Significance
Tripartite motif or TRIM proteins make up the largest superfamily of RING-domain E3 ubiquitin ligases. These enzymes function in a wide variety of important cellular processes, particularly innate antiviral response mechanisms. Dimerization is critical for the function of many TRIM proteins. Here we show how TRIM25 dimerizes and demonstrate that this dimerization mode is apparently conserved across the entire TRIM protein family. Our results reveal how the dimerization domain positions the other TRIM effector domains to recognize and ubiquitylate substrates and how the TRIM5 family can form higher-order hexagonal assemblies that increase the avidity of substrate recognition.
Keywords: antiparallel dimer, disulfide crosslinking, X-ray crystallography
Abstract
Tripartite motif (TRIM) proteins make up a large family of coiled-coil-containing RING E3 ligases that function in many cellular processes, particularly innate antiviral response pathways. Both dimerization and higher-order assembly are important elements of TRIM protein function, but the atomic details of TRIM tertiary and quaternary structure have not been fully understood. Here, we present crystallographic and biochemical analyses of the TRIM coiled-coil and show that TRIM proteins dimerize by forming interdigitating antiparallel helical hairpins that position the N-terminal catalytic RING domains at opposite ends of the dimer and the C-terminal substrate-binding domains at the center. The dimer core comprises an antiparallel coiled-coil with a distinctive, symmetric pattern of flanking heptad and central hendecad repeats that appear to be conserved across the entire TRIM family. Our studies reveal how the coiled-coil organizes TRIM25 to polyubiquitylate the RIG-I/viral RNA recognition complex and how dimers of the TRIM5α protein are arranged within hexagonal arrays that recognize the HIV-1 capsid lattice and restrict retroviral replication.
Tripartite motif (TRIM) proteins make up the largest superfamily of RING E3 ubiquitin ligases, with more than 100 members in the human proteome (1, 2). TRIM proteins function in a variety of cellular pathways, and many regulate innate immunity and/or mediate antiviral responses. Antiviral TRIMs include TRIM25, which regulates the IFN response to RNA viruses (3, 4), and TRIM5α, which senses and inhibits early stages of retroviral replication (5, 6).
TRIM proteins share a common N-terminal domain organization, termed the tripartite or RBCC (RING, B-box, coiled-coil) motif, followed by variable C-terminal protein recognition domains (Fig. 1 A and B). “Linker” segments of unknown structure typically separate both the RING and B-box domains (L1) and the coiled-coil and terminal effector domains (L2). The coiled-coil region mediates oligomerization, and both homooligomeric and heterooligomeric TRIMs have been described (7–13). Furthermore, many TRIM proteins form higher-order assemblies in vitro and form punctate or fibrous structures in cells (14–16). For example, TRIM5α assembly allows the protein to function as a cytosolic pattern-recognition receptor that can intercept the incoming capsids of diverse retroviruses, including HIV-1 (6). This results in species-specific “restriction” of viral replication (5), capsid dissociation (5, 6), and induction of innate immune responses (17). Retroviral capsids are recognized through a remarkable mechanism of multivalent pattern recognition. TRIM5α forms a homodimer (10, 11, 18), which can further assemble into a 2D lattice of linked hexagons (18). The hexagonal TRIM5α net matches the symmetry and spacing of the retroviral capsid surface lattice, thereby positioning multiple C-terminal B30.2/SPRY domains to interact with their repeating binding epitopes on the capsid.
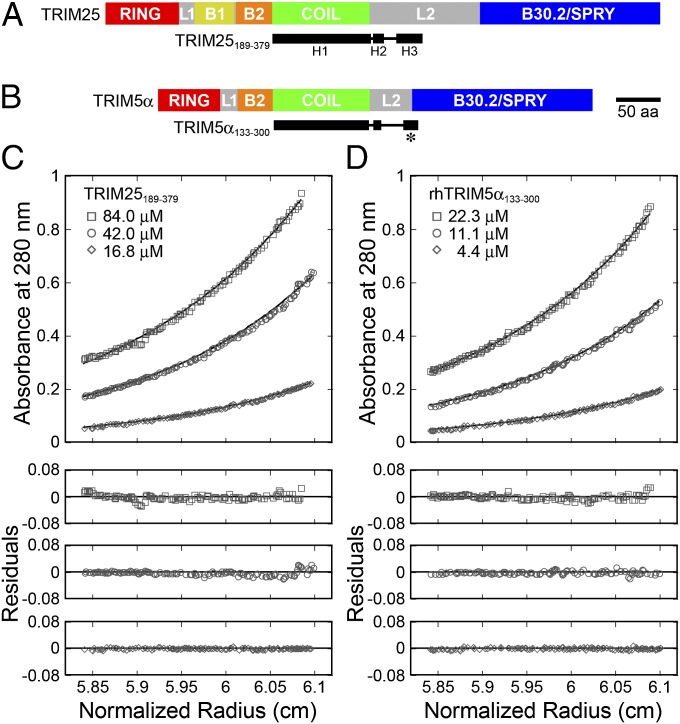
Fig. 1.
Domain organization and dimerization of TRIM proteins. (A) Schematic of the domain structure of TRIM25. The principal domains and linker regions are RING (red), L1 (gray), B-box 1 (yellow), B-box 2 (orange), coiled-coil (green), L2 (gray), and B30.2/SPRY (blue). The TRIM25189–379 construct used in this study is shown beneath (black), with the secondary structure derived from the crystal structure (rectangles represent helices). (B) Analogous schematic of the domain structure of TRIM5α. TRIM5α does not contain a B-box 1 domain and has a shorter L2. The TRIM5α133–300 construct used in this study is shown beneath (black). The asterisk denotes a predicted helix (H3) that crosses from L2 into the B30.2 domain. (C) TRIM25189–379 is a stable dimer in solution. Equilibrium sedimentation distributions of the indicated protein concentrations are shown for the rotor speed of 12,000 rpm. (Upper) Absorbance measurements (open symbols; 280 nm) and best-fit curves (solid lines). (Lower) Residual differences. Equilibrium distributions were also measured at rotor speeds of 17,000 and 23,000 rpm (not shown for clarity), and all of the data were globally fit to a single-species model in which the molecular weight (Mobs) was allowed to float (Mobs = 41,674 Da; Mcalc = 21,835 Da; Mobs/Mcalc = 1.91). Fits in which the molecular weight was fixed to that of a dimer are shown in Fig. S1A. (D) TRIM5α133–300 is also a stable dimer in solution (Mobs = 43,505 Da; Mcalc = 23,038 Da; Mobs/Mcalc = 1.89). See Fig. S1D for fits to a single-species model with a fixed dimer molecular weight.
Structures of isolated RING, B-box, and C-terminal domains of several TRIM proteins have been described, but the molecular details of TRIM oligomerization and high-order assembly have yet to be defined. Here, we report biochemical and crystallographic characterization of a coiled-coil-containing fragment of TRIM25. The crystallized construct forms a stable dimer in solution, and the structure reveals an elongated dimer composed of interdigitating hairpin-shaped subunits. We also present evidence that this dimer architecture is conserved across other TRIM family members, including TRIM5α. Finally, our studies allow us to assign the domain organization in the low-resolution EM reconstruction of the TRIM5α lattice (18) and thereby gain new insights into the mechanism of retroviral capsid pattern recognition.
Results
TRIM25189–379 Forms an Elongated, Antiparallel Dimer.
We chose human TRIM25 for analysis because it lacks the propensity of TRIM5α to form high-order assemblies and is therefore more tractable biochemically. In initial experiments, we found that a series of coiled-coil-containing TRIM25 constructs, including the full tripartite motif and the full-length protein, behaved as single species during purification. All of these constructs eluted rapidly on gel filtration chromatographs, indicating they had elongated shapes and/or were oligomers. The shortest well-behaved construct spanned residues 189–379, which includes the entire coiled-coil region as well as the N-terminal half of the L2 linker region that connects the coiled-coil to the B30.2/SPRY domain (Fig. 1A). Analytical ultracentrifugation experiments revealed that this construct is a stable dimer (Fig. 1C; Fig. S1 A–C), implying that the full-length protein is also probably dimeric.
To determine the molecular basis for TRIM25 dimerization, we crystallized TRIM25189–379 and determined its structure to 2.6 Å resolution (Fig. S2; Table S1; Materials and Methods). The asymmetric unit comprises a single, elongated dimer ∼17 nm in length (Fig. 2). Each subunit in the symmetrical dimer folds back into a hairpin configuration with long and short arms. The elements annotated as the coiled-coil and L2 linker are structurally distinct, with the coiled-coil residues forming the long arm of each subunit (helix H1, colored green in Fig. 2A) and the L2 residues forming the short arm that folds back and packs against H1 (helices H2, H3, and an irregular but well-ordered intervening segment, colored gray). The two subunits dimerize intimately in an antiparallel orientation, similar to two interdigitated bobby pins (Fig. 2B). Almost all hydrophobic side-chains are involved in packing interactions, which occur along the entire length of each hairpin and bury a total surface area of 5,102 Å2. Polar and charged side-chains also form numerous hydrogen bonding and salt bridge interactions.
Fig. 2.
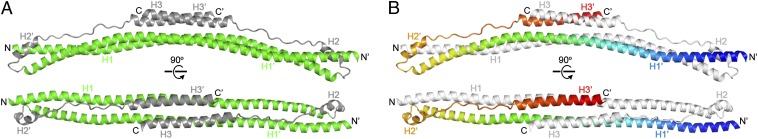
Structure of the TRIM25189–379 dimer. (A) Orthogonal views of the dimer in ribbons representation, with the coiled-coil and L2 segments colored in green and gray, respectively (matching the color scheme of Fig. 1). (B) Orthogonal views of the dimer with one subunit colored in rainbow gradient, with blue at the N terminus and red at the C terminus, and the other subunit in white.
TRIM proteins have been predicted to contain two distinct coiled-coil segments separated by a helical, but noncoil, segment (19, 20). The TRIM25 structure reveals that these segments actually make up a single contiguous coil, helix H1, which forms the long arm of each subunit. The dimeric H1–H1′ interaction is mediated by classic “knobs-into-holes” packing of both heptad repeats (wherein amino acid residue positions in each repeat are denoted by the letters abcdefg) and hendecad repeats (abcdefghijk) (Fig. 3; Fig. S2 C and D). Residues in the h positions of the hendecads also form “knobs-to-knobs” interactions (21). The repeats are arranged in a symmetric 7-7-7-7-11-11-11-11-7-7-7-7 pattern, which produces a supercoil that is canonically left-handed at the ends but is underwound and slightly right-handed in the middle. This unusual configuration likely explains why sequence analysis programs failed to predict H1 as a single, contiguous coil. The hendecads mediate interactions at the center of the coiled-coil, and superhelical underwinding allows H1 and H1′ to sit side by side and form an amphipathic platform. Here, the terminal H3 helices from the short arm of each hairpin pack against one side of the platform to form a 4-helix bundle. Thus, the structure indicates that both ensuing C-terminal B30.2 domains will be centrally located on the same side of the dimer.
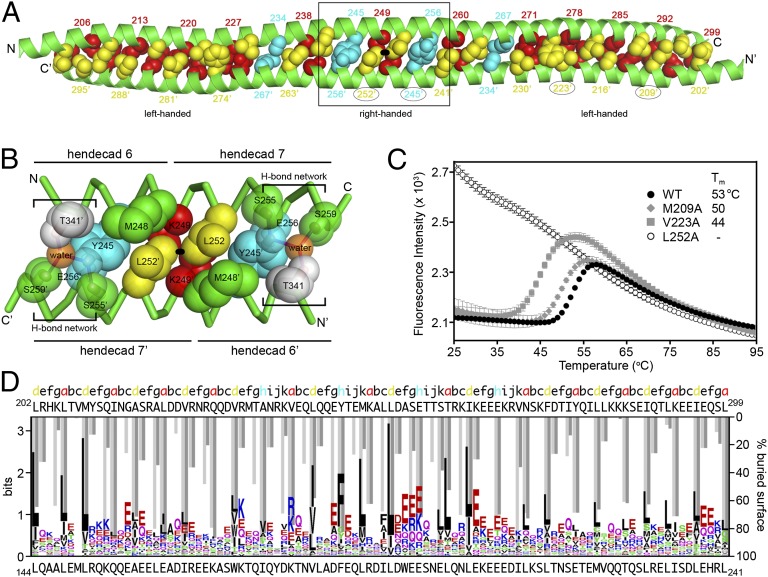
Fig. 3.
Dimeric packing of TRIM coiled-coil helices. (A) Coiled-coil formed by the H1 and H1′ helices in the TRIM25189–379 structure. Side-chains that mediate interhelix packing interactions are numbered and shown as spheres, with the a, d, and h positions colored in red, yellow, and cyan, respectively. Circled numbers indicate mutation sites analyzed in C. The dimer symmetry axis (black oval) runs perpendicular to the page. (B) Expanded view of the central region boxed in A. Side-chains that mediate important packing interactions are shown as spheres, colored as in A and labeled, as are buried water molecules (orange). (C) Thermofluor melting curves of wild-type (filled circles), L252A (open circles), M209A (filled diamonds), and V223A (filled squares). The high fluorescence signal for L252A at 25 °C indicates that the hydrophobic residues of this mutant are already exposed. Error bars represent the SDs from 4 replicates performed in parallel. (D) Structure-to-sequence alignment. The graph shows a multiple sequence alignment of 54 different human TRIM coiled-coil/L2 sequences displayed in logo format (see Fig. S4 for full alignment). The sequence alignment is overlaid with percentage buried surface area plots calculated using the entire TRIM25189–379 structure (light gray bars) or the H1/H1′ helices only (dark gray bars). Heptad/hendecad residue assignments are color coded as in A. The TRIM25 sequence is shown at the top and the aligned TRIM5α sequence at the bottom, with the first and last residue numbers indicated.
TRIM25 Dimerization Requires Hydrophobic Residues at the Center of the Coiled-Coil.
The central region of the coiled-coil platform (boxed in Fig. 3A, expanded in Fig. 3B) has a particularly high density of intermolecular interactions because of tight packing of the H1 helices against one another and against the H3 helices (Fig. 2). Key H1–H1′ interactions in this region include the Leu252 side-chain, which packs against Tyr245′, Met248′, Lys249′, and the symmetry equivalent Leu252′ in the apposing helix (Fig. 3B). This segment is further stabilized by salt bridges between the Lys249 and Asp253 side-chains and is flanked on either end by a buried hydrogen bond network (indicated by square brackets in Fig. 3B) involving the Tyr245 side-chain hydroxyl, a buried water molecule (orange sphere), Ser255 (H1′), Glu256 (H1′), Ser259 (H1′), Thr341 (H3′), and Gln356 (H3, not shown for clarity). This region therefore appears to be particularly important for dimer stability.
Coiled-coils represent a special case of protein folding in which formation of the hydrophobic core is coupled to oligomerization (dimerization in this case). We therefore used differential scanning fluorescence thermal melting assays to examine the coupled folding/dimerization transition. In this assay, the signal comes from a dye that fluoresces on binding hydrophobic side-chains that become exposed as the protein unfolds with increasing temperature (22). As shown in Fig. 3C (filled circles), wild-type TRIM25189–397 displayed a typical coiled-coil differential scanning fluorescence profile with a single transition and an apparent melting temperature (Tm) of 53 °C. Consistent with the structure, the L252A mutant was difficult to purify and did not display a sigmoidal melting curve, indicating it was already unfolded (or misfolded), even at 25 °C (Fig. 3C, open circles). The Y245A mutant was also apparently misfolded and could not even be purified. In contrast, two control proteins with alanine substitutions for completely buried residues elsewhere in H1 (M209A and V223A) were properly folded, albeit with reduced stability (Fig. 3C, gray symbols). These results confirm that the center of the coiled-coil helix is critical for proper folding of the TRIM25 dimer, perhaps making up the “trigger site” that directs coiled-coil formation (23–25).
This central region of TRIM25 is also where the terminal H3 helices pack to form the 4-helix bundle and contribute hydrophobic residues to the compact core. Unlike H1 mutants, however, alanine substitutions in buried hydrophobic H3 residues (T341A, L344A, and L348A) did not prevent TRIM25189–379 coiled-coil formation (Fig. S3A). These results are consistent with the observation that TRIM5 protein dimerization requires only the coiled-coil domain and that both upstream (RING and B-boxes) and downstream (L2 equivalents and beyond) elements are dispensable (8, 19, 26, 27). Thus, even though the coiled-coil and L2 regions appear to form an integrated “domain” in our structure, the L2 segment is apparently not critical for dimerization and may be dynamic. Consistent with this idea, the average temperature factor for the short arm was 15% higher than the long arm in the native structure. In the selenomethionine crystal, one of the L2 arms had extremely poor density, likely because it had dissociated from the coiled-coil (Fig. S3B).
The H1 Coiled-Coil Heptad/Hendecad Pattern Is Conserved in the TRIM Family.
The TRIM25 coiled-coil sequence diverges significantly from other human TRIM proteins (e.g., TRIM25 and TRIM5α share only ∼10% sequence identity in this region). Nevertheless, our analysis of the human TRIM family using the secondary structure prediction program JPRED (28) indicated that the putative coiled-coil regions of most TRIM proteins are embedded within a contiguous helix of about 110 amino acids, consistent with the TRIM25 structure. To align these regions, we performed a structure-to-sequence comparison by first generating a multiple sequence alignment (MSA) of the coiled-coil regions of 54 different human TRIM family members (Fig. S4). The alignment revealed a pattern of conserved hydrophobic amino acids, with leucine being the most highly represented residue. We next calculated and plotted the percentage buried surface area (BSA) for each residue in the H1/H1′ portion of the TRIM25 structure (i.e., not including the L2 arms) and aligned this plot with the MSA. As shown in Fig. 3D, there is excellent correspondence between the pattern of conserved hydrophobic positions in the MSA plot and the a, d, and h positions that mediate formation of the H1/H1′ dimer (dark gray bars; see also Fig. S4). These results indicate that the unusual pattern of heptad and hendecad repeats is conserved across the TRIM protein family and that the structures of other dimeric TRIM coiled-coils likely resemble the TRIM25 structure.
We also calculated a BSA plot for the entire structure (Fig. 3D, light gray bars). Comparison of the two BSA plots revealed that packing of the long and short arms of the TRIM25 subunits is mediated by H1 residues in the c, g, and k positions. Importantly, these residues are also conserved in the MSA, particularly at the center of the dimer (e.g., Glu244, Met248, Leu251). These results indicate that the hairpin configuration of the subunits and the central 4-helix bundle are also likely to be conserved.
TRIM5α and TRIM25 Have Similar Dimer Architectures.
The 17-nm length of the TRIM25189–379 dimer corresponds almost exactly to the length of each edge of the assembled TRIM5α hexagon (18), suggesting that the TRIM25 structure can also inform our understanding of the TRIM5α hexagonal lattice. Intermolecular disulfide bond formation was used to probe and compare the structures of the TRIM25 and TRIM5α dimers in solution. The TRIM25189–379 crystal structure was analyzed using a disulfide prediction program (29) to identify three pairs of residues that are in close proximity in the dimer and are predicted to form intermolecular disulfides when mutated into cysteines (Fig. 4A). Two of the designed disulfides, A216C/K285C and A234C/E267C, probe for packing and phase of the H1/H1′ helices (i.e., antiparallel coiled-coil formation), and the third, S195C/L308C, probes for packing of H1 in one subunit against H2 in the other subunit (i.e., the fold-back configuration). These disulfide bonds collectively sample the entire length of the dimer (Fig. 4A).
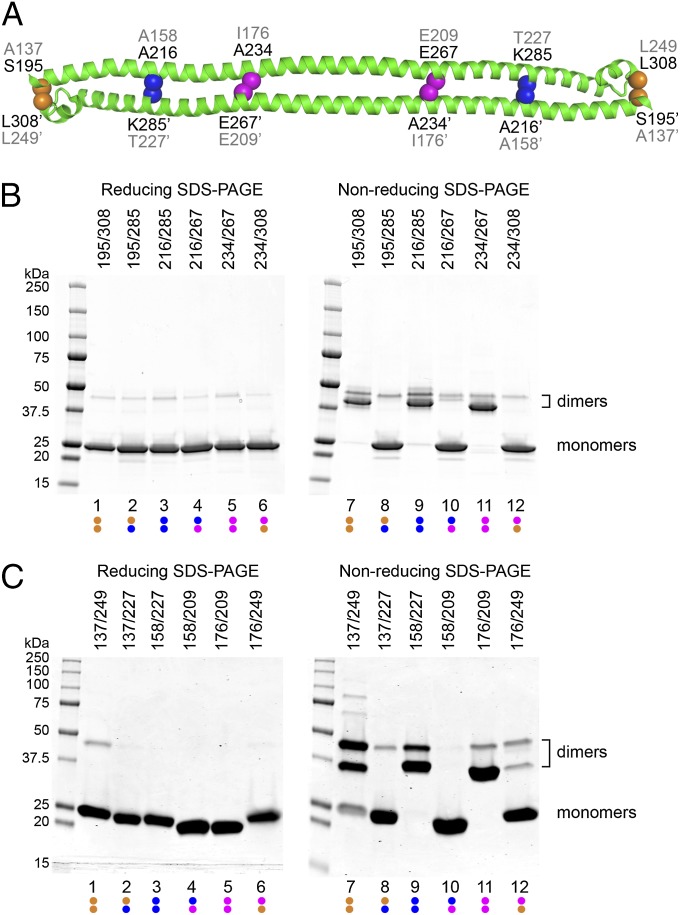
Fig. 4.
Disulfide crosslinking of TRIM25 and TRIM5α dimers. (A) H1 and H2 regions of the TRIM25189–379 structure showing positions of residue pairs chosen for cysteine mutagenesis. Equivalent TRIM25 and TRIM5α residues are labeled in black and gray, respectively. (B) Electrophoretic profiles of purified TRIM25189–379 double-cysteine mutants that were dialyzed under nonreducing conditions, then denatured in SDS-PAGE buffer under reducing (Left) or nonreducing (Right) conditions. Molecular weight marker positions are labeled on the left. Positions of monomers and crosslinked dimers are labeled on the right. Note that the symmetric dimer is expected to produce two types of intermolecular disulfide-crosslinked species: one in which both cysteine pairs are oxidized (lower bands) and another in which one of the pairs is reduced (upper bands). Data are representative of 3 independent experiments. (C) Profiles of rhesus TRIM5α133–300 cysteine mutants that were dialyzed under mildly reducing conditions and then prepared for SDS-PAGE, as described for TRIM25189–379. Data are representative of 3 independent experiments.
As shown in Fig. 4B, all three double-cysteine mutant TRIM25189–379 proteins behaved as designed. Each formed intermolecular disulfide crosslinks very efficiently under nonreducing conditions and migrated exclusively as crosslinked dimers on a denaturing polyacrylamide gel (lanes 7, 9, and 11). In contrast, three negative controls that contained scrambled pairs of cysteines migrated almost exclusively as monomers under the same conditions (Fig. 4B, even-numbered lanes). Thus, disulfide crosslinking can be used as a sensitive probe of the dimeric conformation of TRIM25.
Analogous disulfide crosslinking experiments were performed on rhesus TRIM5α133–300 to test whether the TRIM5α protein also adopts a similar dimeric structure. Equilibrium sedimentation distributions of the wild-type TRIM5α133–300 fit well to a single-species dimer model, confirming that this region was sufficient for dimerization (Fig. 1D; Fig. S1 D–F). Three pairs of TRIM5α133–300 cysteine mutants were then created in positions that were equivalent to the three crosslinking pairs of TRIM25189–379 (colored dots in Fig. S4). As shown in Fig. 4C, these TRIM5α133–300 Cys pairs also formed intermolecular disulfides efficiently (lanes 7, 9, and 11), although the A137C/L249C disulfide crosslink formed somewhat less efficiently than did the A158C/T227C and I176C/E209C crosslinks, suggesting that H2 may not reside in precisely the same position in TRIM25 and TRIM5α. The crosslinks were judged to be stable because the proteins migrated almost exclusively as dimers, even after extended incubation under mildly reducing conditions (2 mM β-mercaptoethanol), consistent with the favorable disulfide geometries predicted by the homology model. We therefore conclude that TRIM5α and TRIM25 form dimers of similar structure.
Discussion
Mechanistic Implications for TRIM25-Mediated Polyubiquitylation of RIG-I.
TRIM25 is an established effector of the RIG-I signaling pathway, which mediates the intracellular innate immune response to RNA viruses. TRIM25 recognizes and catalyzes Lys63-linked polyubiquitylation of the RIG-I/viral RNA recognition complex, thereby activating downstream effectors in the pathway and establishing an antiviral state (3). RIG-I/viral RNA complexes are recognized by the C-terminal B30.2 domain of TRIM25 (3, 30), and ubiquitin transfer is facilitated by the N-terminal RING domain, in cooperation with a ubiquitin E2 ligase. Our structure indicates that in the full-length TRIM25 dimer, the two catalytic RING domains will be separated by at least 17 nm at either end of the elongated dimer (Fig. S5). In this geometry, the two RING domains within one TRIM25 dimer probably could not cooperate during catalysis, at least not in the same manner as well-characterized cooperative homodimeric RING domains such as RNF4 (31) or BIRC7 (32). As illustrated in Fig. S5, the fold-back configuration of the TRIM25 subunits explains how the RING domains can approach the B30.2 domains to enable RIG-I ubiquitylation. It is likely, however, that there is a more precise positioning mechanism than we can currently describe, as TRIM25 has been shown to modify RIG-I at a specific lysine residue (3, 30). We speculate that dynamics of the L2 arm (including possibly the L2 region that is missing from our structure) and other factors (33, 34) may make important contributions in this regard. In addition, RNA-bound RIG-I has been shown to dimerize (35). It will therefore be interesting to learn whether both subunits of the TRIM25 dimer can engage both subunits of the RIG-I dimer simultaneously.
Implications for Dimerization of the Tripartite Motif.
Our analysis indicates that TRIM25 is likely to be an obligate dimer. Furthermore, the distinctive 7-7-7-7-11-11-11-11-7-7-7-7 pattern of heptad and hendecad repeats in the TRIM25 coiled-coil appears to be conserved, and we speculate that it may be a “signature” of the TRIM family. Studies of dimeric coiled-coils have shown that short sequence elements or “trigger sites” of 7–14 amino acids are critically important for proper folding because they are the first segments to become helical, and therefore nucleate dimerization (23–25). Once the initial dimer contact is established, the peripheral residues then “zip up” to form the fully folded coiled-coil. This general model implies that associating helices, whether homodimeric or heterodimeric, must have compatible trigger sites. Our structural and mutational analyses indicate that the center of the H1 helix is likely to be the TRIM25 coiled-coil trigger site. This element includes Tyr245 and Leu252 (hendecads 6 and 7) and is flanked by polar residues that form a buried hydrogen bond network (Fig. 3B). It is likely that these buried polar interactions help to define pairing specificity and helix packing registry in TRIM proteins, as has been seen in SNARE coiled-coil complexes (36). Our sequence analysis indicates that almost every human TRIM protein has a unique central sequence, although there is conservation within the same branches of the TRIM family tree (Fig. S4). This likely explains why TRIM proteins apparently do not form heterodimers promiscuously and why reports of TRIM heterodimerization generally involve closely related TRIM proteins (7, 9, 12, 13).
Implications for Dimerization and High-Order Assembly of TRIM5α.
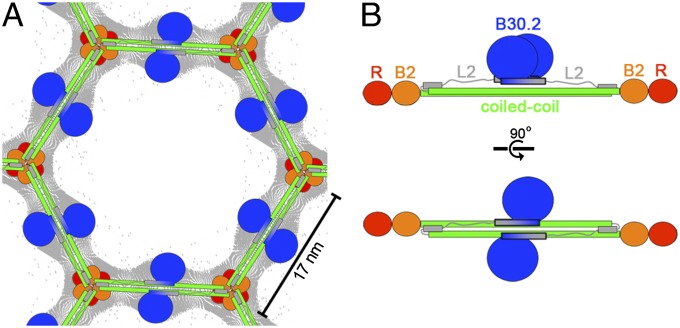
Our structural and biochemical data establish that the coiled-coil and L2 regions of TRIM25 and TRIM5α form similar structures. We have therefore used the TRIM25 structure to interpret the protein density seen in the 2D cryoEM reconstruction of the assembled TRIM5α hexagonal lattice (18) (Fig. 5). This analysis indicates that each edge of the TRIM5α hexagon corresponds to a single coiled-coil dimer (each 17 nm in length). This interpretation, in turn, implies that the threefold symmetric densities observed at each vertex correspond to the N-terminal RING and B-box 2 domains and that the twofold symmetric densities at the midpoint of each hexagon edge correspond to the B30.2/SPRY domains (18) (Fig. 5A). These assignments are consistent with the known requirement for the B-box 2 domain in high-order TRIM5α assembly (18, 37, 38) and could also explain how assembly can activate the RING domains by bringing them into close proximity, consistent with the observation that capsid binding enhances E3 ligase activity (17). We envision two possible subunit configurations for the hexagonal lattice, which differ in domain connectivity at the local threefold vertex (Fig. S6). In one configuration, the intact dimers would interact at the vertex through the RING, B-box 2, and/or ends of the coiled-coil. In the alternative configuration, the associated coiled-coil dimers would “swap” arms in a fashion reminiscent of clathrin triskelion assembly (39). We cannot yet unambiguously discriminate between these different possible assembly modes but note that domain swapping would provide a mechanism for autoinhibition, and thereby prevent unregulated assembly, and would be consistent with recent studies indicating that the L2 linker plays an important role in high-order TRIM5α assembly (40, 41).
Fig. 5.
Models of quaternary TRIM5α interactions. (A) Schematic model of the TRIM5α hexagonal lattice, showing the deduced positions of the different domains and overlaid with the cryoEM projection map (gray contours) (18). Domains are colored as in Fig. 1B. (B) Schematic model of the full-length TRIM5α dimer. The C-terminal B30.2 domains (blue) are shown packed against one side of the coiled-coil domain via a putative extended H3 helix (colored in gray to blue gradient and outlined in black) that spans both L2 and B30.2 sequences and forms a 4-helix bundle with the coiled-coil, as seen in the TRIM25 structure.
Our domain assignments, together with the density distribution in the EM map of the TRIM5α lattice, further suggest that the B30.2 domain may interact with the coiled-coil. The sequences of TRIM5α and TRIM25 diverge considerably beyond the short-arm H2 helix, but secondary structure algorithms predict an α-helix at the TRIM5α L2/B30.2 boundary (residues 283–300) that is equivalent in position to TRIM25 H3 (asterisks in Fig. 1B and Fig. S7). Interestingly, residues 291–300 of rhesus TRIM5α (colored in blue and boxed in Fig. S7) do indeed form a helix in two independent crystal structures of the isolated B30.2 domain (42, 43), although the immediately preceding residues (287–290) adopt a nonhelical loop configuration with high temperature factors in one of the structures (43). These observations lead us to speculate that the N-terminal helix of the TRIM5α B30.2 domain (or the longer, predicted helix) may pack against the center of the upstream coiled-coil to form a 4-helix bundle, as seen in the TRIM25 structure. In support of this idea, alanine substitutions of surface-exposed residues on the TRIM5α B30.2 helix (Arg297, Arg298, and Tyr299) impair both restriction activity and capsid binding. These residues are far removed from the capsid-binding surface (44) but could mediate interaction of the TRIM5α B30.2 domain against its coiled-coil. Such interactions would not only position the B30.2 domains on the same side of the dimer but also possibly define their spacing and orientation, as has been postulated to be a “minimum design feature” of retroviral capsid restriction factors (45). Consistent with this general idea, residues within the TRIM5α coiled-coil domain are under positive selection (46, 47), implying they can influence capsid recognition, and the coiled-coil itself may be a determinant of binding specificity (47).
In summary, we propose that the tripartite motif coiled-coil has a conserved structure and a conserved scaffolding function that organizes the biochemical activities of TRIM proteins, thereby facilitating selective substrate polyubiquitylation by TRIM25 and capsid pattern recognition by TRIM5α. Thus, the TRIM domains are organized spatially, consistent with the idea that they have coevolved and behave as an integrated module, rather than as a collection of independent functional elements (14, 20, 48).
Materials and Methods
Construct Design, Protein Purification, and Characterization.
TRIM25189–379 and TRIM5α133–300 protein expression constructs, purification protocols, analytical ultracentrifugation analyses, and TRIM25189–379 crystallization and structure determination are described in SI Materials and Methods.
Sequence and Structure Analysis.
Sequences aligned in Fig. 3D started from the last zinc-coordinating pair of the B-box 2 domain (typically His-X-X-His) and spanned the subsequent 130 residues. Sixty-seven members of the human TRIM family (table 1 in ref. 20), excluding TRIM25, were initially used to generate a multiple sequence alignment with the ClustalW2 program. Duplicates and sequences with gaps of 3 or more residues within H1 were removed, resulting in a final alignment of 54 sequences (Fig. S4). To facilitate structure-to-sequence comparisons, a consensus sequence plot was generated using the Weblogo program (49), total buried surface areas for each residue in the TRIM25 structure were calculated using the PISA Web server (50), and the Weblogo and buried surface area plots were aligned manually.
Differential Scanning Fluorimetry.
Thermofluor melting assays (22) were performed with a Bio-Rad CFX96 thermal cycler. Proteins in crystallization buffer were mixed with a 1:400 dilution of “10,000×” SYBR Safe dye (Invitrogen). Final protein concentrations were 2 mg/mL, except for the L252A and L348A mutants, which were 1 mg/mL and 2.6 mg/mL, respectively. Samples were held at 20 °C for 5 min, and the temperature was then raised to 100 °C in 1 °C increments every 15 s, taking fluorescence readings at each increment. Each sample was set up in 4 replicates, and melting curves for each protein were determined at least twice, with independent protein preparations. Tm was determined from the maximum of the first derivative of the melting curve. The maximal variation in wild-type TRIM25189–379 Tm was <1 °C in seven independent determinations.
Crosslinking Analysis.
Double-cysteine mutant proteins were reduced by dilution to 30 μM in reducing buffer [50 mM Tris at pH 8.0, 150 mM NaCl, 20 mM β-mercaptoethanol (βME)] and then dialyzed overnight at 4 °C into the same buffer containing 0 mM (TRIM25) or 2 mM (TRIM5α) βME to allow formation of stable disulfide crosslinks. Aliquots were then mixed with the same volume of 2× SDS-PAGE sample buffer containing either 1 M βME (reducing) or no additional βME (nonreducing), incubated for 5 min at 99 °C in a dry bath and immediately analyzed by SDS-PAGE with Coomassie blue staining.
Supplementary Material
Acknowledgments
We thank Barbie Ganser-Pornillos, Chris Hill, and members of the O.P. and W.I.S. laboratories for critical reading of the manuscript; Ed Campbell for helpful discussions; Kate Skorupka and Peter Horanyi for technical support; Debra Eckert and Steve Alam for assistance with the equilibrium sedimentation analyses. We also thank an anonymous reviewer for helpful suggestions on the analysis of the helical repeats in the antiparallel coiled-coil. This project was supported by a Research and Development grant from the University of Virginia School of Medicine (to O.P.) and National Institutes of Health Grant P50 GM082545 (to W.I.S.). J.G.S. is supported by a predoctoral Cell and Molecular Biology Training Grant from the National Institutes of Health (T32 GM008136). K.O. participated in this study while on leave from Jagiellonian University, Krakow, Poland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4LTB).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318962111/-/DCSupplemental.
References
- 1.Han K, Lou DI, Sawyer SL. Identification of a genomic reservoir for new TRIM genes in primate genomes. PLoS Genet. 2011;7(12):e1002388. doi: 10.1371/journal.pgen.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNab FW, Rajsbaum R, Stoye JP, O’Garra A. Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol. 2011;23(1):46–56. doi: 10.1016/j.coi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 4.Oshiumi H, Matsumoto M, Seya T. Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I. J Biochem. 2012;151(1):5–11. doi: 10.1093/jb/mvr111. [DOI] [PubMed] [Google Scholar]
- 5.Stremlau M, et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 6.Grütter MG, Luban J. TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr Opin Virol. 2012;2(2):142–150. doi: 10.1016/j.coviro.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centner T, et al. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306(4):717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 8.Short KM, Cox TC. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem. 2006;281(13):8970–8980. doi: 10.1074/jbc.M512755200. [DOI] [PubMed] [Google Scholar]
- 9.Li X, et al. Unique features of TRIM5α among closely related human TRIM family members. Virology. 2007;360(2):419–433. doi: 10.1016/j.virol.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Kar AK, Diaz-Griffero F, Li Y, Li X, Sodroski J. Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5α protein. J Virol. 2008;82(23):11669–11681. doi: 10.1128/JVI.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langelier CR, et al. Biochemical characterization of a recombinant TRIM5α protein that restricts human immunodeficiency virus type 1 replication. J Virol. 2008;82(23):11682–11694. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herquel B, et al. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci USA. 2011;108(20):8212–8217. doi: 10.1073/pnas.1101544108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napolitano LM, Meroni G. TRIM family: Pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life. 2012;64(1):64–71. doi: 10.1002/iub.580. [DOI] [PubMed] [Google Scholar]
- 14.Reymond A, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20(9):2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javanbakht H, Diaz-Griffero F, Stremlau M, Si Z, Sodroski J. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5α. J Biol Chem. 2005;280(29):26933–26940. doi: 10.1074/jbc.M502145200. [DOI] [PubMed] [Google Scholar]
- 16.Campbell EM, et al. TRIM5 α cytoplasmic bodies are highly dynamic structures. Mol Biol Cell. 2007;18(6):2102–2111. doi: 10.1091/mbc.E06-12-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pertel T, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472(7343):361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganser-Pornillos BK, et al. Hexagonal assembly of a restricting TRIM5α protein. Proc Natl Acad Sci USA. 2011;108(2):534–539. doi: 10.1073/pnas.1013426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javanbakht H, et al. Characterization of TRIM5α trimerization and its contribution to human immunodeficiency virus capsid binding. Virology. 2006;353(1):234–246. doi: 10.1016/j.virol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Sardiello M, Cairo S, Fontanella B, Ballabio A, Meroni G. Genomic analysis of the TRIM family reveals two groups of genes with distinct evolutionary properties. BMC Evol Biol. 2008;8:225. doi: 10.1186/1471-2148-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JH, Cohen C, Parry DA. Heptad breaks in alpha-helical coiled coils: Stutters and stammers. Proteins. 1996;26(2):134–145. doi: 10.1002/(SICI)1097-0134(199610)26:2<134::AID-PROT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Matulis D, Kranz JK, Salemme FR, Todd MJ. Thermodynamic stability of carbonic anhydrase: Measurements of binding affinity and stoichiometry using ThermoFluor. Biochemistry. 2005;44(13):5258–5266. doi: 10.1021/bi048135v. [DOI] [PubMed] [Google Scholar]
- 23.Kammerer RA, et al. An autonomous folding unit mediates the assembly of two-stranded coiled coils. Proc Natl Acad Sci USA. 1998;95(23):13419–13424. doi: 10.1073/pnas.95.23.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kammerer RA, et al. An intrahelical salt bridge within the trigger site stabilizes the GCN4 leucine zipper. J Biol Chem. 2001;276(17):13685–13688. doi: 10.1074/jbc.M010492200. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz MO, et al. Molecular basis of coiled-coil formation. Proc Natl Acad Sci USA. 2007;104(17):7062–7067. doi: 10.1073/pnas.0700321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mische CC, et al. Retroviral restriction factor TRIM5α is a trimer. J Virol. 2005;79(22):14446–14450. doi: 10.1128/JVI.79.22.14446-14450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Caballero D, Hatziioannou T, Yang A, Cowan S, Bieniasz PD. Human tripartite motif 5α domains responsible for retrovirus restriction activity and specificity. J Virol. 2005;79(14):8969–8978. doi: 10.1128/JVI.79.14.8969-8978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36(Web Server issue):W197-201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dombkowski AA. Disulfide by Design: A computational method for the rational design of disulfide bonds in proteins. Bioinformatics. 2003;19(14):1852–1853. doi: 10.1093/bioinformatics/btg231. [DOI] [PubMed] [Google Scholar]
- 30.Gack MU, et al. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci USA. 2008;105(43):16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plechanovová A, Jaffray EG, Tatham MH, Naismith JH, Hay RT. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature. 2012;489(7414):115–120. doi: 10.1038/nature11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dou H, Buetow L, Sibbet GJ, Cameron K, Huang DT. BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat Struct Mol Biol. 2012;19(9):876–883. doi: 10.1038/nsmb.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HM, et al. The mitochondrial targeting chaperone 14-3-3ε regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11(5):528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon SC, et al. The RNA-binding protein repertoire of embryonic stem cells. Nat Struct Mol Biol. 2013;20(9):1122–1130. doi: 10.1038/nsmb.2638. [DOI] [PubMed] [Google Scholar]
- 35.Feng M, et al. Structural and biochemical studies of RIG-I antiviral signaling. Protein Cell. 2013;4(2):142–154. doi: 10.1007/s13238-012-2088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95(26):15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Griffero F, et al. A B-box 2 surface patch important for TRIM5α self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83(20):10737–10751. doi: 10.1128/JVI.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Sodroski J. The TRIM5α B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82(23):11495–11502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fotin A, et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432(7017):573–579. doi: 10.1038/nature03079. [DOI] [PubMed] [Google Scholar]
- 40.Sastri J, et al. Identification of residues within the L2 region of rhesus TRIM5α that are required for retroviral restriction and cytoplasmic body localization. Virology. 2010;405(1):259–266. doi: 10.1016/j.virol.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Yeung DF, Fiegen AM, Sodroski J. Determinants of the higher order association of the restriction factor TRIM5α and other tripartite motif (TRIM) proteins. J Biol Chem. 2011;286(32):27959–27970. doi: 10.1074/jbc.M111.260406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biris N, et al. Structure of the rhesus monkey TRIM5α PRYSPRY domain, the HIV capsid recognition module. Proc Natl Acad Sci USA. 2012;109(33):13278–13283. doi: 10.1073/pnas.1203536109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, et al. Structural insight into HIV-1 capsid recognition by rhesus TRIM5α. Proc Natl Acad Sci USA. 2012;109(45):18372–18377. doi: 10.1073/pnas.1210903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebastian S, et al. An invariant surface patch on the TRIM5α PRYSPRY domain is required for retroviral restriction but dispensable for capsid binding. J Virol. 2009;83(7):3365–3373. doi: 10.1128/JVI.00432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yap MW, Mortuza GB, Taylor IA, Stoye JP. The design of artificial retroviral restriction factors. Virology. 2007;365(2):302–314. doi: 10.1016/j.virol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5α identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci USA. 2005;102(8):2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maillard PV, Ecco G, Ortiz M, Trono D. The specificity of TRIM5 α-mediated restriction is influenced by its coiled-coil domain. J Virol. 2010;84(11):5790–5801. doi: 10.1128/JVI.02413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27(11):1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- 49.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.