the variables explained 21.8% (P = 0.002) of the variance in the outcome. Presence of knee OA and quadriceps intramuscular fat fraction were the only independent variables with a regression coefficient that made a significant contribution to the model (Table 2). For distance covered in the six minute walk test, the regression model with all the variables explained 53.9% (P < 0.001) of the variance in the outcome. Age, BMI, quadriceps intramuscular fat fraction, and quadriceps lean ACSA all made significant contributions to the model (Table 2). For time taken to the complete the stair climbing test, the regression model with all the variables explained 51.5% (P < 0.001) of the variance in the outcome. BMI and quadriceps intramuscular fat fraction significantly contributed to the model (Table 2). Finally for quadriceps isometric peak torque, the regression model with all the variables explained 45.1 % (P < 0.001) of the variance in the outcome. Quadriceps lean ACSA was the only variable which contributed significantly to the model (Table 2).
TABLE 2.
Results from Multiple Linear Regression. Unstandardized regression coefficients (95 % confidence Intevals), Standardized regression coefficients (in standard deviations) and P values.
| Quadriceps intramuscular fat fraction | Quadriceps Lean ACSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstandardized Regression Coefficient |
95 % Confidence Intervals |
Standardized Regression Coefficient |
P value | Unstandardized Regression Coefficient |
95 % Confidence Intervals |
Standardized Regression Coefficient |
P value |
||
| KOOS | Symptoms | −2.25 | −3.75, −0.74 | −0.34 | 0.004 | −0.06 | −0.49, 0.36 | −4.00 | 0.771 |
| Pain | −2.64 | −4.43, −0.84 | −0.32 | 0.004 | 0.07 | −0.44, 0.57 | 3.39 | 0.802 | |
| ADL | −3.31 | −4.71, −1.90 | −0.47 | <0.001 | 0.15 | −0.25,0.55 | 9.27 | 0.452 | |
| 6 Minute Walk Test | −11.79 | −21.70, −1.87 | −0.24 | 0.021 | 4.0 | 1.62,6.37 | 38.01 | 0.001 | |
| Stair Climbing Test | 0.66 | 0.38, 0.95 | 0.47 | <0.001 | −0.04 | −0.10, 0.03 | −12.78 | 0.293 | |
| Quadriceps Isometric Torque | −3.09 | −7.18, 1.0 | −0.17 | 0.135 | 1.39 | 0.53, 2.25 | 43.22 | 0.002 | |
KOOS = Knee injury and Osteoarthritis Outcome Score; ADL = Activities of Daily Living; ACSA = Anatomical Cross-sectional Area
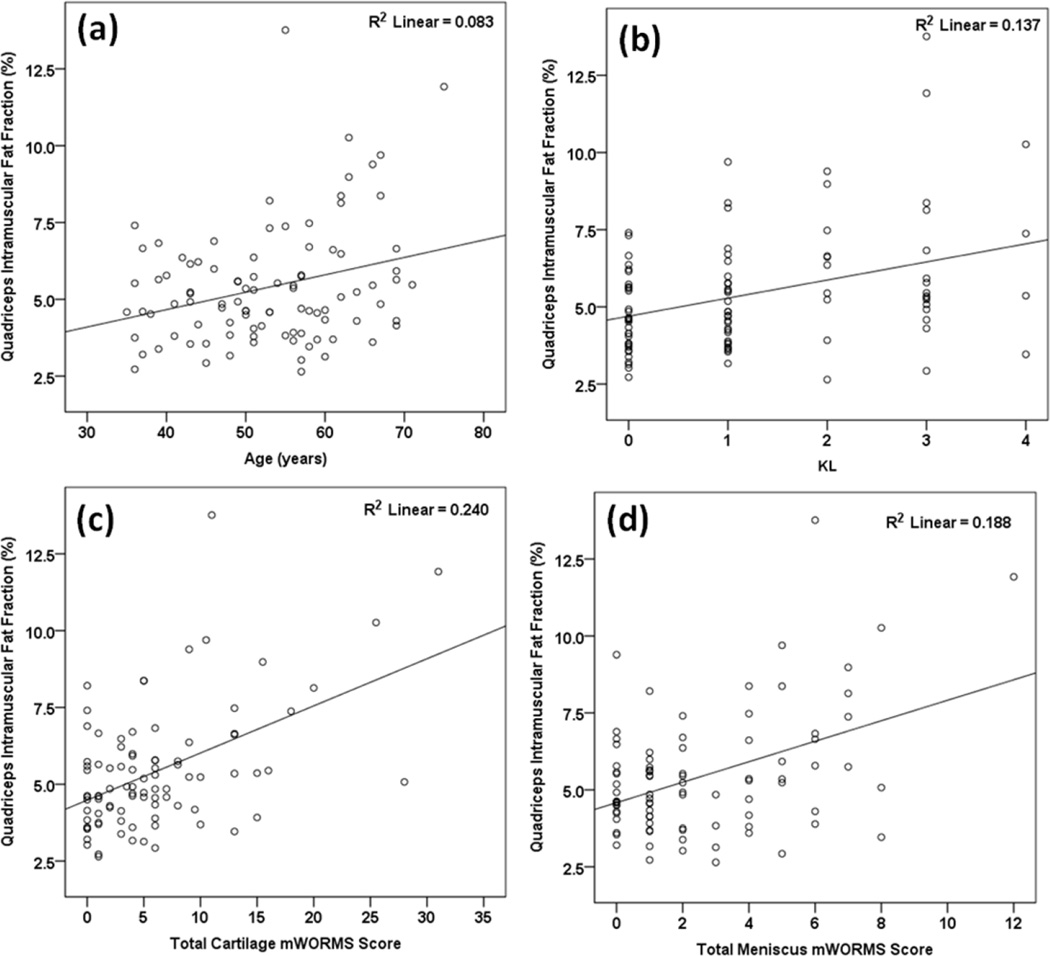
Table 4 and Figure 3 show the results from the correlational analyses. Higher quadriceps intramuscular fat fraction was associated with older age (Figure 3a), worse ROA severity (Figure 3c), and greater total cartilage (Figure 3c) and meniscus (Figure 3d) mWORMS scores. Lower quadriceps lean ACSA was associated with older age, lower BMI, and higher total cartilage mWORMS score. Lower quadriceps torque was associated with lower age, worse ROA severity and greater total cartilage mWORMS score. BML scores were not related to any of the parameters.
FIGURE 3.
Scatter-plots for the association of quadriceps intramuscular fat fraction (y-axes) with (a)age, (b) KL, (c) total cartilage mWORMS score, and (d) total meniscus mWORMS score on the x-axes.
DISCUSSION
In this study we compared thigh muscle intramuscular fat fractions and lean ACSA in people with and without ROA. We found that people with ROA have higher quadriceps fatty infiltration and weaker quadriceps, but muscle area is not different. We also evaluated the relationships between quadriceps intramuscular fat fraction, lean ACSA, strength, patient function and knee joint lesions. The results show that quadriceps intramuscular fat fraction is negatively associated with self-reported and physical measures of function and radiographic and MR severity of knee OA. The study presents novel data on quadriceps adiposity in people with knee OA using quantitative MR techniques.
We found that people with ROA have greater quadriceps fatty infiltration compared to those without radiographic knee OA. Also, greater severity of OA on radiograph and MRI was related to higher quadriceps intramuscular fat fraction. The difference in quadriceps fat fraction between the groups was ~ 1.5 %. It is unlikely that this difference could have been detected with conventional T1-weighted imaging. Furthermore, this difference appears to be clinically meaningful as well, because the ROA group had greater self-reported disability. This was further confirmed by the negative associations between quadriceps fat fractions and function in these individuals. Similar findings have been observed at the shoulder in individuals with symptomatic rotator cuff tears.[18] Longitudinal studies would be needed to understand if increase in quadriceps fat fraction occurs prior to or follows symptomatic and structural decline. We observed greater fatty infiltration for all thigh muscles in people with knee OA compared to the control group. However, after adjusting for age, BMI, and gender, the differences were only significant for the quadriceps. Hence, it may be possible that the quadriceps muscle is affected to a greater extent than other muscles in people with knee OA. This is supported by the ubiquitous finding of quadriceps weakness in people with knee OA.[20] Data from the OAI have also shown that in people with bilateral ROA, the knees with pain had lower quadriceps ACSA but no difference in the flexor or adduction ACSAs.[35] On the other hand, it could also be a limitation of the sample size in our study. However, due to the cross-sectional design of our study, it is not possible to confirm these speculations. Hence, future work is needed to understand if the quadriceps muscle is preferentially affected in people with knee OA.
The observed differences in quadriceps fatty infiltration could be related to systemic inflammatory processes or to muscle disuse. Previous studies have shown that skeletal adipose tissue may be related to systemic inflammatory processes since higher skeletal muscle fat is associated with higher insulin resistance, cholesterol and fasting sugar. [36, 37] IMAT of the thigh is considered a peripheral ectopic fat depot and shares a direct vascular connection with the muscles it infiltrates. [5] It has been suggested that this relationship may be analogous to that of visceral adipose tissue in the liver and liver vasculature where visceral adipose tissue is known to be related to impaired fat oxidation [38] and unfavorable lipoprotein profiles. [39] This was confirmed in a study which reported that in men, a reduction in IMAT with targeted aerobic activity had a stronger and significant association with less atherogenic lipoprotein profile compared to visceral adipose tissue. [5] With aging and disuse, there is a loss of lean muscle tissue (sarcopenia) and an increase in skeletal muscle fat. [40] Physical activity can increase lean muscle mass and decrease IMAT [41], which can additionally impact systemic metabolic profile. [5] Hence, impact of a physical activity intervention on quadriceps intramuscular fat fraction in people with knee OA needs to be assessed.
Prior studies have reported no differences in the quadriceps muscle mass between people with and without knee ROA. [7, 8, 42] Our findings are in accordance with these earlier reports. Furthermore, our results suggest that loss of lean quadriceps mass may not be as significant as an increase in quadriceps intramuscular fat fraction in knee OA. This is further corroborated by a lack of significance in the relationships between quadriceps lean ACSA, self-reported disability and most metrics of structural severity of knee OA. However as expected, the lean ACSA was a significant predictor of quadriceps strength. We did not observe a significant difference in quadriceps lean ACSA but did observe people with ROA to have weaker quadriceps. Besides atrophy [20, 43, 44], quadriceps weakness could be related deficits in central activation [20], and inhibition due to pain and effusion [45, 46]. Furthermore, lower quadriceps torque could also be an artifact of greater antagonist co-contraction during MVIC testing [47] It has been shown that there is significant antagonist activity during quadriceps strength testing. [47] However, we did expect to find an independent association between quadriceps intramuscular fat fraction and strength. Although quadriceps intramuscular fat fraction was negatively correlated with isometric torque (r = −0.41, P = 0.014), this association was not significant after accounting for age, BMI, gender, presence of knee OA and quadriceps lean ACSA. Further work is needed to elucidate these relationships in people with knee OA.
Earlier reports from our group have shown that a fat fraction of 6.5±4% corresponds to semi-quantitative Goutallier grade of 1. [19] Since both groups in this study had a mean quadriceps fat fraction of ≤ 6.5 %, both groups would on average be considered a Goutallier grade 1. Hence, Goutallier grading would fail to detect clinically meaningful differences between the groups. In individuals with symptomatic rotator cuff tears at the shoulder, quantitative fatfraction measures, but not semi-quantitative Gotuallier grading, demonstrated significant negative correlations with patient pain and range of motion. [18] Traditionally, Goutallier grades 2 and greater are considered pathologic. [48] However, our results suggest that in individuals with ROA, even smaller amounts of fatty infiltration may relate to significant pathology and functional decline.
We did not find significant differences in intermuscular fat or SAT volume between the groups. Earlier studies using OAI datasets have reported higher intermuscular fat volume in women with ROA. [8, 49] However, in these studies the authors were unable to differentiate adipose tissue from non-adipose tissue in the intermuscular compartment and hence did not truly assess intermuscular fat volume. To compare our results with theirs, we analyzed the differences in the volume of the entire intermuscular compartment (excluding the muscles) between women with (n = 14) and without ROA (n = 38), adjusting for age. We did not find any significant differences (P = 0.194) which could be due to differences in the sample size, and region of interest. However, Maly et al. only found weak negative relationships between intermuscular fat volume and function in women with ROA.[8] Our results demonstrate strong associations between intramuscular fat fraction and function suggesting that intramuscular fatty infiltration may be more important towards functional decline than intermuscular fatty infiltration. However, further longitudinal analyses will be needed to fully understand the roles of different fat depots in the thigh in people with knee OA.
The study has limitations which need to be considered when interpreting the results. The region of interest for analysis for measures of muscle adiposity and morphology consisted of a 2 cm section of the thigh 10–12 cm proximal to the superior pole of the patella. This convention allowed us to be consistent across all subjects, and a similar approach has been used in previous studies. [8, 49] However, it may have an impact on the quantification of muscle adiposity since different parts of the muscles along their length may have been evaluated depending on the height of the person and length of their thigh muscles. Overall the heights were not different between the groups in this study. To address this limitation further, we estimated femur lengths for each participant using gender specific equations.[50] Average femur length was 18.2±1.7 in. for the control group and 18.4±1.7 in. for the ROA group. Assuming a proportional difference in thigh muscle length between the groups, it is unlikely that this difference would cause significant effect on the parameters studied. Secondly, this cohort of subjects may be considered relatively high functioning and our findings may not be generalizable to people with knee OA with worse functional limitations. Lastly, we did not adjust for multiple comparisons in the statistical analyses, and did not account for the use of pain relieving medications, which could affect the results.
In conclusion, we found that subjects with ROA have greater intramuscular fat in the quadriceps muscle and weaker quadriceps but the lean ACSA is no different when compared to individuals without ROA. Quantitative measures of quadriceps fat fraction had significant negative associations with patient reported function, physical function and severity of structural damage to the knee joint but quadriceps lean ACSA did not.
Table 3.
Pearson’s r correlation with 95% confidence intervals and P values for associations of quadriceps intramuscular fat fraction, Lean ACSA, and strength with age and BMI. Kendall’s τ correlation with 95% confidence intervals and P values for associations of quadriceps intramuscular fat fraction, Lean ACSA, and strength with KL grade, and mWORMS scores for cartilage, meniscus and BMLs.
| Age | BMI | KL | Total Cartilage mWORMS |
Total Meniscus mWORMS |
Total BML mWORMS |
|
|---|---|---|---|---|---|---|
| Quadriceps intraMF Fraction |
0.29 (0.09, 0.46) 0.004 |
0.16 (−0.04, 0.35) 0.112 |
0.25 (0.11, 0.37) 0.002 |
0.26 (0.12, 0.38) 0.001 |
0.18 (0.04, 0.31) 0.024 |
0.14 (0.0002, 0.28) 0.077 |
| Quadriceps Lean ACSA |
−0.32 (−0.48, −0.12) 0.002 |
0.22 (0.02, 0.40) 0.035 |
− 0.07 (−0.21, 0.06) 0.339 |
−0.17 (−0.30, −0.03) 0.024 |
−0.13 (−0.27, 0.01) 0.087 |
−0.03 (−0.17, 0.11) 0.737 |
| Quadriceps Isometric Torque |
−0.36 (−0.54, −0.16) 0.001 |
0.22 (0.0003, 0.42) 0.050 |
−0.23 (−0.36, −0.08) 0.007 |
−0.16 (−0.31, −0.01) 0.044 |
−0.13 (−0.27, 0.03) 0.133 |
−0.13 (−0.28, 0.28) 0.136 |
ACSA = Lean Anatomical Cross-Sectional Area; BML = Bone Marrow Lesion; mWORMS = modified Whole Organ Magnetic Resonance Score
ACKNOWLEDGEMENT
The authors would like to thank Melissa Guan for assistance with patient recruitment, Justin Singh and Joseph Schooler for assistance with data acquisition, and Alexander Valentinitsch, K. Subburaj and Megan Marsh for software support. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Numbers 2 R01 AR046905 and 1 R01 AR062370. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conception and Design: Kumar, Karampinos, Li, Majumdar, Souza
Acquisition of data: Kumar, Karampinos, MacLeod
Analysis and Interpretation of Data: Kumar, Karampinos, Lin, MacLeod, Nardo, Li, Link, Majumdar, Souza
Drafting of article or revising it critically for important intellectual content: Kumar, Karampinos, Lin, MacLeod, Nardo, Li, Link, Majumdar, Souza
Final approval of the version of the article to be published: Kumar, Karampinos, Lin, MacLeod, Nardo, Li, Link, Majumdar, Souza
Authors Kumar (Deepak.kumar@ucsf.edu) and Souza (Richard.souza@ucsf.edu) take full responsibility for the integrity of this work as a whole.
CONFLICT OF INTEREST: No conflict of interest for any of the authors.
Contributor Information
Dimitrios C. Karampinos, Email: dimitrios.karampinos@tum.de.
Toran D. MacLeod, Email: toran.macleod@ucsf.edu.
Wilson Lin, Email: Wilson.lin@ucsf.edu.
Lorenzo Nardo, Email: Lorenzo.nardo@ucsf.edu.
Xiaojuan Li, Email: xiaojuan.li@ucsf.edu.
Thomas M Link, Email: Thomas.link@ucsf.edu.
Sharmila Majumdar, Email: sharmila.majumdar@ucsf.edu.
Richard B Souza, Email: Richard.souza@ucsf.edu.
REFERENCES
- 1.Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260:332–354. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 5.Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab. 2008;295:E407–E412. doi: 10.1152/ajpendo.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenfeld O, Williams FM, Hart DJ, Arden NK, Spector TD, Livshits G. Lower limbs composition and radiographic knee osteoarthritis (RKOA) in Chingford sample--a longitudinal study. Arch Gerontol Geriatr. 2013;56:148–154. doi: 10.1016/j.archger.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Conroy MB, Kwoh CK, Krishnan E, Nevitt MC, Boudreau R, Carbone LD, et al. Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:15–21. doi: 10.1002/acr.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maly MR, Calder KM, Macintyre NJ, Beattie KA. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Stenger VA, Boada F, McKolanis T, Davis D, Ross R, et al. Skeletal muscle lipid concentration quantified by magnetic resonance imaging. Am J Clin Nutr. 2004;79:748–754. doi: 10.1093/ajcn/79.5.748. [DOI] [PubMed] [Google Scholar]
- 10.Schick F, Machann J, Brechtel K, Strempfer A, Klumpp B, Stein DT, et al. MRI of muscular fat. Magn Reson Med. 2002;47:720–727. doi: 10.1002/mrm.10107. [DOI] [PubMed] [Google Scholar]
- 11.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 12.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 13.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 14.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido-Gamio J, et al. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012;35:899–907. doi: 10.1002/jmri.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardo L, Karampinos DC, Krug R. Quantitative Assessment of Fat Infiltration in the Rotator Cuff Muscles using water-fat MRI. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, et al. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22:1592–1600. doi: 10.1007/s00330-012-2404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menashe L, Hirko K, Losina E, Kloppenburg M, Zhang W, Li L, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;20:13–21. doi: 10.1016/j.joca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Charles HC, Kraus VB, Ainslie M, Hellio Le Graverand-Gastineau MP. Optimization of the fixed-flexion knee radiograph. Osteoarthritis Cartilage. 2007;15:1221–1224. doi: 10.1016/j.joca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25:644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- 26.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:727–735. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahl R, Luke A, Ma CB, Krug R, Steinbach L, Majumdar S, et al. Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects-a 3.0 T magnetic resonance imaging study. Skeletal Radiol. 2008;37:627–638. doi: 10.1007/s00256-008-0491-y. [DOI] [PubMed] [Google Scholar]
- 28.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Valentinitsch A, D CK, Alizai H, Subburaj K, Kumar D, T ML, et al. Automated unsupervised multi-parametric classification of adipose tissue depots in skeletal muscle. J Magn Reson Imaging. 2013;37:917–927. doi: 10.1002/jmri.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 31.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985;28:542–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- 32.Rejeski WJ, Ettinger WH, Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157–167. doi: 10.1016/s1063-4584(05)80050-0. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord. 2005;6:3. doi: 10.1186/1471-2474-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field A. Discovering Statistics Using SPSS. Second Edition. London, UK: Sage Publications Ltd.; 2005. [Google Scholar]
- 35.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sanger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012;20:532–540. doi: 10.1016/j.joca.2012.02.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 37.Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 39.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–179. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 40.Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55:217–223. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruhdorfer A, Dannhauer T, Wirth W, Hitzl W, Kwoh CK, Guermazi A, et al. Thigh muscle cross-sectional areas and strength in advanced versus early painful osteoarthritis: an exploratory between-knee, within-person comparison in osteoarthritis initiative participants. Arthritis Care Res (Hoboken) 2013;65:1034–1042. doi: 10.1002/acr.21965. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda S, Tsumura H, Torisu T. Age-related quadriceps-dominant muscle atrophy and incident radiographic knee osteoarthritis. J Orthop Sci. 2005;10:121–126. doi: 10.1007/s00776-004-0876-2. [DOI] [PubMed] [Google Scholar]
- 44.Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen-Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis Rheum. 2007;56:3626–3633. doi: 10.1002/art.22960. [DOI] [PubMed] [Google Scholar]
- 45.Hassan BS, Doherty SA, Mockett S, Doherty M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis. 2002;61:422–428. doi: 10.1136/ard.61.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torry MR, Decker MJ, Viola RW, O'Connor DD, Steadman JR. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000;15:147–159. doi: 10.1016/s0268-0033(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 47.Kubo K, Tsunoda N, Kanehisa H, Fukunaga T. Activation of agonist and antagonist muscles at different joint angles during maximal isometric efforts. Eur J Appl Physiol. 2004;91:349–352. doi: 10.1007/s00421-003-1025-x. [DOI] [PubMed] [Google Scholar]
- 48.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 49.Beattie KA, MacIntyre NJ, Ramadan K, Inglis D, Maly MR. Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:22–29. doi: 10.1002/acr.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NSBRI. Predicting Height from the Length of Limb Bones. http://www.nsbri.org/humanphysspace/focus6/student1.html. [Google Scholar]