Abstract
Coronary heart disease is a global malady and it is the leading cause of death in the United States. Chronic stable angina is the most common manifestation of coronary heart disease and it results from the imbalance between myocardial oxygen supply and demand due to reduction in coronary blood flow. Therefore, in addition to lifestyle changes, commonly used pharmaceutical treatments for angina (nitrates, β-blockers, Ca2+ channel blockers) are aimed at increasing blood flow or decreasing O2 demand. However, patients may continue to experience symptoms of angina. Ranolazine is a relatively new drug with anti-anginal and anti-arrhythmic effects. Its anti-anginal mechanism is not clearly understood but the general consensus is that ranolazine brings about its anti-anginal effects by inhibiting the late Na+ current and the subsequent intracellular Ca2+ accumulation. Recent studies suggest other effects of ranolazine that may explain its anti-anginal and anti-arrhythmic effects. Nonetheless, clinical trials have proven the efficacy of ranolazine in treating chronic angina. It has been shown to be ineffective, however, in treating acute coronary syndrome patients. Ranolazine is a safe drug with minimal side effects. It is metabolized mainly in the liver and cleared by the kidney. Therefore, caution must be taken in patients with impaired hepatic or renal function. Due to its efficacy and safety, ranolazine was approved for the treatment of chronic angina by the Food and Drug Administration (FDA) in 2006.
Keywords: ranolazine, chronic angina, late Na+ current, mitochondria
Introduction
Cardiovascular diseases are the number one cause of death globally. According to the World Health Organization, an estimated 17.3 million people died from cardiovascular diseases in 2008, representing 30% of all global deaths. Of these deaths, an estimated 7.3 million were due to coronary heart disease. Coronary heart disease, along with stroke, is projected to remain the single leading cause of death in the future. Chronic stable angina is the most prevalent manifestation of coronary artery disease (CAD) with an incidence of 320,000 cases in males and 180,000 cases in females every year in the US.1 Stable angina is a clinical syndrome characterized by discomfort in the chest, jaw, shoulder, back, or arms, typically elicited by exertion or emotional stress and relieved by rest or nitroglycerin,2 and it is caused by an imbalance between myocardial oxygen supply and myocardial oxygen consumption. Management of chronic stable angina includes lifestyle changes (eg, eating a heart-healthy diet, lowering cholesterol, getting regular exercise, cessation of smoking, and controlling diabetes and high blood pressure), pharmacological treatment (β-blockers, Ca2+ channel blockers, nitrates, and antiplatelet medications), and revascularization procedures when indicated (percutaneous coronary intervention (PCI), coronary artery bypass grafting).2 Despite the effectiveness of the current management strategies, episodes of angina may still persist or even worsen3,4 and many patients cannot tolerate a combination of anti-anginal drugs due to their many side effects. Therefore, a new treatment that provides an alternative option for patients who continue to suffer from symptoms of angina despite using other anti-anginal drugs would be helpful. Ranolazine is a relatively new drug for the treatment of chronic angina that was approved by the Food and Drug Administration (FDA) and has been in use in the US since 2006.
Metabolism and Pharmacokinetic Profile
Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl], with the empirical formula of C24H33N3O4.5 It contains two enantiomeric forms (S-ranolazine and R-ranolazine).6 Ranolazine was initially manufactured in an immediate-release formulation but it was discontinued due to the short elimination half life.7 Ranolazine is currently manufactured by Gilead Sciences in an extended (sustained)-release tablets of 500 mg and 1000 mg for clinical use and it is sold under the trade name Ranexa®. It is extensively absorbed after oral administration and it reaches peak plasma concentrations within 2–5 hours5,7 after a single dose where it binds mainly to α-1 acid glycoprotein and weakly to albumin. With a dose of 500–1000 mg given twice daily, the peak to trough difference is only 1.6 fold. Steady state is usually reached within 3 days of multiple dosing. The half-life of ranolazine at steady state after oral administration is nearly 7 hours. Less than 5% of ranolazine is excreted unchanged by the kidney while the remainder is extensively and rapidly metabolized mainly by CYP3A4 enzymes in the liver and, to a lesser extent, by CYP2D6. Following a single oral dose of ranolazine, total elimination is 75% in urine and 25% in feces. These pharmacokinetics are not affected by age, gender, or food.5
Mechanism of Action
Although the precise mechanisms for the anti-anginal effects of ranolazine are not well understood, several pharmacological activities of ranolazine have been described. Of these, two distinct mechanisms appear to primarily promote its cardiac protection against ischemic injury. These include its effect as a metabolic modulator and as a late Na+ current inhibitor. Therefore, in this section we will summarize the findings of the many studies in which attempts were made to elucidate the mechanism of action of ranolazine. Table 1 summarizes some of the mechanisms of ranolazine that could be responsible for its anti-anginal effects.
Table 1.
A summary of the possible mechanisms of ranolazine as an anti-anginal drug.
| Mechanism | Details | Notes |
|---|---|---|
| Effects on metabolism |
|
|
| Effects on late Na+ current (INaL) |
|
|
| Effects on mitochondrial complex I |
|
|
| Effects on myofilaments Ca2+ sensitivity |
|
|
Ranolazine as a metabolic modulator
In general, drugs such as β-blockers, Ca2+ channel blockers, and long acting nitrates exert their anti-anginal effects by causing hemodynamic changes such as drop in blood pressure, heart rate, and/or contractility which decreases cardiac work.8 On the other hand, ranolazine as many trials showed, does not have any clinically significant effect on these hemodynamic parameters.9–14 Initially it was reported that ranolazine was effective as an anti-anginal drug by acting as a metabolic modulator.15 For this mechanism, ranolazine was proposed to stimulate glucose oxidation at the expense of fatty acid oxidation. Under non-ischemic conditions, myocytes ATP requirement is met by both glucose and fatty acid oxidation with the majority (about 90%) coming from fatty acid oxidation.16 Fatty acid oxidation is more efficient, energy-wise, than glucose oxidation in ATP production. However as the supply of O2 dwindles during ischemia, glucose becomes the preferred substrate for ATP production because it is more efficient oxygen-wise (for more details on this topic refer to the review by Cheng et al16). Therefore, by switching substrate utilization to glucose oxidation, ranolazine may provide a non-hemodynamic mechanism for preserving cardiac myocytes during ischemia and reperfusion injury. It is not clear how ranolazine promotes glucose oxidation, but in perfused guinea pig hearts subjected to low-flow ischemia it was shown that ranolazine increased the amount of active dephosphorylated pyruvate dehydrogenase15 which is thought to play a key role in determining the rate of carbohydrate utilization.17,18
Ranolazine as a late Na+ current inhibitor
The effect of ranolazine as a modulator of metabolism occurs at concentrations greater than 10 μM. However, ranolazine exerts its effects as an anti-anginal drug at lower concentrations, ie, 2–6 μM.19 This implies that a mechanism other than promoting glucose oxidation is probably responsible for the anti-anginal characteristics of ranolazine. Indeed, in a comprehensive study19 it was shown that ranolazine produces several ion channel effects. Of particular interest was the inhibitory effect of ranolazine on the late Na+ current (INaL) with an IC50 = 5.9 μM.19 This inhibitory effect was also observed in other studies in which the INaL was pharmacologically induced by the Anemonia sulcata toxin (ATX)-II.20,21 A later study showed that ranolazine blocked both the peak Na+ current (INa) and the INaL with a nine-fold selectivity for INaL vs. INa,22 and that this blockade occurred at the same binding site where other local anesthetics, such as lidocaine, interacts with Na+ channels. Although the inhibitory effect of ranolazine on Na+ current and especially on the INaL may suggest an anti-arrhythmic effect, it could also explain its mechanism as an anti-anginal drug on the basis of preventing Na+ overload and the subsequent increase in intracellular Ca2+.
Cytosolic Ca2+ overload is a key event in the injury sustained during ischemia. It occurs due to activation of the Na+/Ca2+ exchanger in the reverse mode as a result of the increase in cytosolic Na+ which results from impaired Na+/K+ ATPase pump activity, coupled with an increased pH gradient between the intracellular and extracellular spaces and concomitant activation of the Na+/H+ exchanger.23 However, increased cytosolic Na+ may also arise in part from activation of the INaL by toxic ischemic metabolites24,25 and by reactive oxygen species that are generated during reperfusion.26 Thus ranolazine, an inhibitor of INaL, could reduce cytosolic Na+ during ischemia and subsequently reduce cytosolic Ca2+. Indeed, Song et al27 reported that ranolazine attenuated H2O2-induced intracellular Na+ and Ca2+ overload in rabbit isolated ventricular myocytes. We also showed that ranolazine reduced cytosolic Ca2+ in intact beating guinea pig hearts undergoing global no flow ischemia, and that this led to a decrease in mitochondrial Ca2+ overload and reactive oxygen species generation during ischemia.28 These effects of ranolazine on mitochondria may underlie, in part, its cardio-protective effects against ischemia. What remains unclear is the exact mechanism by which ranolazine inhibits INaL.
Recent studies have focused on the pore-forming subunit of the cardiac Na+ channel, Nav1.5, which is the main ion channel that conducts Na+ in cardiac cells. One interesting study showed mechano-sensitive properties for INa in isolated mouse cardiomyocytes.29 This implies that the function of Nav1.5, among many other described regulatory mechanisms, is also modulated by the mechanical stretch of the membrane in which it is embedded. However, and more importantly, the novelty of the study came from the finding that ranolazine inhibited the mechano-sensitivity of Nav1.5 in a dose dependent manner, and that this inhibition did not require the established binding site. This study also suggested that it is only the neutral form of ranolazine that is capable of inhibiting the mechano-sensitivity of Nav1.5, thus leading the authors to speculate that ranolazine obtains access to the channel by partitioning within the lipid bilayer of the cell membrane. Ranolazine inhibited the mechano-sensitivity of Nav1.5 with an IC50 = 54 μM which is about 10-fold higher than the therapeutic concentration. Nonetheless, the authors suggest two reasons not to dismiss these findings. First, the results were obtained in an experimental model that does not mimic exactly the true mechanical environment of the channels in situ where other mechano-relevant elements likely mediate the mechano-sensitivity of the Nav1.5. Second, ranolazine inhibits the mechano-sensitivity of Nav1.5 by partitioning into the bilayer hydrophobic core29 in its neutral (effective) form. However, this partitioning increases with increasing temperatures and since the experiments were conducted at room temperature, one can expect that the effective membrane concentration of ranolazine will increase at the higher physiological temperatures of the clinical setting.
Ranolazine and mitochondrial complex I
As mentioned earlier, the effect of ranolazine as a metabolic modulator is probably related to increasing the amount of the active dephosphorylated pyruvate dehydrogenase. It is worth noting, however, that the pyruvate dehydrogenase complex is exclusively intramitochondrial in mammalian tissues, which may suggest that ranolazine has another site of action that is intra-mitochondrial.8 Interestingly, Wyatt et al30 showed that ranolazine inhibited mitochondrial respiration of NADH-linked substrates and that this was due to inhibition of the mitochondrial electron transport chain at the level of complex I. Furthermore, the inhibition by ranolazine resembled that of rotenone and amobarbital which are specific complex I blockers. The significance of this is that inhibiting complex I with amobarbital was protective against ischemic injury as we and others have shown.31,32 We postulated that inhibiting complex I during ischemia decreases electron transfer from complex I to complex III, thereby reduces electron leak, and superoxide (O2•−) generation at complex III during late ischemia. So, if ranolazine inhibits complex I in a manner similar to that of rotenone and amobarbital, then it could very well promote its anti-anginal effect in part by a mitochondrial mediated mechanism, which involves the reduction in reactive oxygen species generation during ischemia and subsequently on reperfusion.
Indeed, ranolazine was shown to reduce reactive oxygen species generation during ischemia.28 Furthermore, in a recent study Gadicherla et al showed that in hearts exposed to global ischemia, the addition of ranolazine on reperfusion improved mitochondrial complex I activity, restored electron transfer through Fe-S clusters of complex I, and preserved supercomplex assembly and cardiolipin integrity.33 However, there was no evidence that these observations were due to direct effects of ranolazine on complex I or even on mitochondria. Therefore, it was concluded that ranolazine indiscriminately reduced oxidative damage to complex I and its supportive structures and thus maintained optimal electron flow through complex I and reduced the tendency for electron leak and the subsequent generation of O2•−. It is important to mention that in the study by Wyatt et al30 ranolazine was shown to be a weak inhibitor of complex I when compared with rotenone or amobarbital, especially in energetically coupled mitochondria. However, in uncoupled or broken mitochondria (sub-mitochondrial particles) ranolazine exerted a more potent inhibitory effect on complex I. The authors suggested that this inhibitory effect by ranolazine occurred because of the lower membrane potential in uncoupled mitochondria coupled with a more acidic environment which favors greater protonation and uptake of ranolazine, or that it is only the charged form of ranolazine that is inhibitory. Nonetheless, regardless of the conditions of energisation of the mitochondrial inner membrane, the IC50 for inhibition of mitochondrial complex I by ranolazine was much higher than its therapeutic concentrations.
Ranolazine modulates myofilaments Ca2+ sensitivity
The general consensus so far is that ranolazine acts as an anti-anginal and anti-ischemic drug primarily by inhibiting INaL. In doing so, ranolazine prevents cytosolic Na+ overload and the subsequent excess in cytosolic Ca2+. The increase in cytosolic Ca2+ plays a key role in ischemic injury by causing mitochondrial Ca2+ overload, which then triggers a cascade of events that eventually leads to apoptosis/necrosis and cell death.34,35 Increase in cytosolic Ca2+ may induce damage by non-mitochondrial mechanisms such as by activating Ca2+-dependent phospholipases and proteases with the subsequent loss of structure and function of the myocyte.36 Ca2+ also may induce diastolic dysfunction which leads to a sustained contracture during diastole, an increase in myocardial O2 consumption and compression of intramural small vessels, thus reducing nutritive blood flow to the ischemic territory.37 Interestingly ranolazine was found to attenuate the diastolic dysfunction in several animal studies38–43 and in human studies.44,45 While ranolazine may reduce diastolic dysfunction by inhibiting INaL and the subsequent increase in cytosolic Ca2+, a very recent study may shed some light on a different mechanism for ranolazine.
In this study,46 the authors used a hypertensive mouse model (deoxycorticosterone acetate [DOCA]-salt) of diastolic dysfunction. It is important to mention that, in this study, no INaL was noted, nor were there changes in Ca2+ cycling to indicate significant alterations in Ca2+ handling in this form of diastolic dysfunction. Yet ranolazine was effective in abating this form of diastolic dysfunction. Myofilaments isolated from mice with diastolic dysfunction exhibited increased maximal tension and sensitivity to Ca2+ as well as a slowing of cross-bridge exit kinetics compared with sham mice, and these changes were normalized with ranolazine treatment. This suggested that ranolazine improved diastolic function at the cardiomyocyte level through the modulation of myofilament cross-bridge kinetics and sensitivity to Ca2+. Additionally, ranolazine, acting directly on myofilaments, improved diastolic dysfunction in the DOCA-salt sensitive mice with the same concentrations used clinically with no significant effects on hemodynamic parameters. The results obtained from the DOCA-salt sensitive mice may not be transferable to humans. Nonetheless, the finding of this study may hint to a new possible mechanism of ranolazine as an anti-anginal drug which deserves further exploration.
Clinical Studies
Several clinical trials have studied the effects of ranolazine in patients with coronary heart disease. Of these, three major trials focused on the role of ranolazine in treating chronic stable angina and one trial study focused on the role of ranolazine in treating acute coronary syndrome. A recent clinical trial focused on the role of ranolazine in PCI, and one more study assessed the cost of using ranolazine. There are no further studies assessing the effects of ranolazine on cardiovascular morbidity in patients with chronic angina. These studies are summarized below.
MARISA trial
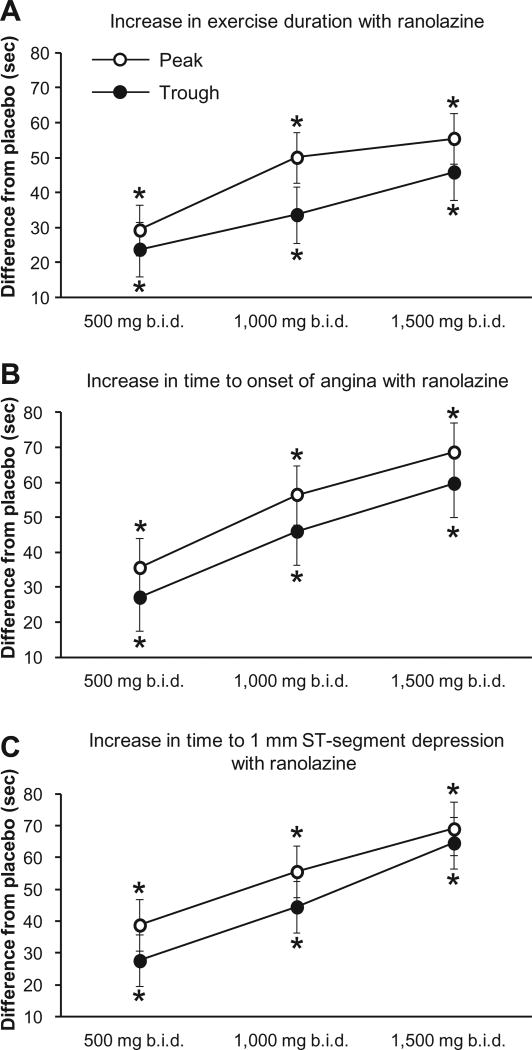
Before the MARISA (Monotherapy Assessment of Ranolazine In Stable Angina) trial, clinical studies focused on the immediate release formulation of ranolazine in combination with other anti-anginal drugs. The MARISA trial was the first study to focus on the sustained release formulation of ranolazine as a sole treatment for angina. The purpose of the study was to compare, in a dose dependent manner, the effects of the sustained release formulation of ranolazine on treadmill exercise performance and to provide long-term survival data in patients who completed the trial.10 In this study, 191 patients with documented CAD and treated with anti-anginal drugs were enrolled. These patients had to discontinue their anti-anginal treatments during the study except for sublingual nitroglycerin as needed. Randomized patients received a double-blind treatment with the sustained release formulation of ranolazine at doses of 500, 1000, and 1500 mg or a placebo, each administered twice daily for one week, according to a four-period, balanced Latin Square crossover design. At the end of each treatment period, exercise treadmill tests were performed at 4 h (peak) and later at 12 h (trough) after dosing. Compared to placebo, treatment with ranolazine significantly improved total exercise duration, time to angina, and time to 1 mm ST-segment depression at both peak and trough time points in a dose dependent manner (Fig. 1). Increase in ranolazine plasma concentrations was associated with an increase in mean exercise durations, but the increase in exercise duration appeared to plateau at the higher ranolazine concentrations. Further analysis showed that gender, age, diabetes, and prior history of heart failure did not significantly modify the treatment effects of ranolazine. Some patients from the MARISA trial were still receiving ranolazine at one year and two years after their first doses. Their one-year and two-year survival rates on ranolazine were 96.3% and 93.6%, respectively. The MARISA study showed also that ranolazine was a safe drug with side effects usually occurring at higher dosage. Common side effects included constipation, nausea, dizziness, and asthenia. Ranolazine also prolonged the corrected QT (QTc) interval in a dose dependent manner. In summary, the MARISA trial showed that ranolazine is an effective and safe drug when used alone as an anti-anginal treatment.
Figure 1.
Summary of the effects of three doses of ranolazine on exercise treadmill test parameters.
Notes: values shown represent difference from placebo in seconds. Data are mean ± standard error. *P < 0.05 in treatment with ranolazine vs. placebo. b.i.d. = twice daily.
CARISA trial
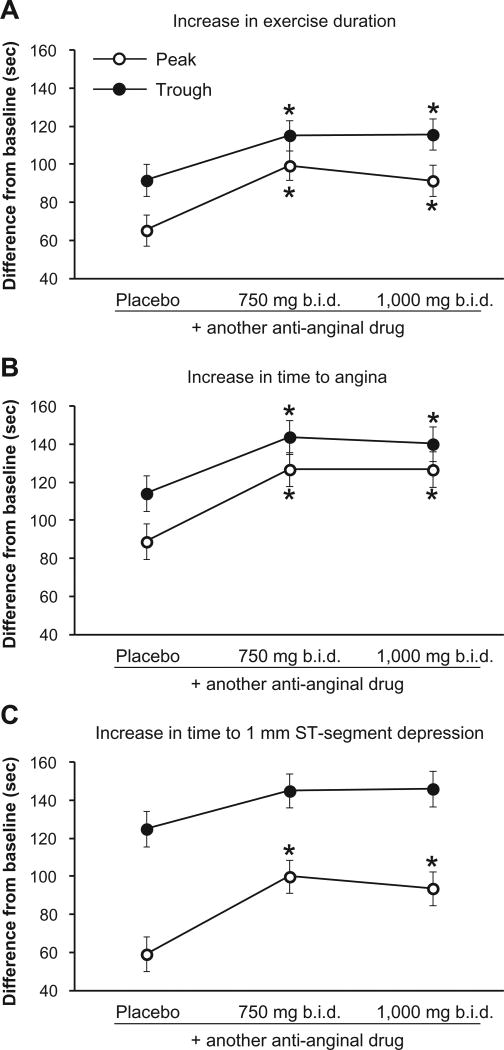
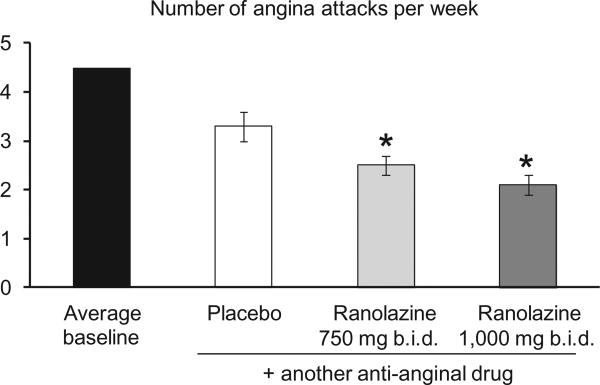
The purpose of the CARISA (Combination Assessment of Ranolazine In Stable Angina) trial was to assess the anti-anginal and anti-ischemic effects of ranolazine in symptomatic chronic angina patients with severe CAD when combined with standard doses of a typical anti-anginal drug, ie, atenolol, amlodipine, or diltiazem.9 The efficacy end points included treadmill exercise duration, time to angina, time to 1 mm ST-segment depression at peak and trough, and the number of angina attacks and sublingual nitroglycerin uses reported by the patients. In this study, 823 patients were assigned randomly to receive placebo, 750 mg ranolazine, or 1000 mg ranolazine twice daily for 12 weeks in addition to receiving another typical anti-anginal drug. The major finding was that ranolazine at the two doses significantly increased exercise duration at both the trough and the peak concentrations (Fig. 2). More importantly, treatment with the other anti-anginal drugs did not significantly modify the response to ranolazine. Ranolazine decreased the number of angina attacks and subsequently it decreased nitroglycerin consumption (Fig. 3). Some patients from the CARISA trial were still receiving ranolazine at one year and two years after their first doses. Their one-year and two-year survival rates on ranolazine were 98.4% and 95.9%, respectively. As in the MARISA trial, common side effects included constipation, nausea, dizziness, and asthenia with very few patients using 1000 mg ranolazine reporting episodes of syncope. Ranolazine also caused small increases in QTc interval. In summary, the CARISA trial showed that ranolazine can provide an additional anti-anginal effect in patients treated with the classical anti-anginal drugs.
Figure 2.
Summary of the effects of two doses of ranolazine on exercise treadmill test parameters.
Notes: values shown represent difference from baseline values in seconds. placebo indicates treatment only with another anti-anginal drug (atenolol 50 mg, amlodipine 5 mg, diltiazem 180 mg). Data are mean ± standard error. *P < 0.05 in treatment with ranolazine vs. placebo. b.i.d. = twice daily.
Figure 3.
Summary of the effects of two doses of ranolazine on number of angina attacks per week.
Notes: Placebo indicates treatment only with another anti-anginal drug (atenolol 50 mg, amlodipine 5 mg, diltiazem 180 mg). Data are mean ± standard error. *P < 0.05 in treatment with ranolazine vs. placebo. b.i.d. = twice daily.
ERICA trial
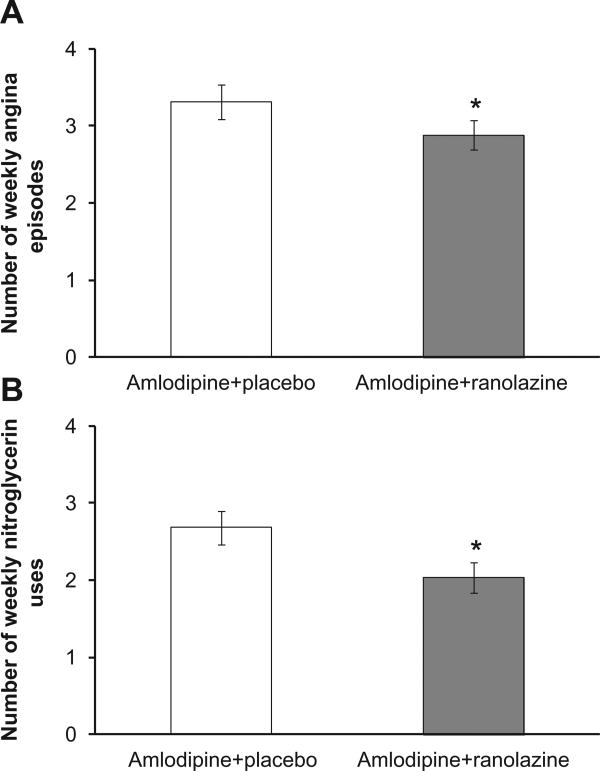
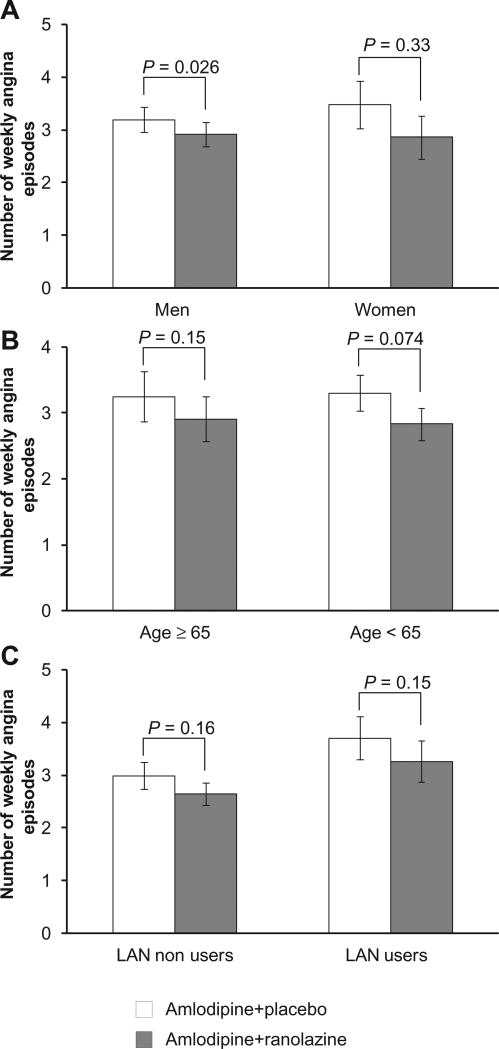
Prior to the ERICA (Efficacy of Ranolazine In Chronic Angina) trial, ranolazine was shown to be effective as an anti-anginal treatment when used alone or in combination with other anti-anginal drugs at sub-maximal dosage, but its efficacy when combined with a maximum recommended dosage of another conventional anti-anginal treatment had not been investigated. Therefore, the goal of the ERICA trial was to determine if ranolazine could reduce angina in patients with persistent angina despite treatment with the maximal recommended daily dosage of amlodipine.14 The efficacy of ranolazine was assessed by the weekly average frequency of angina episodes, the average weekly nitroglycerin consumption rate, and the change from baseline of the 5 dimensions of the Seattle Angina Questionnaire (SAQ). Safety was assessed by evaluating reported adverse effects, hemodynamics, laboratory measures, and ECG. In this study, 565 patients with ≥3 episodes of angina per week were receiving a maximum dosage of amlodipine at 10 mg/day. In addition to amlodipine, patients were randomized to also receive either 1000 mg ranolazine or placebo twice daily for 6 weeks. Adding ranolazine reduced the weekly rate of angina episodes (Fig. 4A), and it reduced the average weekly rate of nitroglycerin consumption (Fig. 4B), but it improved only the angina frequency dimension of the SAQ. The treatment effect of ranolazine in different subgroups was numerically similar to that in the population as a whole (Fig. 5), however the study was not powered to test the treatment effects within subgroups. Ranolazine did not induce any significant changes in heart rate or blood pressure when compared to placebo. The adverse effects occurred in 35.3% of amlodipine+placebo and 39.9% of amlodipine+ranolazine-treated patients with women and patients older than 65 reporting more adverse effects. In summary, the ERICA trial demonstrated that ranolazine provided additional anti-anginal benefit in patients who remained symptomatic despite being treated with the maximal dosage of the Ca2+ channel blocker amlodipine.
Figure 4.
Summary of the effects of ranolazine when added to a maximum dose of amlodipine (10 mg) on the number of angina episodes and nitroglycerin uses per week.
Notes: Data are mean ± standard error. *P < 0.05 in treatment with ranolazine vs. placebo.
Figure 5.
Summary of the effects of ranolazine when added to a maximum dose of amlodipine (10 mg) on the number of angina episodes per week in three subgroups.
Note: Data are mean ± standard error.
Abbreviation: LAN, long acting nitrate.
MERLIN-TIMI 36 trial
The goal of the MERLIN (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome)-TIMI 36 trial was to evaluate the efficacy and safety of ranolazine in the short and long term in patients with acute coronary syndromes who were also receiving standard therapy.47 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or recurrent ischemia through the end of the study. The major safety end points were assessed as death from any cause and the incidence of symptomatic documented arrhythmia. In this study, 6560 patients who were receiving standard treatment for non-ST elevation acute coronary syndrome were also randomized to additionally receive ranolazine or placebo. Ranolazine 200 mg (or matching placebo) was given first intravenously within 1 h followed by slower intravenous infusion over the next 12–96 h. Then, ranolazine extended-release (or matching placebo) tablet was given orally at a dose of 1000 mg twice daily for the remainder of the study (up to 24 months with a median of 348 days). Ranolazine did not reduce the rate of cardiovascular death, myocardial infarction, or recurrence of ischemia (ranolazine 21.8% vs. placebo 23.5%, P = 0.11), all considered as the primary endpoints of the study. However, the effect of long term treatment with ranolazine on angina was evident as it reduced worsening angina (ranolazine 4.2% vs. placebo 5.9%, P = 0.02). Moreover, an increase in or addition of an anti-anginal drug was less frequent (ranolazine 10.6% vs. placebo 13%, P = 0.003). There was no significant heterogeneity in the effect of ranolazine on the primary endpoints across the major subgroups examined. Death from any cause did not differ among patients treated with ranolazine compared with patients treated with placebo. The incidence of symptomatic documented arrhythmias throughout the duration of the study was similar in patients treated with ranolazine compared with placebo. However, the frequency of clinically significant arrhythmias observed during Holter monitoring during the first 7 days was lower in the ranolazine group vs. in the placebo group. Adverse events (dizziness, nausea, constipation, syncope) were more frequent with ranolazine vs. placebo. In summary, this study showed no significant benefit of ranolazine compared with placebo with respect to the primary efficacy endpoints. Also, ranolazine appeared to be safe but with no difference from placebo with respect to the safety endpoints.
Role of ranolazine during percutaneous coronary intervention
Myocardial injury may occur in patients undergoing PCI and this may lead to higher mortality. Therefore, the goal of this study was to determine if pretreatment with ranolazine before PCI has any protective role on periprocedural myocardial damage.48 The primary endpoint was defined as the postprocedural increase in creatine kinase-MB (CK-MB) ≥3 times of the upper limit of normal indicating periprocedural myocardial damage. Other cardiac markers such as Troponin I and myoglobin levels were also measured. In this study, 70 patients who fulfilled the inclusion criteria and scheduled for elective PCI were included and randomly divided into two groups, 35 patients each. One group received placebo while the other received ranolazine 1000 mg twice daily for 7 days before the planned intervention. Ranolazine reduced the periprocedural myocardial infarction (ranolazine 6% vs. placebo 22%, P = 0.041), which is the primary endpoint. Overall, there were fewer patients in the ranolazine group compared to the placebo group that showed increases above the upper limit of normal in CK-MB (ranolazine 23% vs. placebo 40%, P = 0.01), troponin I (ranolazine 31% vs. placebo 48%, P = 0.011), and myoglobin (ranolazine 28% vs. placebo 43%, P = 0.033). Further, ranolazine reduced the incidence of death, myocardial infarction, or target-vessel revascularization at 30 days after the procedure (ranolazine 9% vs. placebo 28%, P = 0.03), but this was mainly influenced by the periprocedural events because when periprocedural infarctions were not counted there was no significant difference between the two groups (ranolazine 3% vs. placebo 6%, P = 1). In summary, this study showed that pretreatment with ranolazine is effective in decreasing the incidence of myocardial infarction during PCI.
Costs and clinical outcome
Ranolazine per dose is more expensive than the other traditional anti-anginal treatments. However, the focus should be on total costs of care and not on the cost of single treatment. Although some studies analyzed the costs for treating angina, the study by Phelps et al49 was the first chronic angina medication-specific cost comparative study. In this study, a large managed care administrative claims database was analyzed to better understand the effect of ranolazine versus alternative anti-anginal drugs on health care use and total costs of care. Specifically, the primary dependent variables for this study were total costs of care and rate of revascularization procedures and services. A retrospective administrative claims-based analysis from a large, geographically diverse US health insurance plan with 11.2 million commercial enrollees was performed. First, patients who were identified to have angina were selected. Then of these, only patients who were prescribed a new anti-anginal drug (index event) that was not used within the previous 6 months (pre-index period) and continued using the new treatment for 6 more months (post-index period) were selected. This was to insure that only patients who had uncontrolled angina or had side effects that required their physician to alter their medication plan were enrolled. After further exclusion, 4545 patients were left for final analysis and were divided into 3 groups. Newly prescribed ranolazine, long acting nitrates, or β-blockers/Ca2+ channel blockers. In the 6 months pre-index period, there were no significant differences among all groups in total costs of care and in the rates of revascularization. Interestingly, even though ranolazine is more expensive per dose than the other anti-anginal drugs, the unadjusted total costs of care were lower for ranolazine than for the other groups. This is likely a result of fewer hospitalizations in patients treated with ranolazine compared to the other non-ranolazine treated groups. The ranolazine treated group also had significantly lower revascularization rates in the 6 months post-index period. Furthermore, a multivariate analysis was performed to control for differences in age, gender, and presence of co-morbid conditions that can affect total costs of care. But even then, the adjusted total costs of care and revascularization rates were significantly lower in the ranolazine treated group compared to the other non-ranolazine treated groups. Therefore, it was concluded that adding ranolazine to the therapy of patients with poorly controlled angina could lead to better outcome and lower costs compared with the other alternatives. These finding are all summarized in Table 2.
Table 2.
A summary of the differences between ranolazine and the other traditional anti-anginal drugs in total costs of care and rate of revascularization in the pre- and post-index period.
| Ranolazine (n = 881) | Nitrates (n = 1788) | β blockers/Ca2+ channel blockers (n = 1876) | |
|---|---|---|---|
| pre-index total cost $ | 19199 | 19841 (P = 0.603 vs. ranolazine) |
18152 (P = 0.410 vs. ranolazine) |
| % pre-index revascularization | 24.97 | 26.51 (P = 0.394 vs. ranolazine) |
25.16 (P = 0.915 vs. ranolazine) |
| Unadjusted post-index total cost $ | 14781 | 17773 (P = 0.022 vs. ranolazine) |
16785 (P = 0.120 vs. ranolazine) |
| % unadjusted post-index revascularization | 9.88 | 20.25 (P < 0.001 vs. ranolazine) |
15.51 (P < 0.001 vs. ranolazine) |
| adjusted post-index total cost $ | 13961 | 18166 (P < 0.001 vs. ranolazine) |
17612 (P = 0.002 vs. ranolazine) |
| % adjusted post-index revascularization | 9.9 | 20.4 (P < 0.001 vs. ranolazine) |
15.4 (P < 0.001 vs. ranolazine) |
Table assembled from Phelps et al. Clinical Therapeutics/volume 34, Number 6, 2012.
Safety
From all the 4 major clinical trials, ranolazine, at any dose, did not induce clinically significant changes in heart rate or blood pressure during rest or exercise. Overall, the side effects event rate increased with higher doses. The most common side effects of ranolazine are dizziness, nausea, vomiting, constipation, headache, and asthenia. However, all these side effects are mild to moderate in general, occur soon after using the drug, and reverse promptly with reducing the dose or discontinuation of treatment.50 The increase in adverse events seen with the 1500 mg dose in MARISA is disproportionately larger than the increase in anti-anginal efficacy; thus, the 1500 mg twice-daily dose is not recommended for clinical use. Postural hypotension and syncope were observed in a small number of patients taking ≥ 1000 mg ranolazine, most likely due to concomitant usage of other medications that are vasoactive or known to increase the plasma concentration of ranolazine. However, this can be prevented by starting ranolazine at a low dose (500 mg) and slowly increasing the dose as needed. Ranolazine may cause some small changes in laboratory parameters such as an increase in eosinophil count, an increase in creatinine, a decrease in hematocrit, and a decrease in hemoglobin A1C in diabetic patients. Ranolazine may prolong the QTc interval in a dose dependent manner,9,10 therefore it is recommended to avoid ranolazine in patients with known long QTc and in patients taking drugs that may prolong QTc.
Ranolazine is metabolized primarily in the liver by the enzyme CYP3A4, therefore caution should be taken in those who use other drugs that are known to interact with this enzyme or in patients with hepatic dysfunction as this may cause accumulation of ranolazine.51 Furthermore, ranolazine is cleared mainly by the kidneys and so careful dose titration is recommended in patients with mild to moderate renal impairment, whereas it is completely contraindicated in patients with severe renal impairment.52 Ranolazine is transported by P-glycoprotein7 and therefore it must be used cautiously with drugs that inhibit P-glycoprotein such as verapamil, as this may increase the absorption and subsequently the plasma concentration of ranolazine.
Efficacy
Early on, ranolazine was manufactured in an immediate release form and several clinical trials tested its efficacy for the treatment of chronic angina.11–13,53,54 These trials yielded results ranging from negative effects to studies showing benefits of ranolazine as an anti-anginal treatment. This range of effects likely stems from the different dosages utilized. Eventually, the immediate release form was replaced by the more favorable extended (sustained) release form which is now commercially available for treatment. The efficacy of this formulation was evaluated in the 4 large clinical trials summarized above.9,10,14,47 MERLIN-TIMI 36 was the only study to assess the efficacy of the extended formulation of ranolazine for treating non-ST-elevation acute coronary syndrome and it showed no benefit for ranolazine in this population. However, the other three studies (MARISA, CARISA, ERICA) demonstrated beneficial effects of ranolazine in treating chronic angina. For example, ranolazine alone (MARISA) increased the treadmill exercise performance similar to that observed with other anti-anginal drugs.55,56 However, when ranolazine was combined with other anti-anginal drugs in some trials (CARISA, ERICA), the beneficial effects of ranolazine seemed initially limited, ie, it reduced angina episodes by nearly 1 per week, and it increased exercise duration by nearly 24–34 s at trough concentrations (MARISA) compared to placebo. Nonetheless, one cannot underestimate these findings because a small increase in exercise duration translates into the ability of patients to carry on more daily living activities without being symptomatic. Therefore, and based on these findings, it is safe to say that ranolazine is recommended for the therapy of chronic angina as the sole drug, or combined with other treatments.
Patient Preference
Based on federal published guidelines, pharmacological management of chronic angina includes using short acting nitrates for treating the episode of angina, and then combined treatment with long acting nitrates, β-blockers, and Ca2+ channel blockers.57 As an alternative, ranolazine can also be used by itself or in combination with other anti-anginal drugs. Each one of these drugs has its share of side effects. Ranolazine, however, is the exception in that it brings about its anti-anginal effects without significant clinical changes in hemodynamics (blood pressure, heart rate). Therefore, it may be well suited for patients with lower blood pressures or heart rates in whom the addition or the gradual increase in the dose of anti-anginal drugs with important hemodynamic effects may not be tolerated.9 Moreover, ranolazine was shown to have anti-arrhythmic58 and anti-diabetic effects.59 The latter is of a particular interest since many patients who suffer from chronic angina already have diabetes mellitus. Ranolazine in fact was shown to reduce HbA1C in patients with diabetes mellitus and to possibly mitigate new hyperglycemia in patients at risk for diabetes mellitus. These findings make ranolazine an attractive anti-anginal treatment for patients with chronic angina and impaired glucose metabolism.59
Ranolazine costs considerably more than the other anti-anginal drugs per dose. However, and as discussed earlier, a comprehensive retrospective claims analysis49 showed that adding ranolazine as a replacement for another ineffective anti-anginal drug was associated with lower rates of prescription fills for short acting nitrates, reduced revascularization rates, and lower total cost of care greater than with other anti-anginal therapies (discussed above, see section Costs and Clinical Outcome). Therefore, the associated reductions in non-pharmacy costs of care when using ranolazine more than offset the higher prescription drug costs.49
Place in Therapy
The FDA approved the use of ranolazine as a first line drug for the treatment of chronic angina. However, the European Society of Cardiology (ESC) still recommends using ranolazine as an add-on therapy or as a substitution therapy when conventional drugs are not tolerated.2 Recent guidelines from the American College of Cardiology Foundation and the American Heart Association (ACCF/AHA, etc) also recommends using ranolazine in circumstances in which other anti-anginal drugs are not adequately effective or are not tolerated.60 Specifically, ranolazine can be used to relieve symptoms in patients with stable ischemic heart disease in two cases: (1) as a substitute for β-blockers if initial treatment with β-blockers leads to unacceptable side effects or is ineffective or if initial treatment with β-blockers is contraindicated; (2) in combination with β-blockers if the initial treatment with β-blockers is not successful. Ranolazine is available as extended-release tablets at 500 mg and 1000 mg, and a recommendation is to start ranolazine at 500 mg twice daily and increase to 1000 mg twice daily, as needed, based on clinical symptoms. The MERLIN-TIMI 36 trial showed no benefit of ranolazine in treating acute coronary syndrome; therefore ranolazine is not approved for treating these conditions. Clinical trials did not show significant differences in the efficacy of ranolazine between younger and older patients. However, older patients may have a greater frequency of decreased hepatic or renal function and therefore it is recommended to start ranolazine at the lowest dosing range in this population. Ranolazine should not be used in patients with cirrhosis, severe renal insufficiency, preexisting long QTc, or in patients using drugs that are known to prolong QTc or inhibit CYP3A4.
Conclusions
Coronary heart disease remains a global malady despite the advancement in its management. Chronic angina is one manifestation of CAD. Traditional anti-anginal therapy includes drugs that decrease O2 demand and increase blood flow. Ranolazine, however, belongs to a different class of anti-anginal drugs. It antagonizes the disturbance in ion homeostasis, specifically Na+ and subsequently Ca2+ that may occur during ischemia by inhibiting the INaL. Other possible mechanisms including its direct effect on cardiac myofilaments, or indirectly preserving mitochondrial complex I, may in part mediate its effects as an anti-anginal drug. Although ranolazine is more expensive than other traditional treatments per dose, it actually cuts the non-pharmacy costs of care in the long run. However, it remains important to compare total costs of care of ranolazine with the other anti-anginal drugs when ranolazine is used as a new, first line, anti-anginal drug and not only as an alternative therapy when the other treatments fail to control angina. Clinical trials showed ranolazine to lack many of the hemodynamic side effects observed with the other anti-anginal drugs, and more importantly to be an effective anti-anginal treatment when used alone or in combination with other conventional treatments. Yet, a direct comparison between ranolazine and the other conventional anti-anginal drugs (β-blockers, Ca2+ channel blockers, nitrates) is warranted before ranolazine can seriously be recommended as a first line therapy for chronic angina.
Acknowledgments
The authors wish to thank Dr. Edward J. Lesnefsky and Dr. Jennifer Strande for their help in the current guidelines for using ranolazine.
The authors grant exclusive rights to all commercial reproduction and distribution to Libertas Academica. Commercial reproduction and distribution rights are reserved by Libertas Academica. No unauthorised commercial use permitted without express consent of Libertas Academica. Contact tom.hill@la-press.com for further information.
Funding: This work was supported by grants from the National Institutes of Health (R01 HL095122, A.K.S. Camara/R.K. Dash; R01 HL089514, D.F. Stowe; P01 GM066730, Z.J. Bosnjak).
Footnotes
Author Contributions: Wrote the first draft of the manuscript: MA. Contributed to the writing of the manuscript: MA, DFS, AKSC. Agree with manuscript results and conclusions: DFS, AKSC. Jointly developed the structure and arguments for the paper: MA, AKSC. Made critical revisions and approved final version: MA, DFS, AKSC. All authors reviewed and approved of the final manuscript.
Competing Interests: Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics: As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006 Jun;27(11):1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 3.Holubkov R, Laskey WK, Haviland A, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002 Nov;144(5):826–33. doi: 10.1067/mhj.2002.125505. [DOI] [PubMed] [Google Scholar]
- 4.Hueb W, Soares PR, Gersh BJ, et al. The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004 May 19;43(10):1743–51. doi: 10.1016/j.jacc.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 5.Gilead S. Ranexa® (Ranolazine Extended-Release Tablets) [Accessed Jul 27, 2012]; http://www.Gilead.Com/Pdf/Ranexa_Pi.Pdf.Pdf.
- 6.Reddy BM, Weintraub HS, Schwartzbard AZ. Ranolazine: a new approach to treating an old problem. Tex Heart Inst J. 2010;37(6):641–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45(5):469–91. doi: 10.2165/00003088-200645050-00003. [DOI] [PubMed] [Google Scholar]
- 8.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996 Jan 1;93(1):135–42. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004 Jan 21;291(3):309–16. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 10.Chaitman BR, Skettino SL, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004 Apr 21;43(8):1375–82. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Cocco G, Rousseau MF, Bouvy T, et al. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol. 1992 Jul;20(1):131–8. [PubMed] [Google Scholar]
- 12.Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional anti-anginal agents. Ranolazine Study Group. Am J Cardiol. 1999 Jul 1;84(1):46–50. doi: 10.1016/s0002-9149(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005 Feb 1;95(3):311–6. doi: 10.1016/j.amjcard.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006 Aug 1;48(3):566–75. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Clarke B, Spedding M, Patmore L, McCormack JG. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol. 1993 Jul;109(3):748–50. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JW. Ranolazine for the management of coronary artery disease. Clin Ther. 2006 Dec;28(12):1996–2007. doi: 10.1016/j.clinthera.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 18.Randle PJ. Fuel selection in animals. Biochem Soc Trans. 1986 Oct;14(5):799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- 19.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiological effects of ranolazine, a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004 Aug 24;110(8):904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004 Aug;44(2):192–9. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004 Aug;310(2):599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 22.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006 May;148(1):16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teshima Y, Akao M, Jones SP, Marbán E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003 Nov 4;108(18):2275–81. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 24.Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992 Nov;71(5):1231–41. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol. 1994 Mar;266(3 Pt 2):H1034–46. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- 26.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997 May 1;500(Pt 3):631–42. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006 Jul;318(1):214–22. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 28.Aldakkak M, Camara AK, Heisner JS, Yang M, Stowe DF. Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol Res. 2011 Oct;64(4):381–92. doi: 10.1016/j.phrs.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyder A, Strege PR, Reyes S, et al. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel Na(v)1.5: a novel mechanism of drug action. Circulation. 2012 Jun 5;125(22):2698–706. doi: 10.1161/CIRCULATIONAHA.112.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyatt KM, Skene C, Veitch K, Hue L, McCormack JG. The anti-anginal agent ranolazine is a weak inhibitor of the respiratory complex I, but with greater potency in broken or uncoupled than in coupled mitochondria. Biochem Pharmacol. 1995 Nov 9;50(10):1599–606. doi: 10.1016/0006-2952(95)02042-x. [DOI] [PubMed] [Google Scholar]
- 31.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008 Jan 15;77(2):406–15. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006 Jan;316(1):200–7. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 33.Gadicherla AK, Stowe DF, Antholine WE, Yang M, Camara AK. Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim Biophys Acta. 2012 Mar;1817(3):419–29. doi: 10.1016/j.bbabio.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 35.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004 Feb 15;61(3):372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 36.Nayler WG. The role of calcium in the ischemic myocardium. Am J Pathol. 1981 Feb;102(2):262–70. [PMC free article] [PubMed] [Google Scholar]
- 37.Reuter H, Schwinger RH. Calcium handling in human heart failure—abnormalities and target for therapy. Wien Med Wochenschr. 2012 Jul;162(13–4):297–301. doi: 10.1007/s10354-012-0117-9. [DOI] [PubMed] [Google Scholar]
- 38.Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res. 1994 Aug;28(8):1231–7. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- 39.Hwang H, Arcidi JM, Jr, Hale SL, et al. Ranolazine as a cardioplegia additive improves recovery of diastolic function in isolated rat hearts. Circulation. 2009 Sep 15;120(Suppl 11):S16–21. doi: 10.1161/CIRCULATIONAHA.108.844167. [DOI] [PubMed] [Google Scholar]
- 40.Rastogi S, Sharov VG, Mishra S, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2149–55. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabbah HN, Chandler MP, Mishima T, et al. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail. 2002 Dec;8(6):416–22. doi: 10.1054/jcaf.2002.129232. [DOI] [PubMed] [Google Scholar]
- 42.Sossalla S, Wagner S, Rasenack EC, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008 Jul;45(1):32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006 May;17(Suppl 1):S169–77. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994 Oct;8(5):741–7. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- 45.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008 Dec;19(12):1289–93. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovelock JD, Monasky MM, Jeong EM, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012 Mar 16;110(6):841–50. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007 Apr 25;297(16):1775–83. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 48.Pelliccia F, Pasceri V, Marazzi G, Rosano G, Greco C, Gaudio C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. Am Heart J. 2012 Jun;163(6):1019–23. doi: 10.1016/j.ahj.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Phelps CE, Buysman EK, Gomez Rey G. Costs and clinical outcomes associated with use of ranolazine for treatment of angina. Clin Ther. 2012 Jun;34(6):1395–407. doi: 10.1016/j.clinthera.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Chaitman BR. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. J Cardiovasc Pharmacol Ther. 2004 Sep;9(Suppl 1):S47–64. doi: 10.1177/107424840400900105. [DOI] [PubMed] [Google Scholar]
- 51.Abdallah H, Jerling M. Effect of hepatic impairment on the multiple-dose pharmacokinetics of ranolazine sustained-release tablets. J Clin Pharmacol. 2005 Jul;45(7):802–9. doi: 10.1177/0091270005276739. [DOI] [PubMed] [Google Scholar]
- 52.Aslam S, Gray D. Ranolazine (Ranexa) in the treatment of chronic stable angina. Adv Ther. 2010 Apr;27(4):193–201. doi: 10.1007/s12325-010-0018-5. [DOI] [PubMed] [Google Scholar]
- 53.Jain D, Dasgupta P, Hughes LO, Lahiri A, Raftery EB. Ranolazine (RS-43285): a preliminary study of a new anti-anginal agent with selective effect on ischaemic myocardium. Eur J Clin Pharmacol. 1990;38(2):111–4. doi: 10.1007/BF00265967. [DOI] [PubMed] [Google Scholar]
- 54.Thadani U, Ezekowitz M, Fenney L, Chiang YK. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Ranolazine Study Group. Circulation. 1994 Aug;90(2):726–34. doi: 10.1161/01.cir.90.2.726. [DOI] [PubMed] [Google Scholar]
- 55.Parker JO, Amies MH, Hawkinson RW, et al. Intermittent transdermal nitroglycerin therapy in angina pectoris. Clinically effective without tolerance or rebound. Minitran Efficacy Study Group. Circulation. 1995 Mar 1;91(5):1368–74. doi: 10.1161/01.cir.91.5.1368. [DOI] [PubMed] [Google Scholar]
- 56.Pratt CM, McMahon RP, Goldstein S, et al. Comparison of subgroups assigned to medical regimens used to suppress cardiac ischemia (the Asymptomatic Cardiac Ischemia Pilot [ACIP] Study) Am J Cardiol. 1996 Jun 15;77(15):1302–9. doi: 10.1016/s0002-9149(96)00196-8. [DOI] [PubMed] [Google Scholar]
- 57.Management of Stable Angina. A National Clinical Guideline. [Accessed Aug 6, 2012]; http://www.Guideline.Gov/Content.Aspx?Id=34825&Search=Chronic+Angina.
- 58.Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an anti-anginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007 Oct 9;116(15):1647–52. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 59.Morrow DA, Scirica BM, Chaitman BR, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009 Apr 21;119(15):2032–9. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- 60.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012 Nov 19; Epub ahead of print. [Google Scholar]