Abstract
Therapeutic Abs possess several clinically relevant mechanisms of action including perturbation of tumor cell signaling, activation of complement-dependent cytotoxicity, Ab-dependent cellular cytotoxicity (ADCC), Ab-dependent cellular phagocytosis (ADCP), and induction of adaptive immunity. In view of the important role of phagocytic lineage cells in the mechanism of action of therapeutic Abs, we analyzed FcγR receptor-dependent effector functions of monocytes and macrophages triggered by glycoengineered (GE) Abs (having enhanced FcγRIIIa [CD16a] binding affinity) versus their wild-type (WT) counterparts under different experimental conditions. We first defined the precise FcγR repertoire on classical and nonclassical intermediate monocytes—M1 and M2c macrophage populations. We further show that WT and GE Abs display comparable binding and induce similar effector functions (ADCC and ADCP) in the absence of nonspecific, endogenous IgGs. However, in the presence of these IgGs (i.e., in a situation that more closely mimics physiologic conditions), GE Abs display significantly superior binding and promote stronger monocyte and macrophage activity. These data show that in addition to enhancing CD16a-dependent NK cell cytotoxicity, glycoengineering also enhances monocyte and macrophage phagocytic and cytotoxic activities through enhanced binding to CD16a under conditions that more closely resemble the physiologic setting.
Introduction
Over the past decades, several anticancer Ab therapeutic agents have been developed and tested in the clinic, and 12 are currently approved for use in oncology (1–6). Many of these agents affect tumor growth by interfering with receptor signaling. For example, trastuzumab (targeting Her2/neu) and cetuximab (targeting EGFR) counteract tumor progression by blocking receptor downstream signaling, whereas rituximab (targeting CD20) and alemtuzumab (targeting CD52) induce direct cell death or apoptosis (3, 7, 8).
In addition to direct antitumor effects, therapeutic mAbs control tumor progression through additional mechanisms, including complement-dependent cytotoxicity, and immune cell effector functions such as Ab-dependent cellular cytotoxicity (ADCC) and Ab-dependent cellular phagocytosis (ADCP) (3). ADCC and ADCP are triggered through interaction of the Ab fragment crystallizable (Fc) domain with the corresponding Fcγ receptors (FcγRs) expressed on NK cells, neutrophils, monocytes, macrophages, dendritic cells (DCs), and eosinophils (3, 9). Once engaged, FcγRs transduce activating signals through ITAMs or inhibitory signals through ITIMs. Most Fc-dependent inhibitory signals are transduced via FcγRIIb (CD32b), whereas most stimulatory signals are transduced by FcγRI (CD64) and CD16a (10). CD64 is a high-affinity receptor expressed by macrophages, DCs, neutrophils, and eosinophils, whereas CD16a is a low-affinity receptor expressed by NK cells, DCs, macrophages, and mast cells; it is required for NK cell–mediated ADCC (10).
Various engineering technologies have recently been developed to modulate the binding affinity of the Fc region of therapeutic Abs to different FcγRs to enhance or suppress FcγR-dependent immune effector functions. This has been achieved either by modifying the structure of the Fc-attached oligosaccharides (glycoengineering) or by engineering the Fc-polypeptide backbone. In particular, glycoengineered (GE) Abs enriched in glycoforms lacking core fucose residue from oligosaccharides attached at Asn297 of the Fc have significantly enhanced binding affinity to CD16a and are more potent and efficacious at mediating ADCC (6, 11–14)
Although removing the core fucose residue from Fc-oligosaccharides increases the affinity of all IgG subclasses to human activating CD16a (and to its mouse ortholog FcγRIV) by 10–50-fold, binding to other human activating and inhibitory receptors, including CD32a or CD32b, remains largely unchanged. Despite the overall similarity in protein structure, individual FcγRs have unique binding patterns. In the case of the CD16a-IgG Fc interaction, the carbohydrate attached to CD16a at its Fc binding interface, a structural element unique to CD16a among FcγRs, is required for the increased binding affinity to afucosylated, GE Abs (10, 15–17).
Glycoengineering confers two clinically relevant properties: 1) the ability to achieve high levels of ADCC, even in individuals harboring the low-affinity CD16a allotype (158Phe), potentially overcoming the problem of individual heterogeneity in CD16a polymorphism and therapy response and 2) the ability to preserve activity in the presence of high concentrations of nonspecific serum IgGs (>10 mg/ml) that compete with conventional therapeutic Abs for FcγRs and impair their activity. In line with these properties, a number of preclinical studies demonstrated that Ab Fc-afucosylation translates into significantly enhanced activity in vivo (6, 9, 17), and this has led to the approval of new therapeutic GE Abs. GA101 (obinutuzumab) is a GE, type II anti-CD20 mAb that has recently been approved by the U.S. Food and Drug Administration for the first line treatment of patients with chronic lymphocytic leukemia (CLL) in combination with chlorambucil (18–20). In Japan, the GE CCR4 Ab mogamulizumab has been approved for treatment of patients with relapsed or refractory CCR4+ T cell leukemia-lymphoma (21).
The effector cells contributing to the efficacy of therapeutic Abs and their relative contribution in vivo have not been clearly defined. NK cells are considered as one of the players because they rapidly elicit potent ADCC in vitro in comparison with freshly isolated monocytes or granulocytes (6, 17, 22, 23) and infiltrate tumors in trastuzumab-treated patients (24). On the other hand, several preclinical studies in murine models convincingly demonstrated that macrophage subsets are primarily responsible for eliminating circulating and tissue B cells upon anti-CD20 mAb therapy and essentially excluded a role for NK cell-, T cell- or perforin-dependent mechanisms in vivo in those particular models (25–29). In addition, it has been shown recently that glycoengineering through its enhanced affinity to CD16b on neutrophils results in enhanced neutrophil mediated ADCP and neutrophil activation as compared with the respective wild type (WT) Abs (30).
In view of the potential important role of phagocytic lineage cells in the mechanism of action of therapeutic Abs, we investigated whether Ab glycoengineering enhances their cytotoxic (ADCC) and phagocytic (ADCP) activity in comparison with conventional Abs. To reflect the plasticity of myeloid lineage cells better we evaluated the activities on classical (CD14++/CD16−) monocytes, nonclassical/intermediate (CD14+/CD16+) monocytes, classically activated type I macrophages (M1) and alternatively activated type II macrophages (M2). In the current study, we report a quantitative assessment of FcγRs expression on different monocyte and macrophage subsets and provide evidence that GE, afucosylated Abs display enhanced binding to CD16+ monocytes and macrophages and induce significantly higher levels of ADCC and ADCP than the respective WT Abs do under conditions that more closely reflect the natural ones.
Materials and Methods
Abs and reagents
GA101 GE and WT, GA201 GE and WT, trastuzumab GE and WT, and rituximab were obtained from F. Hoffmann-La Roche AG. Ofatumumab was obtained from a local pharmacy. Cetuximab (commercial grade) was obtained from Merck, and the nonspecific total human IgG Redimune was obtained from CSL Behring. PKH67 (PKH67GL), PKH26 (PKH26GL) and CFSE (#21888) dyes were obtained from Sigma-Aldrich. The characteristics of the WT and GE variants of the therapeutic Abs evaluated in the current study were as follows: GA101 (Gazyva [obinutuzumab]; GE type II anti-CD20 Ab), MabThera (rituximab; type I anti-CD20 Ab approved in non-Hodgkin's lymphoma, CLL, and rheumatoid arthritis), Arzerra (ofatumumab; type I anti-CD20 Ab approved in CLL), GA201 (GE Ab targeting EGFR, clinical trial phase II in colorectal carcinoma and head and neck carcinoma (currently on clinical hold), Erbitux (cetuximab; targeting EGFR in colorectal carcinoma and squamous cell carcinoma), Herceptin (trastuzumab; targeting Her2 in breast carcinoma).
Cell culture
Raji and WIL2S cells (Ref: 85011429 and 90112121, respectively; European Collection of Cell Cultures [ECACC]) as well as A549 (Ref: 86012804, ECACC) and KPL-4 (Kawasaki Medical School) were cultivated in DMEM containing 10% FCS and N-acetyl-l-alanyl-l-glutamine (2 mM). MKN45 (DSMZ Ref: ACC 409) were cultivated in RPMI 1640 containing 10% FCS and N-acetyl-l-alanyl-l-glutamine (2 mM). 1833-PPOP233 cells (provided by Thomas Weber, Roche Diagnostics, Penzberg, Germany) were cultivated in DMEM containing 10% FCS and N-acetyl-l-alanyl-l-glutamine (2 mM) plus 200 μg/ml G418.
Monocyte isolation and generation of polarized macrophage subsets
PBMCs were isolated from buffy coats obtained from the Blutspendezentrale Zürich (Switzerland). Depending on the assay requirements, monocytes were isolated using the Pan Monocyte Isolation Kit (for pan monocyte isolation), the Monocyte-Isolation Kit II (for classical, CD16 negative monocyte isolation) or CD16+ Monocyte Isolation Kit (for intermediate, nonclassical, CD16+ monocyte isolation), all from Miltenyi Biotech. For monocyte-derived macrophage (MDM) generation, pan monocytes or classical monocytes were incubated in RPMI 1640 supplemented with human macrophage CSF (M-CSF) (30 ng/ml), 10% FCS, 2 mM glutamine for 6–7 d. After removal of M-CSF, M1 macrophage subtype was generated by polarizing MDMs with human IFN-γ (100 ng/ml) and LPS (100 ng/ml) for an additional 24 h; M2c was generated by polarizing MDMs with human IL-10 (10 ng/ml) for an additional 48 h; M2a was generated by polarizing MDMs with IL-4 (20 ng/ml) for an additional 24 h; and M2b was generated by polarizing MDMs with freshly prepared immune complexes (10 μg/ml) and LPS (100 ng/ml) for an additional 24 h. All cytokines used were from Peprotech. Immune complexes have been prepared using OVA (Sigma Aldrich, #A5503) and anti–chicken egg white antiserum (Sigma Aldrich, #C6534) (31). LPS was obtained from Sigma Aldrich (#L5629).
Quantification of FcγRs on monocytes and macrophages
Freshly isolated human classical and intermediate, nonclassical monocytes or polarized macrophages were stained with anti-human CD16-FITC (BD Bioscience; clone 3G8, slightly higher affinity for CD16a compared with CD16b) or anti-human CD32-FITC (BD Bioscience; clone FLI8.26, higher affinity and avidity for CD32a compared with CD32b) or anti-human CD64-FITC (BD Bioscience, clone 10.1) for 30 min at 4°C, washed and measured immediately by flow cytometry (BD FACSCanto II) in parallel with calibration beads consisting of Ab-coated Quantum Simply Cellular microspheres (Bang Laboratories) preincubated with the same Abs according to the manufacturer’s instructions. The Ab-binding capability (ABC) for each Ab was calculated for monocytes and M1 and M2c macrophage subsets according to the manufacturer’s instructions.
Labeling of Abs
Labeling with Alexa Fluor 647 (Alexa Fluor 647 carboxylic acid, succinimidyl ester [Invitrogen], 5 mg, A20106, dissolved in DMSO with concentration 10 mg/ml) was performed in PBS (Life Technologies DPBS1×, Ref no 14190-136) at a molar ratio of 7.5 (dye to protein), with final 0.1 M NaHCO3 and a final protein concentration of 4 mg/ml. After 1 h incubation at room temperature (end-over-end shaking), the free dye was removed on a Zeba-Spin 0.5-ml column (40 kDa cutoff; Thermo scientific, No 87766). Absorption A280 and A650 was determined and concentration was calculated:
Determination of labeling efficiency was calculated:
Binding of therapeutic Abs to monocytes and macrophages
Fluorescently labeled Abs were generated in house using AlexaFluor647 fluorochrome (see Materials and Methods). AlexaFluor647-labeled GE (GA101 and GA201) and wild-type Abs (GA101 WT, GA201 WT, and ofatumumab; dye/protein ratio 5.3–7.6) were incubated with human monocytes or polarized macrophage subsets for 30 min at 4°C in the presence or absence of 10 mg/ml Redimune. For the assessment of the role of CD16, M1 and M2c macrophages were preincubated with saturating concentration of the blocking CD16 Ab (BD; FITC-labeled clone 3G8) for 45 min at 37°C.
ADCP
CFSE-labeled target cells (Raji or KPL4) were incubated with monocytes, M1 or M2c macrophages (E:T 3:1), and increasing concentrations of anti-CD20 (Raji) or anti-Her2 Abs (KPL-4) for 1–24 h at 37°C in the presence or absence of competing unspecific human IgGs (10 mg/ml Redimune). Typical IgG concentrations in human plasma cover 8–18 mg/ml. Cells were subsequently stained with anti-CD206–FITC or anti-CD206–PE and anti-human CD22-APC (Raji) or anti-human Epcam-APC (KPL-4) Abs (all from BioLegend) and subject for FACS analysis. ADCP by monocytes was determined by gating total target cells and determining the percentages of CFSE+/CD206+ double-positive target cells in a child gate. ADCP by macrophages was determined by gating total target cells and determining the percentages of CFSE+/CD206+ double-positive target cells in a child gate, creating another child gate of this to distinguish phagocytosed targets from effector attached targets by determining the percentages of Epcam-negative, CFSE+/CD206+ targets.
ADCC
Target cells (A549 or MKN45) were incubated with human monocytes (E:T ratio, 4:1) or macrophages (E:T ratio, 3:1) for 24 h in the presence of increasing concentrations of anti-EGFR Abs (GA201 GE, GA201 WT, cetuximab). Lactate dehydrogenase (LDH) release was measured using the LDH Cytotoxicity Detection Kit (Roche Applied Science). Target cell killing was calculated using the following formula:
Spontaneous release, corresponding to LDH released by target cells without Ab and effectors, was defined as 0% cytotoxicity. Maximal release (corresponding to LDH released by target cells lysed with 2% Triton X-100) was defined as 100% cytotoxicity.
In addition to LDH release, ADCC was determined by luminescence in an assay based on the assessment of active caspase-3/7 pathway in tumor target cells using 1833-POPP233 cells stably transfected with plasmid coding for split luciferase (C-Luc and N-Luc domain fused with intervening caspase-3/7 cleavage motif [DEVD]). The cleavage of the reporter molecule by activated caspase-3/7 enables interaction of C-Luc and N-Luc and reconstitutes luciferase activity (32, 33). 1833-POPP233 target and effector cells (human M1 or M2c macrophages of CD16+ monocytes) were incubated for 8 or 24 h in the presence or absence of WT or GE GA201 (1 μg/ml), and luminescence was quantified by the addition of D-Luciferin (Biosynth).
Overall monocyte and macrophage activity (cell ELISA)
MKN45 or KPL4 cells were incubated with human macrophages (E:T ratio, 3:1) and 1 μg/ml anti-EGFR (GA201 versus cetuximab) or anti-Her2 Abs (trastuzumab GE versus WT) in the presence or absence of competing IgGs (10 mg/ml Redimune) for 48 h. Raji cells were incubated with human isolated monocytes of monocyte-derived macrophages (E:T ratio, 1:1) and 1 μg/ml of anti-CD20 Abs (GA101, rituximab, ofatumumab) in the presence or absence of competing IgGs (10 mg/ml Redimune) for 24 h. At the end of the incubation time, coculture was washed and fixed with PFA. Intact target cells, which escaped macrophage ADCC and ADCP, were quantified using anti-human Epcam (MKN45, KPL-4) or anti-human CD19 (Raji) Abs followed by detection using HRP-labeled secondary Ab (all Abs derived from BioLegend). The OD of 540–570 nm was determined after incubation with TMB (3,3′,5,5′ tetramethylbenzidine from BioLegend). The reaction was stopped using H2SO4 2N (Sigma-Aldrich). Ab-dependent killing of targets by macrophages was calculated using the following formula:
Spontaneous release, corresponding to target cells incubated with effector cells without Ab was defined as 0% cytotoxicity, with maximal release (target cells lysed with 2% Triton X-100) defined as 100% cytotoxicity. The mean and SD of quadruplicates of each experiment were calculated.
NO release
Supernatants were collected from different ADCC and cell ELISA assays described above, corresponding to cocultures of human monocytes or macrophages with Raji or WIL2S target cells (E:T ratio, 1:1) in the presence of anti-CD20 Abs (GA101 GE or WT, ofatumumab or rituximab). NO release was detected in cell supernatants using the Griess Reagent (Molecular Probes). The amount of NO in the supernatants was calculated based on the standard curve generated by the supplier.
Wide-field fluorescence microscopy
PKH26-labeled MKN45 cells were incubated with PKH67-labeled M2c macrophages in the presence of anti-EGFR Abs (GA201 GE, cetuximab or isotype control GA101 GE at 1 μg/ml) and 10 mg/ml competing IgGs (Redimune). Cell culture medium was exchanged before image acquisition to Leibovitz L-15 without phenol red supplemented with 10% FBS. Cells were imaged using a 100× oil immersion objective, 1.3 numerical aperture on an Axio Observer inverted microscope with stage-top incubator with support for 35 mm dish with CO2 (5%) and temperature (37°C) controllers (CTI Controller 3700 and Temp Control 37-2, respectively). Fluorescence time-lapse images were acquired using a CoolSNAPHQ2 camera every 5 min for 3 h and 14 h (overnight) on multiple positions per dish. Exposure times were 30 ms for PKH67, 100 ms for PKH26, and 10 ms for bright field.
Results
Isolation of monocytes and generation of macrophage subsets
Classical and intermediate, nonclassical monocytes were isolated from buffy coats derived from healthy donors (see Materials and Methods). Macrophages were generated by culturing pan monocytes in the presence of M-CSF for 6–7 d before polarization to M1, M2a, M2b, and M2c macrophage subsets (see Materials and Methods) (34–36). The purity of isolated monocytes and the efficiency of macrophage polarization were confirmed by analysis of cell surface markers and cytokines known to be expressed or secreted by the above-mentioned subsets (Supplemental Fig. 1). In agreement with literature data, classical monocytes were highly positive for CD14 and negative for CD16, whereas intermediate and nonclassical monocytes express CD14 and CD16. M1 macrophages expressed high levels of CD80, MHC class II and secreted IL-12, TNF-α, IL-6, and IP10. M2a expressed CD206 and MHC class II and secreted predominantly CCL-22. M2b expressed MHC class II and secreted predominantly TNF-α, IL-10, and IL-6, whereas M2c were MHC class II negative, expressed CD163 and IL-1RII, and secreted high levels of IL-10, confirming the validity of polarization methods applied in the current study (7, 35–37).
Expression of CD16, CD32, and CD64 and binding of therapeutic Abs
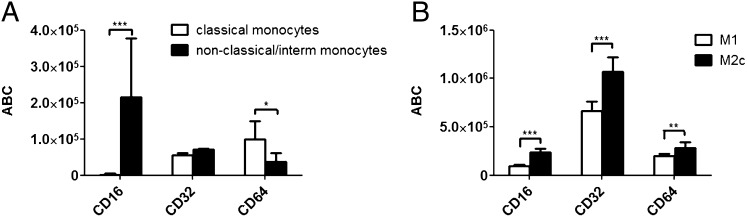
The quantitative assessment of the expression of CD16a,CD16b, CD32a, CD32b, and CD64 on monocytes and M1 and M2c macrophages was performed using quantum beads FACS-based method (see Materials and Methods). Despite the variability between donors, CD16a and CD16b displayed a high level of expression on intermediate, nonclassical monocytes and, in agreement with literature, were absent on classical monocytes. CD32a and CD32b had similar expression on both monocyte subsets, whereas CD64 was higher on classical monocytes (Fig. 1A). Interestingly, all three FcγRs displayed higher levels on M2c macrophages than on M1 (Fig. 1B). CD32a and CD32b were the most abundantly expressed Fcγ receptors on both macrophage subsets, followed by CD64, CD16a, and CD16b (Fig. 1B). When comparing monocytes with macrophages, we found that the expression of CD16a and CD16b was comparable between intermediate, nonclassical monocytes and macrophages, whereas the levels of CD32a, CD32b, and CD64 were 5–10-fold higher on macrophages (Fig. 1A, 1B).
FIGURE 1.
Quantitative assessment of FcγRs surface expression on human monocytes and M1 and M2c macrophages. (A) Freshly isolated monocytes or (B) M1- and M2c-polarized macrophages were analyzed with flow cytometry to quantify the surface expression of CD64, CD32, and CD16. Ordinate denotes the ABC obtained for each receptor. Data represent the mean ABC fluorescence ±SD derived from analysis of four different donors. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001.
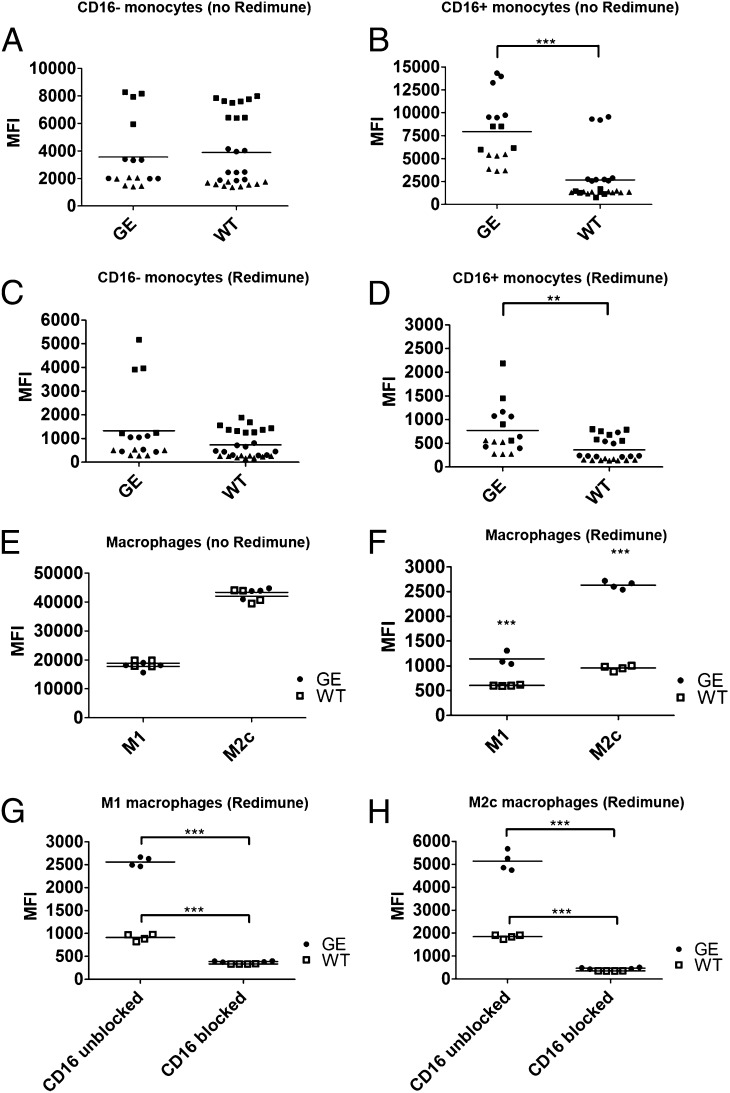
The binding of therapeutic mAbs to monocytes and macrophages was assessed using WT and GE Fc variants of therapeutic Abs targeting CD20 (GA101 and ofatumumab) and EGFR (GA201; Fig. 2). To more accurately reproduce physiologic conditions, Ab binding was also assessed in the presence of physiologic concentrations of normal human endogenous IgGs (10 mg/ml Redimune), which in general caused a 10-fold reduction in Ab binding (compare mean fluorescence in Fig. 2A and 2B to Fig. 2C and 2D and Fig. 2E to Fig. 2F). WT and GE Abs displayed comparable binding pattern on classical (CD16−) monocytes independently of the presence of competing IgGs (Fig. 2A, 2C). Interestingly, CD16+ intermediate, nonclassical monocytes bound significantly higher amounts of GE Abs in the absence and presence of competing IgGs, underscoring the key role of CD16 in binding of GE Abs (Fig. 2B, 2D).
FIGURE 2.
Binding of therapeutic Abs to human monocytes and M1 and M2c macrophages. The binding of AlexaFluor647 directly labeled WT and GE Abs was assessed using classical (CD16−) monocytes (A, C), nonclassical intermediate (CD16+) monocytes (B, D) and M1 and M2c macrophages (E, F). The binding of each Ab was assessed in absence (A, B, E) or presence (C, D, F) of competing endogenous human IgGs (10 mg/ml Redimune). Each symbol in (A)–(D) corresponds to median fluorescence intensity of single data points derived from three independent experiments (donors). Donors are identified by the following symbols: donor 1 = black circles; donor 2 = black squares; donor 3 = black triangles. Two GE Abs were used (GA101 and GA201) as duplicates or triplicates, therefore resulting in four to six symbols per donor per condition. Three WT Abs were used as duplicates or triplicates (GA101 WT, GA201 WT, and ofatumumab), resulting in six to nine symbols per donor per condition. (G and H) Binding of directly labeled WT and GE Abs in the presence of saturating concentrations of blocking CD16 Ab and 10 mg/ml Redimune to M1 (G) and M2c (H) macrophages. Data in (E)–(H) correspond to median fluorescence intensity of duplicates derived from one representative experiment out of a total of three independent ones using GA101 GE, GA201 GE, GA101 WT, and ofatumumab. Two of these experiments included the blocking anti-CD16 Ab. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001.
The binding of GE and WT Abs to M1 and M2c macrophages was undistinguishable in the absence of competing IgGs (Fig. 2E). However, in the presence of competing IgGs, GE Abs displayed a clearly superior binding to both macrophage subsets (Fig. 2F). This difference was even more accentuated on M2c macrophages compared with M1, most likely because of their higher CD16 expression (Fig. 1B). To confirm that CD16 is the Fcγ receptor responsible for the superior binding of GE Abs compared with their WT counterparts, the binding to M1 and M2c macrophages was assessed in the presence of blocking CD16 Abs and competing endogenous IgGs (Fig. 2G, 2H). Blocking of CD16 abolished binding differences between GE and WT Abs, confirming its central role in mediating the binding superiority of GE Abs, as also supported by findings on CD16+ monocytes.
ADCP
The ADCP of monocytes and the four different macrophage subsets (M1, M2a, M2b, and M2c) was initially assessed in a 1-h coculture assay with tumor cells and GA101 (Supplemental Fig. 2). Overall, monocytes mediated the highest maximal ADCP (87%), followed by M2c, M2a (62–70%), and M1 (42%) macrophages. M2b macrophages displayed poor ADCP activity (21%) most likely because of occupancy of their FcγRs by immune complexes used for their generation. In general, the addition of therapeutic Ab led to a dose-dependent enhancement of monocyte and macrophage phagocytic activity of all myeloid subsets that were tested.
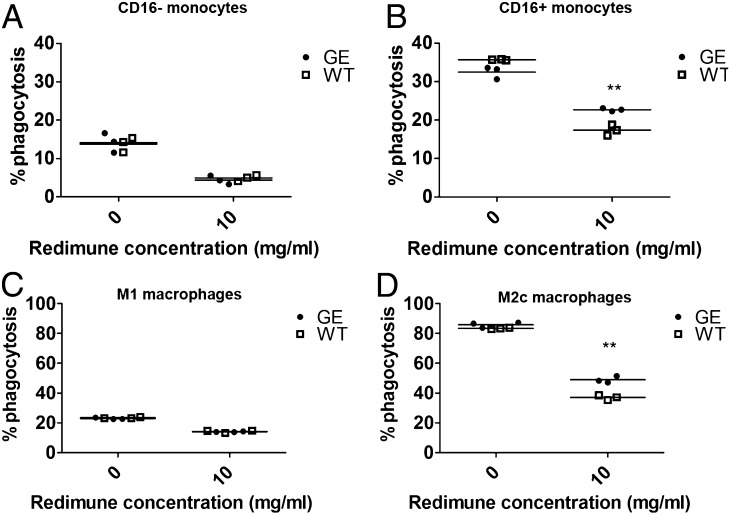
To investigate differences in the activity of WT and GE Abs, ADCP was assessed in the absence and presence of competing endogenous IgGs (10 mg/ml Redimune). In agreement with binding data, the activity of WT and GE Abs was comparable using classical (CD16−) monocytes under all experimental conditions (Fig. 3A). The activity of GE and WT Abs was also comparable on intermediate, nonclassical (CD16+) monocytes in the absence of competing IgGs (Fig. 3B; 0 mg/ml Redimune), despite higher binding of GE Abs under the same experimental conditions (Fig. 2B). Notably, the superiority of GE Abs using intermediate, nonclassical (CD16+) monocytes became clearly detectable upon the addition of competing IgGs (Fig. 3B; 10 mg/ml Redimune).
FIGURE 3.
Ab-dependent phagocytosis. CFSE-labeled Raji cells were cocultured for 4 h with isolated human classical monocytes (A) or nonclassical, intermediate monocytes (B) (E:T ratio, 3:1) in presence of GA101 GE or WT Abs and in the absence or presence of 10 mg/ml Redimune. KPL-4 cells were cocultured for 4 h with M1 (C) or M2c macrophages (D) (E:T ratio, 3:1) in the presence of GE or WT trastuzumab and 10 mg/ml Redimune. The percentage of phagocytized target cells was determined with flow cytometry as described in Materials and Methods. Triplicates of representative experiments (performed using different targets and GE/WT Ab pairs (GA101, trastuzumab) are shown. Statistical analysis, unpaired t test: **p < 0.01.
Similar studies were performed using M1 and M2c macrophages (Fig. 3C, 3D). As for binding, the presence of competing IgGs significantly reduced the overall ADCP, particularly on M1 macrophages on which we could not detect significant differences between Abs (Fig. 3C; compare ADCP at 0 mg/ml to 10 mg/ml Redimune). On the other hand, the phagocytic activity of M2c macrophages was found to be significantly higher than that of M1 macrophages and was better preserved in the presence of competing IgG, allowing solid differentiation of GE and WT Ab-mediated ADCP (Fig. 3D). In the presence of competing IgGs, GE Abs display superior binding to CD16+ monocytes and M2c macrophages, and they mediate higher levels of ADCP compared with their WT counterparts.
ADCC
The cytotoxic activity of monocytes and M1 and M2c macrophages was detected by quantifying LDH released in cell culture supernatants (Fig. 4). In contrast to phagocytosis, which occurs rapidly and is efficiently quantified after 1–4 h, ADCC was found to be a less efficacious process exerted by monocytes and macrophages, which required longer incubation times of 24 h to be detected. In addition, whereas ADCP (despite being significantly reduced) was still measurable in the presence of competing IgGs, the weak cytotoxicity of monocytes and macrophages was completely abolished. For that reason, data related to monocyte and macrophage cytotoxicity are reported only in the absence of competing IgGs.
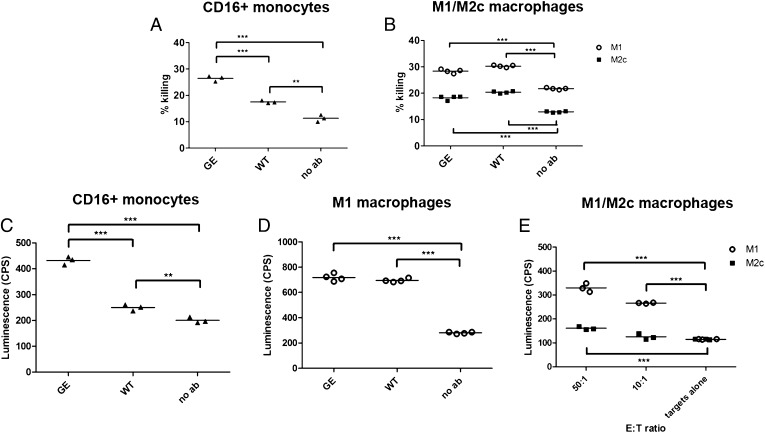
FIGURE 4.
Macrophage-mediated cytotoxicity. (A) A549 cells were incubated with isolated human intermediate, nonclassical (CD16+) monocytes (E:T ratio, 4:1) for 24 h in the presence of 1 μg/ml GA201 GE or WT Abs. (B) MKN45 cells were incubated with M1 or M2c macrophages (E:T ratio, 3:1) for 24 h in the presence of 1 μg/ml GA201 GE or cetuximab Abs. The percent killing was determined by setting the LDH released from target cells to 0% and LDH released by target cells lysed with 2% Triton X-100 to 100%. Triplicates and quadruplicates referring to one representative experiment out of three are shown. (C) 1833-PPOP233 cells were incubated with isolated human intermediate, nonclassical (CD16+) monocytes (E:T ratio, 30:1) for 8 h in the presence of 1 μg/ml GA201 GE or WT Abs. (D) 1833-PPOP233 cells were incubated with polarized human M1 macrophages (E:T ratio, 30:1) for 24 h in the presence of 1 μg/ml GA201 GE or WT Abs. (E) 1833-PPOP233 cells were incubated with polarized human M1 or M2c macrophages (E:T ratio, 10:1 or 50:1) for 24 h in the presence of 1 μg/ml GA201 GE. Triplicates and quadruplicates referring to one representative experiment out of three are shown. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001.
Similar to ADCP, the addition of therapeutic Abs enhanced monocyte and macrophage cytotoxicity as seen by a slight increase in the percent killing (compare the “no Ab” control, corresponding to killing in absence of therapeutic Abs, to “WT” or “GE” samples, corresponding to killing obtained in the presence of 1 μg/ml Abs). In agreement with binding data, the intermediate, nonclassical (CD16+) monocytes induced a significantly higher level of tumor cell killing when triggered with GE than with WT Abs (Fig. 4A). As expected, the classical (CD16−) monocytes were devoid of any cytotoxic activity (data not shown).
Interestingly, M1 macrophages displayed superior cytotoxicity compared with M2c macrophages (Fig. 4B). As expected, the addition of therapeutic Abs enhanced macrophage cytotoxicity (ADCC) from 22% (no Ab) to 29% (M1) and 13% (no Ab) to 19% (M2c; Fig. 4B). In line with phagocytosis, ADCC mediated by GE and WT Abs in the absence of competing human IgGs was undistinguishable (Fig. 4B). In addition to LDH, ADCC was assessed by detection of caspase-3/7 activation in tumor cells (split luciferase assay). In line with LDH data, CD16+ monocytes promptly induced higher levels of tumor cell killing when triggered with GE than with WT Abs (Fig. 4C). In line with LDH, the activity of GE and WT Abs was undistinguishable in the absence of competing human IgGs on the macrophage subsets (Fig. 4D, 4E). As before, M1 macrophages mediated higher levels of ADCC when compared with M2c macrophages (Fig. 4E). Taken together, CD16+ monocytes triggered with GE Abs mediate higher levels of ADCC than when triggered with WT Abs. M1 macrophages are more potent in inducing target cell cytotoxicity than M2c macrophages; the cytotoxic activity of both macrophage subsets is enhanced by the addition of therapeutic Abs.
NO release by human monocytes, M1 and M2c macrophages
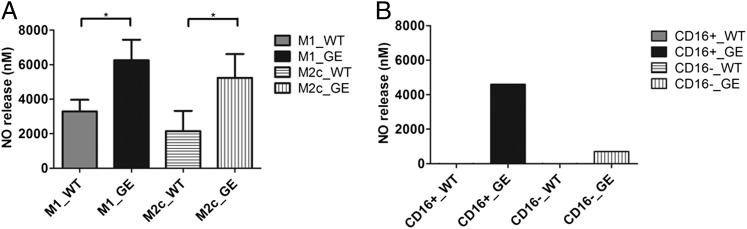
NO release was measured in supernatants recovered from the different macrophage activity assays (as above). The level of NO secreted after GE Ab-mediated ADCC/ADCP was significantly higher than that secreted after WT treatment on both M1 and M2c macrophage subsets already in the absence of competing IgGs (Fig. 5A). In addition, there was more NO release in the presence of intermediate, nonclassical (CD16+) monocytes compared with CD16− monocytes, particularly when triggered with GE Abs (Fig. 5B).
FIGURE 5.
NO release. (A) Raji or WIL2S cells incubated with human M1 or M2c macrophages (E:T ratio, 1:1) for 24 or 48 h in the presence of 1 μg/ml GA101 GE or WT Abs and ofatumumab. NO release in culture supernatants was assessed with Griess reagent and corresponds to the average of pooled triplicates derived from three independent experiments (two using Raji and one using WIL2S as targets). (B) Raji cells incubated with human classical or CD16+ monocytes (E:T ratio, 10:1) for 22 h in the presence of 1 μg/ml GA101 GE or WT Abs. NO release in culture supernatants was assessed by Griess reagent using pooled quadruplicates derived from one experiment. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001.
Assessment of the overall Ab-mediated activity using monocytes and macrophages
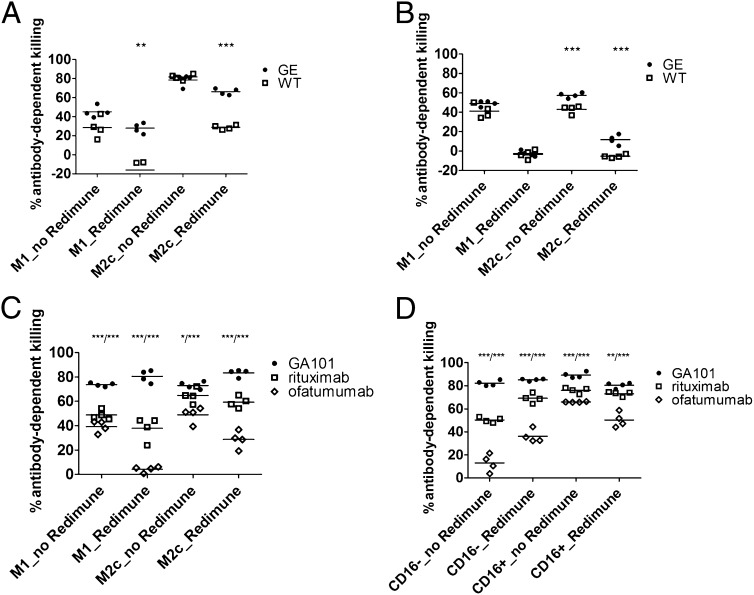
The antitumor activity of Abs in vivo is a combination of direct cell death induction and Fc-related phagocytic and cytotoxic activities mediated by engagement effector cells. We therefore set up an in vitro assay that takes into account all mechanisms of action, thus enabling the assessment of the overall antitumor activity (direct cell death induction plus ADCC and ADCP). The assay is based on the detection of tumor cells (by ELISA) that escaped to Ab-mediated monocyte or macrophage-induced killing or phagocytosis after 24 or 48 h of coculture of tumor cells with monocytes or macrophages in the presence of WT or GE therapeutic Abs and competing human IgG (Fig. 6). In agreement with previous findings, GE Abs (GA201 and GE trastuzumab) were significantly more potent than WT Abs (cetuximab and trastuzumab), particularly in the presence of competing human IgGs and M2c macrophages (Fig. 6A, 6B). As GA201/cetuximab and GE/WT trastuzumab are comparable in direct-cell death induction, we conclude that the difference in their overall tumor-killing activity is solely due to Ab glycoengineering. As third set of WT and GE Abs, we compared the activity of type I and type II anti-CD20 Abs, which notably differ significantly in their capacity to induce direct cell death (5). Type I, WT anti-CD20 Abs (rituximab and ofatumumab) were therefore compared with the type II, GE, anti-C20 Ab GA101 using M1 and M2c macrophages and CD16+ and CD16− monocyte subsets from three different donors (Fig. 6C, 6D). As before, GA101 mediated significantly stronger elimination of CD20-expressing tumor cells in all experimental conditions, further underscoring the relevance of combination of glycoengineering and direct cell death induction for the monocyte- and macrophage-mediated killing of tumor cells.
FIGURE 6.
Assessment of the overall macrophage activity. (A) The overall macrophage activity was assessed in a 48-h coculture assay of MKN45 tumor cells with human M1 or M2c macrophages (E:T ratio, 3:1) in the presence of GA201 or cetuximab and Redimune (10 mg/ml). (B) The overall macrophage activity assessed in a 48-h coculture assay of KPL-4 tumor cells with human M1 or M2c macrophages (E:T ratio, 4:1) in the presence of trastuzumab GE and WT Abs and Redimune (10 mg/ml). Quadruplicates of two representative experiments out of a total of four with different Abs and tumor target cells are shown. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001. The overall macrophage (C) or monocyte (D) activity in combination with direct Ab effects was assessed in a 24-h coculture assay of Raji tumor cells with human isolated CD16+ or CD16− monocytes or monocyte-derived M1 or M2c macrophages (E:T ratio, 3:1) in the presence of GA101, rituximab and ofatumumab, and Redimune (10 mg/ml). Quadruplicates of two representative experiments out of a total of six (three with monocytes and three with macrophages as effectors) from different donors are shown. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001, comparison of GA101 to rituximab/comparison GA101 to ofatumumab indicated on top of the graphs.
Live-cell imaging of macrophage phagocytic and cytotoxic activity
The ADCP activity was further monitored by live-cell imaging of cocultures consisting of MKN45 tumor cells (red), M2c macrophages (green), WT or GE Abs (cetuximab and GA201, respectively), and competing IgGs (10 mg/ml). Images were acquired every 5 min for a total of 3 h (Supplemental Videos 1–3) or 14 h (Supplemental Videos 4, 5). Under control conditions, performed using GA101 that cannot bind to MKN45 cells and thus cannot crosslink macrophages to tumor cells, macrophages migrated nearby tumor cells and sensed them several times but were unable (or weakly able) to phagocytize them, even after overnight incubation (Supplemental Videos 1, 4). Interestingly, phagocytosis was readily induced by the addition of therapeutic Abs, which bound to MKN45 cells and thus cross-linked macrophages to tumor cells (cetuximab [Supplemental Video 2] and GA201 [Supplemental Videos 3, 5]). In addition to increasing ADCP, several interesting differences could be detected between WT and GE Abs: 1) the degree of phagocytized cells was higher with GE than with WT (in line with quantitative FACS data), 2) GE Ab-treated macrophages phagocytized tumor cells faster than those treated with cetuximab (several cells were readily phagocytized within 30 min, rarely seen with cetuximab), and 3) GE Ab-treated macrophages performed serial phagocytosis of more than one tumor cell and frequently lead to macrophages bearing two or three engulfed tumor cells (a phenomenon rarely seen with cetuximab; Supplemental Video 5).
Discussion
FcγR-expressing macrophages have recently been identified as important immune effectors contributing to the activity of mAbs in vivo (25–28). Macrophage depletion by treatment with liposome-encapsulated clodronate or tissue-specific loss of macrophage subpopulations because of CSF-1 deficiency (38) significantly impaired elimination of circulating and spleen B cells by anti-CD20 Ab treatment (25). On the contrary, athymic nude and LAT−/− mice lacking functional T cells, beige and perforin−/−mice with defective NK cell function (39), or mice depleted in NK cells or neutrophils (28) maintained the capacity to eliminate most of blood and spleen B cells upon Ab treatment. Therefore, macrophages have been proposed as primary effector cells involved in the activity of mAbs in murine models in vivo (25, 29).
In view of the important role of phagocytic lineage cells in the mechanism of action of therapeutic Abs, we analyzed the FcγR-dependent effector functions mediated by human monocytes and macrophages upon triggering with WT and GE. Our focus was to assess the contribution of different phagocytic lineage cells and to investigate whether glycoengineering of therapeutic Abs enhances the phagocytic and cytotoxic activity of monocyte and macrophages subsets, similarly to what has been shown for NK cells.
Given the important role of the FcγR expression for the Ab Fc-mediated effector functions, we first performed a detailed quantitative analysis of the expression of Fcγ receptors on classical and nonclassical, intermediate monocytes, in addition to M1 and M2c macrophage populations derived from four different healthy human donors. Overall, macrophages expressed 5–10-fold higher levels of CD64, CD32a, and CD32b than monocytes, whereas the level of CD16a and CD16b was comparable to intermediate, nonclassical monocytes. As expected, CD16a and CD16b were not detected on classical monocytes. Between macrophages, M2c expressed overall higher levels of FcγRs than M1 with expression pattern being CD32a,b > CD64 > CD16a,b.
The Fc-dependent binding of Abs to phagocytic lineage cells was in line with the FcγR expression pattern: M2c macrophages bound the highest amounts of therapeutic Abs (∼10-fold higher than monocytes), followed by M1 macrophages and monocytes. In the presence of nonspecific competing IgGs (i.e., under conditions that more closely resemble the natural setting), the binding of GE Abs was significantly higher than that of their WT counterparts, indicating that glycoengineering also enhances the binding of therapeutic Abs to myeloid lineage cells. We also provided evidence that the superiority acquired by glycoengineering is attributable to CD16 as: 1) the binding of GE Abs was reduced to that of WT ones by CD16 blocking, 2) the binding of GE Abs was comparable to WT ones on CD16− monocytes, and 3) GE Abs were bound in significantly higher amounts to CD16+ monocytes even in absence of nonspecific competing IgGs. Overall, the monocyte and macrophage binding data corroborate previous studies that elucidated the structural features underlying enhanced Ab affinity to CD16 given by glycoengineering (14). As reported, glycoengineering specifically enhances Ab affinity to CD16, whereas the affinity to CD32 and CD64 remains unaltered. In line with this, WT and GE Abs perform equally on monocyte and macrophage effector cells expressing low levels (or not expressing) CD16.
Upon Ab binding, monocytes and macrophages exert both cytotoxic (ADCC) and phagocytic (ADCP) activity. Interestingly, we found that, under our experimental in vitro conditions, both monocytes and macrophages preferentially eliminate tumor cells via ADCP as quantitative assessments of both biological processes and live-cell imaging indicated that ADCP occurs rapidly after Ab addition (15–30 min) and results in 40–85% phagocytized tumor cells within 1 h (as quantified by FACS). On the contrary, ADCC appeared as a less efficient mechanism exploited by macrophages in the presence of therapeutic Abs as only ∼5–30% of tumor cells were eliminated via ADCC after 24 h. Interestingly, M2 macrophages displayed the highest ADCP activity, followed by monocytes and M1 macrophages. On the other hand, M1 macrophages were superior to M2c macrophages and monocytes in mediating ADCC.
A side-by-side comparison of different sets of WT and GE Abs targeting Her2, EGFR, and CD20 revealed that GE Abs triggered more potent ADCP and overall ADCC and ADCP activity (as assessed by cell ELISA) when CD16+ monocytes and M2c macrophages are used, whereas their activity was comparable using CD16− monocytes and M1 macrophages under the same experimental conditions. A direct comparison of the three anti-CD20 Abs (GA101, rituximab, and ofatumumab) in the cell ELISA assay further indicated the superiority of GA101 over both rituximab and ofatumumab resulting from the combination of glycoengineering and its strong direct-cell death induction. These findings differ from the ones of Rafiq et al. (40), who reported that ofatumumab elicited superior ADCP when using MDMs and that GA101 treatment results in less potent activation of monocytes with diminished pERK, TNF-α release, and FcgRIIa recruitment to lipid rafts when exposing monocytes and MDMs to surface-immobilized Abs. However, the different experimental conditions used in the two studies (30 min ADCP, E:T ratio of 1:5 in Rafiq et al. versus 4 h ADCP, E:T ratio of 3:1 in the current study) and differences in phagocytic effector cells (monocytes and MDMs derived from patients with CLL in Rafiq et al. versus CD16+/CD16− monocytes and M1/M2c macrophages in the current study) might have led to different results. Importantly, the elimination of leukemic B cells in whole blood from patients with CLL is consistently superior with GA101 (41, 42).
The functional superiority of GE Abs was further confirmed by live-cell imaging of cocultures of tumor cells and M2c macrophages treated with WT or GE Abs in the presence of competing IgGs. Data collected by imaging indicated that the degree of phagocytized cells is higher in the presence of GE Abs (in line with quantitative FACS data) and that GE Ab-treated M2c macrophages phagocytize tumor cells faster than those treated with WT Abs and perform serial phagocytosis resulting in engulfment of two to three tumor cells.
Our data show that in the presence of nonspecific competing IgGs (i.e., as it occurs in patients) GE Abs have an advantage over the WT ones because they display superior binding to CD16+ monocytes and M2c macrophages and induce them to eliminate tumor cells more efficiently. The current findings potentially shed new light on the activity of M2c macrophages and the traditional way these are viewed within tumors. The conclusions from our in vitro data indicate that, in the context of therapeutic IgG1 mAbs, the presence of M2c macrophages might not necessarily be associated with immunosuppression and poor patient outcome as suggested previously (43–45). On the contrary, our in vitro data suggest that M2c macrophages can readily be engaged by therapeutic Abs (especially when GE), and can be induced to phagocytize tumor cells more efficiently than M1. Recent clinical reports further support our findings indicating that a high tumor-associated macrophage score is associated with favorable prognosis of patients with follicular lymphoma treated with rituximab and chemotherapy (44), and that rituximab is able to circumvent the unfavorable outcome associated high intratumor macrophage count patients with follicular lymphoma (45). Ultimately, the study shows that therapeutic mAbs can engage circulating monocytes and different types of macrophages present within tumors, with M1 displaying more cytotoxic and M2c displaying more phagocytic activity. The Ab-mediated tumor-cell eradication therefore results from a joint contribution of NK cells and different myeloid effector subsets in contact with tumor cells.
Acknowledgments
We thank Dr. Al Rehemtulla, Dr. Stefanie Galbán (Center for Molecular Imaging, University of Michigan, Ann Arbor, MI), and Thomas Weber (Roche Diagnostics) for providing the split luciferase cells (1833-PPOP233 cells) and Dr. Mark S. Cragg for sharing data on CD32a and CD32b on different monocyte subsets, MDMs, and M1 and M2 macrophages.
The online version of this article contains supplemental material.
- ABC
- Ab-binding capability
- ADCC
- Ab-dependent cellular cytotoxicity
- ADCP
- Ab-dependent cellular phagocytosis
- CLL
- chronic lymphocytic leukemia
- DC
- dendritic cell
- LDH
- lactate dehydrogenase
- M1
- type I macrophage
- M2
- type II macrophage
- M-CSF
- macrophage CSF
- MDM
- monocyte-derived macrophage
- WT
- wild type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Shuptrine C. W., Surana R., Weiner L. M. 2012. Monoclonal antibodies for the treatment of cancer. Semin. Cancer Biol. 22: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner L. M., Murray J. C., Shuptrine C. W. 2012. Antibody-based immunotherapy of cancer. Cell 148: 1081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner L. M., Surana R., Wang S. 2010. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichert J. M. 2011. Antibody-based therapeutics to watch in 2011. MAbs 3: 76–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herter S., Herting F., Mundigl O., Waldhauer I., Weinzierl T., Fauti T., Muth G., Ziegler-Landesberger D., Van Puijenbroek E., Lang S., et al. 2013. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 12: 2031–2042. [DOI] [PubMed] [Google Scholar]
- 6.Mössner E., Brünker P., Moser S., Püntener U., Schmidt C., Herter S., Grau R., Gerdes C., Nopora A., van Puijenbroek E., et al. 2010. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams G. P., Weiner L. M. 2005. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23: 1147–1157. [DOI] [PubMed] [Google Scholar]
- 8.Jaglowski S. M., Alinari L., Lapalombella R., Muthusamy N., Byrd J. C. 2010. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood 116: 3705–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimmerjahn F., Ravetch J. V. 2005. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310: 1510–1512. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F., Ravetch J. V. 2006. Fcgamma receptors: old friends and new family members. Immunity 24: 19–28. [DOI] [PubMed] [Google Scholar]
- 11.Sburlati A. R., Umaña P., Prati E. G., Bailey J. E. 1998. Synthesis of bisected glycoforms of recombinant IFN-beta by overexpression of beta-1,4-N-acetylglucosaminyltransferase III in Chinese hamster ovary cells. Biotechnol. Prog. 14: 189–192. [DOI] [PubMed] [Google Scholar]
- 12.Shields R. L., Lai J., Keck R., O’Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277: 26733–26740. [DOI] [PubMed] [Google Scholar]
- 13.Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., et al. 2003. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278: 3466–3473. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., et al. 2011. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 108: 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara C., Brünker P., Suter T., Moser S., Püntener U., Umaña P. 2006. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol. Bioeng. 93: 851–861. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara C., Stuart F., Sondermann P., Brünker P., Umaña P. 2006. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281: 5032–5036. [DOI] [PubMed] [Google Scholar]
- 17.Gerdes C. A., Nicolini V. G., Herter S., van Puijenbroek E., Lang S., Roemmele M., Moessner E., Freytag O., Friess T., Ries C. H., Bossenmaier B., Mueller H. J., Umana P. 2013. GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin. Canc. Res. 19: 1126–1138. [DOI] [PubMed] [Google Scholar]
- 18.Salles G., Morschhauser F., Lamy T., Milpied N., Thieblemont C., Tilly H., Bieska G., Asikanius E., Carlile D., Birkett J., et al. 2012. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 119: 5126–5132. [DOI] [PubMed] [Google Scholar]
- 19.Sehn L. H., Assouline S. E., Stewart D. A., Mangel J., Gascoyne R. D., Fine G., Frances-Lasserre S., Carlile D. J., Crump M. 2012. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 119: 5118–5125. [DOI] [PubMed] [Google Scholar]
- 20.Illidge T. M. 2012. Obinutuzumab (GA101)—a different anti-CD20 antibody with great expectations. Expert Opin. Biol. Ther. 12: 543–545. [DOI] [PubMed] [Google Scholar]
- 21.Ishii T., Ishida T., Utsunomiya A., Inagaki A., Yano H., Komatsu H., Iida S., Imada K., Uchiyama T., Akinaga S., Shitara K., Ueda R. 2010. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin. Canc. Res. 16: 1520–1531. [DOI] [PubMed] [Google Scholar]
- 22.Kubota T., Niwa R., Satoh M., Akinaga S., Shitara K., Hanai N. 2009. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 100: 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf S. Q., Umana P., Mössner E., Ntouroupi T., Brünker P., Schmidt C., Wilding J. L., Mortensen N. J., Bodmer W. F. 2009. Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis. Br. J. Cancer 101: 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gennari, R., S. Menard, F. Fagnoni, L. Ponchio, M. Scelsi, E. Tagliabue, F. Castiglioni, L. Villani, C. Magalotti, N. Gibelli, et al. 2004. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Canc. Res. 10: 5650-5655. [DOI] [PubMed]
- 25.Uchida J., Hamaguchi Y., Oliver J. A., Ravetch J. V., Poe J. C., Haas K. M., Tedder T. F. 2004. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 199: 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minard-Colin V., Xiu Y., Poe J. C., Horikawa M., Magro C. M., Hamaguchi Y., Haas K. M., Tedder T. F. 2008. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood 112: 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tedder T. F., Baras A., Xiu Y. 2006. Fcgamma receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin. Immunopathol. 28: 351–364. [DOI] [PubMed] [Google Scholar]
- 28.Gong Q., Ou Q., Ye S., Lee W. P., Cornelius J., Diehl L., Lin W. Y., Hu Z., Lu Y., Chen Y., et al. 2005. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J. Immunol. 174: 817–826. [DOI] [PubMed] [Google Scholar]
- 29.Montalvao F., Garcia Z., Celli S., Breart B., Deguine J., Van Rooijen N., Bousso P. 2013. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J. Clin. Invest. 123: 5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golay J., Da Roit F., Bologna L., Ferrara C., Leusen J. H., Rambaldi A., Klein C., Introna M. 2013. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 122: 3482–3491. [DOI] [PubMed] [Google Scholar]
- 31.Luo C., Chen M., Madden A., Xu H. 2012. Expression of complement components and regulators by different subtypes of bone marrow-derived macrophages. Inflammation 35: 1448–1461. [DOI] [PubMed] [Google Scholar]
- 32.Galbán S., Jeon Y. H., Bowman B. M., Stevenson J., Sebolt K. A., Sharkey L. M., Lafferty M., Hoff B. A., Butler B. L., Wigdal S. S., et al. 2013. Imaging proteolytic activity in live cells and animal models. PLoS ONE 8: e66248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber T. G., Pöschinger T., Galbán S., Rehemtulla A., Scheuer W. 2013. Noninvasive monitoring of pharmacodynamics and kinetics of a death receptor 5 antibody and its enhanced apoptosis induction in sequential application with doxorubicin. Neoplasia 15: 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas S. K., Mantovani A. 2010. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11: 889–896. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 36.Kou P. M., Babensee J. E. 2011. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J. Biomed. Mater. Res. A. 96: 239–260. [DOI] [PubMed] [Google Scholar]
- 37.Lolmede K., Campana L., Vezzoli M., Bosurgi L., Tonlorenzi R., Clementi E., Bianchi M. E., Cossu G., Manfredi A. A., Brunelli S., Rovere-Querini P. 2009. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J. Leukoc. Biol. 85: 779–787. [DOI] [PubMed] [Google Scholar]
- 38.Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. 1994. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120: 1357–1372. [DOI] [PubMed] [Google Scholar]
- 39.Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369: 31–37. [DOI] [PubMed] [Google Scholar]
- 40.Rafiq S., Butchar J. P., Cheney C., Mo X., Trotta R., Caligiuri M., Jarjoura D., Tridandapani S., Muthusamy N., Byrd J. C. 2013. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J. Immunol. 190: 2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patz M., Isaeva P., Forcob N., Müller B., Frenzel L. P., Wendtner C. M., Klein C., Umana P., Hallek M., Krause G. 2011. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br. J. Haematol. 152: 295–306. [DOI] [PubMed] [Google Scholar]
- 42.Laprevotte E., Voisin G., Ysebaert L., Klein C., Daugrois C., Laurent G., Fournie J. J., Quillet-Mary A. 2013. Recombinant human IL-15 trans-presentation by B leukemic cells from chronic lymphocytic leukemia induces autologous NK cell proliferation leading to improved anti-CD20 immunotherapy. J. Immunol. 191: 3634–3640. [DOI] [PubMed] [Google Scholar]
- 43.Pander J., Heusinkveld M., van der Straaten T., Jordanova E. S., Baak-Pablo R., Gelderblom H., Morreau H., van der Burg S. H., Guchelaar H. J., van Hall T. 2011. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin. Can. Res. 17: 5668–5673. [DOI] [PubMed] [Google Scholar]
- 44.Canioni D., Salles G., Mounier N., Brousse N., Keuppens M., Morchhauser F., Lamy T., Sonet A., Rousselet M. C., Foussard C., Xerri L. 2008. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J. Clin. Oncol. 26: 440–446. [DOI] [PubMed] [Google Scholar]
- 45.Taskinen M., Karjalainen-Lindsberg M. L., Nyman H., Eerola L. M., Leppa S. 2007. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin. Cancer Res. 13: 5784–5789. [DOI] [PubMed] [Google Scholar]