Significance
Rab GTPases are key regulators of intracellular vesicular transport in eukaryotic cells, for example in neurotransmission and endocytosis. Each of more than 60 Rabs in human cells regulates these processes at a specific subcellular membrane. The hypervariable C-terminal domain (HVD) of Rab has been proposed to contain a targeting signal, but recent studies have argued this model. However, the role of the Rab HVD and the mechanism of Rab membrane targeting remain elusive. In this study, using a combination of unique synthetic protein probes and cell biological tools, we dissect the role of the HVD for subcellular Rab targeting. We find that the HVD can act as a structurally flexible spacer, a subcellular localization determinant, and/or an enhancer of membrane affinity.
Keywords: chemical protein modification, synthetic protein probe, RILP
Abstract
Intracellular membrane trafficking requires correct and specific localization of Rab GTPases. The hypervariable C-terminal domain (HVD) of Rabs is posttranslationally modified by isoprenyl moieties that enable membrane association. A model asserting HVD-directed targeting has been contested in previous studies, but the role of the Rab HVD and the mechanism of Rab membrane targeting remain elusive. To elucidate the function of the HVD, we have substituted this region with an unnatural polyethylenglycol (PEG) linker by using oxime ligation. The PEGylated Rab proteins undergo normal prenylation, underlining the unique ability of the Rab prenylation machinery to process the Rab family with diverse C-terminal sequences. Through localization studies and functional analyses of semisynthetic PEGylated Rab1, Rab5, Rab7, and Rab35 proteins, we demonstrate that the role of the HVD of Rabs in membrane targeting is more complex than previously understood. The HVD of Rab1 and Rab5 is dispensable for membrane targeting and appears to function simply as a linker between the GTPase domain and the membrane. The N-terminal residues of the Rab7 HVD are important for late endosomal/lysosomal localization, apparently due to their involvement in interaction with the Rab7 effector Rab-interacting lysosomal protein. The C-terminal polybasic cluster of the Rab35 HVD is essential for plasma membrane (PM) targeting, presumably because of the electrostatic interaction with negatively charged lipids on the PM. Our findings suggest that Rab membrane targeting is dictated by a complex mechanism involving GEFs, GAPs, effectors, and C-terminal interaction with membranes to varying extents, and possibly other binding partners.
Rab proteins are key regulators of intracellular vesicle transport in eukaryotic cells (1, 2). They comprise the largest subgroup of the Ras superfamily of small GTPases, with more than 60 members in humans and 11 members in yeast (3). Interacting with a complex network of Rab regulators and effectors, Rab GTPases regulate these processes through a spatiotemporally controlled GTPase cycle and their distribution in cells. The GTPase cycle is strictly regulated by guanine nucleotide exchange factors (GEFs) that mediate GDP/GTP exchange and by GTPase-activating proteins (GAPs) that accelerate the hydrolysis of GTP. Active (GTP-bound) Rab proteins associate with distinct intracellular compartments and direct vesicular transport by recruiting a multitude of Rab-specific effectors, including tethering complexes and motor proteins.
Rab proteins are posttranslationally modified at the C terminus with prenyl groups that function as membrane anchors. Rab prenylation involves covalent attachment of the geranylgeranyl (C-20 isoprenyl) moiety to one or two C-terminal cysteine residues of the protein substrate via a stable thioether linkage (4). Unlike other protein prenyltransferases that recognize the C-terminal CaaX motif of protein substrates (e.g., Ras and Rho), Rab geranylgeranyl transferase (RabGGTase) does not recognize its protein substrates (Rab proteins) directly but requires the adaptor Rab escort protein (REP). Rab prenylation requires the formation of a ternary catalytic Rab:REP:RabGGTase complex (5–7). It remains elusive how the single Rab prenylation machinery can process the whole Rab family with diverse C termini.
Cycling between the cytosol and membranes is an essential feature of the mode of action of Rabs and is made possible by reversible interaction with GDP dissociation inhibitor (GDI), which can solubilize the otherwise water-insoluble geranylgeranylated Rab molecules (8). Membrane-bound GDI displacement factors were proposed to disrupt GDI:Rab complexes, leading to insertion of the prenylated Rab into the membrane in the GDP form and release of GDI into the cytosol (9, 10). One of the most perplexing questions is how Rab proteins are specifically targeted to their cognate membranes. The hypervariable C-terminal domain (HVD) was proposed to function as a signal for targeting Rab proteins to specific subcellular membranes (11). However, later studies suggested that several features of Rab molecules, Rab effectors, and GEFs are involved in the targeting process (12–15). The role of the Rab HVD in membrane targeting is controversial, because contradictory results were obtained by swapping the hypervariable domains of Rab proteins (11–13). Thus, the function of the Rab HVD and a complete model for Rab membrane targeting remain to be established. To further understand the significance of the HVD in Rab membrane targeting and prenylation, unique methods are needed to manipulate the structure of Rab C terminus.
In this study, we replaced the Rab C-terminal sequence with the polyethylenglycol (PEG) linker as a nonpeptidic chain. The PEG chain containing two cysteine residues or a simple thiol group at one end was coupled to truncated Rab proteins by oxime ligation. We found that the PEGylated Rab proteins undergo normal prenylation in vitro, confirming that the Rab prenylation machinery does not require a specific C-terminal sequence but rather outsources the specificity to the REP molecule. By combining this semisynthetic strategy with cell imaging, we elucidate the role of the hypervariable C-terminal domain for subcellular Rab targeting. In some instances, the HVD is dispensable for correct subcellular localization (Rab1, Rab5), but is essential in other cases because of specific interactions with effectors (Rab7) or electrostatic interactions with membranes (Rab35). The results further elaborate the model for Rab prenylation and membrane targeting.
Results
Construction of PEGylated Rab Protein Probes.
To further understand the function of the Rab HVD in membrane targeting and prenylation, other methods are needed to manipulate the structure of the Rab C terminus. Advances in protein chemical methods allow for site-specific modification of proteins (16, 17). Recently, we reported a facile method for protein C-terminal modification through intein-mediated incorporation of a (bis)oxyamine moiety into the C terminus of proteins, which is then available for subsequent conjugation under mild conditions with a fluorophore containing a ketone functionality (18). We chose to equip Rab1, Rab5, Rab7, and Rab35, which localize on the Golgi apparatus, early endosome, late endosome/lysosome, and plasma membrane, respectively, with synthetic nonnative C termini and analyze the effect on subcellular localization. Based on sequence alignments, a dibasic repeat (KK) that is conserved in Rab1, Rab5, and Rab35 sequences was defined as the hypervariable domain junction (11, 12, 19).
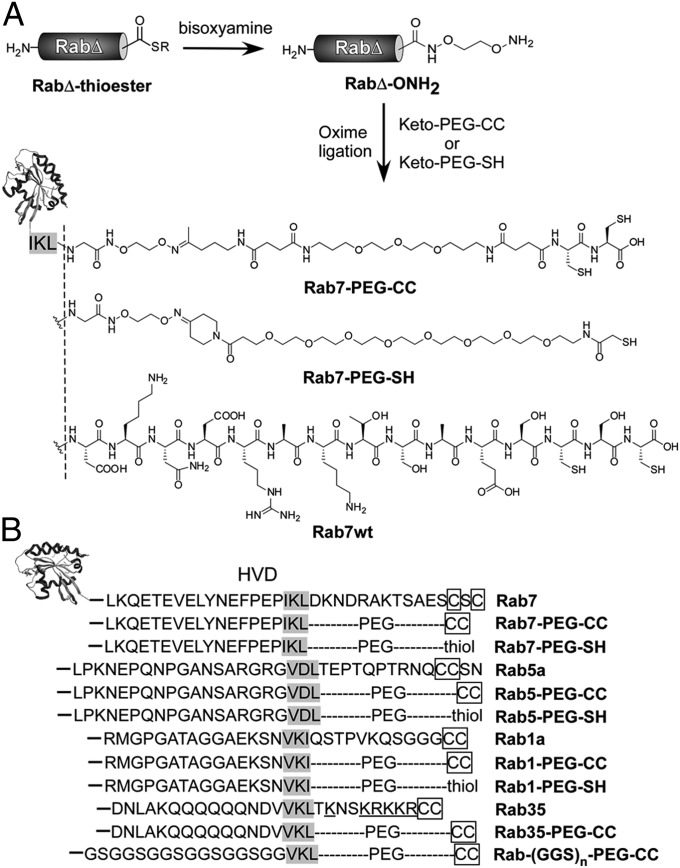
Initially, we investigated to what extent the Rab prenylation machinery could tolerate changes in the Rab C-terminal structure. The hypervariable domain is largely unstructured. Only N-terminal residues of the hypervariable domain are visible in the crystal structure of Rab:GDI and Rab:REP complexes, where a hydrophobic interaction of the C-terminal interacting motif (CIM) within the hypervariable domain with REP and GDI is observed (6, 20). Because the CIM is important for binding to REP and GDI, we made two kinds of structural changes in the HVD flanking the CIM (7). The fragment C-terminal to the CIM was replaced with a PEG linker containing prenylatable cysteines or a thiol group using the oxime ligation strategy. The upstream fragment was replaced by flexible GGS repeats (Fig. 1). The (GGS)n-peptide linker has a random-coil conformation and has been used as a flexible peptide linker in proteins (21). In the construct referred to as Rab-PEG-CC, the C-terminal sequence downstream of the CIM was replaced by a PEG-CC moiety. In Rab-(GGS)n-PEG-CC, the whole hypervariable domain was substituted by a GGS repeat and a PEG-CC moiety (Fig. 1).
Fig. 1.
Modification of Rab HVD. (A) Semisynthesis of Rab-PEG-CC and Rab-PEG-SH and comparison of the C-terminal structures with that of wild-type protein. (B) Substitution of the Rab HVD with different C-terminal structures. The CIM and prenylatable cysteines are highlighted.
To this end, truncated Rab7-thioester proteins were produced via intein-mediated protein splicing by using 2-mercaptoethanesulfonate (MESNA) as the thiol reagent. Subsequently, Rab7-thioester proteins were treated with (bis)oxyamine at pH 7.5 on ice for 4 h, converting them to oxyamine conjugates, Rab7Δ-ONH2, which are competent for oxime ligation with a compound containing a ketone moiety (Fig. 1 and SI Appendix, Fig. S3A).
Keto-PEG-C(StBu)C(StBu) was synthesized by stepwise solid-phase (the synthetic procedure is described in SI Appendix). Incubation of Keto-PEG-C(StBu)C(StBu) and Rab7Δ15-ONH2 on ice overnight led to ca. 90% conversion of the oxyamine to an oxime adduct, as shown by electrospray ionization (ESI)-MS (SI Appendix, Fig. S3B). Subsequently, the thiol protecting groups StBu were removed by treatment with 500 mM MESNA to yield Rab7-PEG-CC. A dimeric (Keto-PEG-SH)2 is connected by a disulfide bond that can be easily cleaved by using 5 mM Dithioerythritol (DTE) during protein conjugation. Ligation of Keto-PEG-SH with Rab7Δ15-ONH2 yielded Rab7-PEG-SH (SI Appendix, Fig. S3C).
PEGylated Rab Proteins Undergo Prenylation in Vitro.
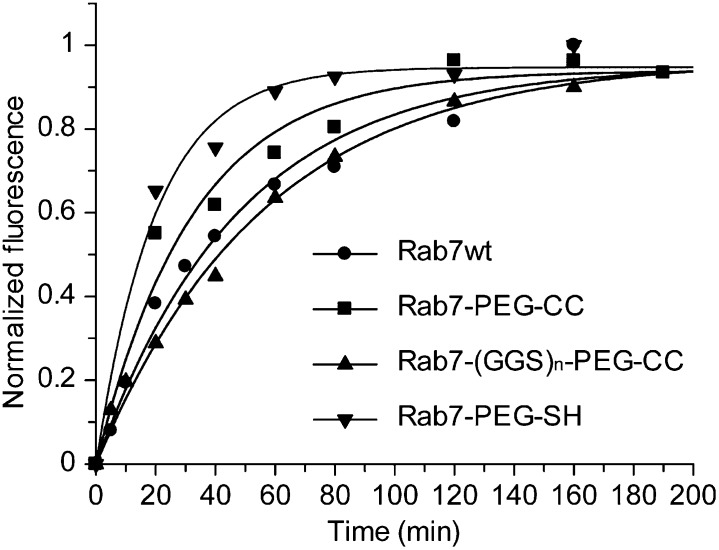
The obtained PEGylated Rab7 proteins were tested in an in vitro prenylation assay by using NBD-FPP, a fluorescent analog of the lipid substrate GGPP (22). Rab7-PEG-CC and Rab7-(GGS)n-PEG-CC protein conjugates display identical prenylation efficiency to that of the wild-type Rab7 protein (Fig. 2). In keeping with NBD-FPP prenylation results, prenylation of Rab7-PEG-CC and Rab7-(GGS)n-PEG-CC using GGPP leads to the doubly geranylgeranylated protein, as shown by mass spectrometry (SI Appendix, Fig. S4). Next, we further examined whether intact cysteine residues are indispensable for prenylation. A Rab7-PEG-SH protein conjugate with two cysteines being replaced by a simple thiol group was subjected to in vitro prenylation (Fig. 1). Rab7-PEG-SH undergoes prenylation with an observed rate that is ca. twofold higher than that of wild-type protein (Fig. 2 and SI Appendix, Fig. S4). These findings suggest that Rabs do not require a specific sequence in the HVD for prenylation, except for the CIM and an SH group as an isoprenyl acceptor. Instead, the amino acids downstream of the CIM can be replaced by a nonpeptidic PEG linker containing cysteines or thiol groups without perturbing Rab prenylation in vitro.
Fig. 2.
Reaction kinetics of prenylation from the SDS/PAGE assay using the fluorescent analog of GGPP, NBD-FPP. The solid lines show single exponential fits for Rab7 wild type (kobs = 0.021 min−1), Rab7-PEG-CC (kobs = 0.031 min−1), Rab7-(GGS)n-PEG-CC (kobs = 0.018 min−1), and Rab7-PEG-SH (kobs = 0.050 min−1).
To gain insights into the role of the Rab C terminus in membrane targeting, we prepared PEGylated EGFP-Rab1, EGFP-Rab5, EGFP-Rab7, and EGFP-Rab35 proteins by ligating Keto-PEG-CC to the truncated EGFP-Rab and EGFP-Rab-(GGS)n proteins, as done for the PEGylated Rab7 conjugates (Fig. 1). These protein conjugates were subjected to in vitro prenylation. As shown in SI Appendix, Fig. S5, all PEGylated Rab proteins undergo prenylation in vitro.
The HVD Sequence of Rab1 and Rab5 Is Not Required for Correct Subcellular Localization and Function.
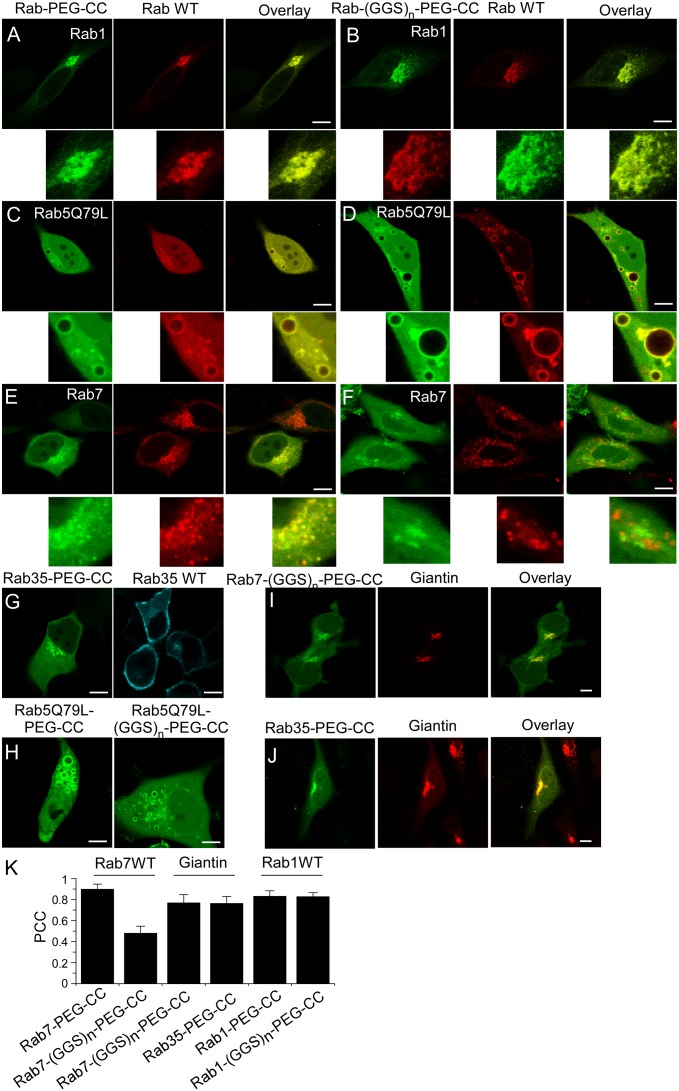
Next, we microinjected the PEGylated fluorescent Rab proteins into HeLa or MDCK cells expressing Cherry-Rab wild-type proteins to examine their subcellular localizations. All of the PEGylated Rab proteins with a dicysteine motif localized to membranes, suggesting that PEGylated Rab proteins are prenylated in the cell (Fig. 3 and SI Appendix, Fig. S6). Both Rab1-PEG-CC and Rab1-(GGS)n-PEG-CC colocalize with the wild-type Rab1 on the Golgi apparatus (Fig. 3 A and B). To demonstrate that the PEGylated Rab proteins are functional, we prepared Rab5Q79L mutants that are deficient in GTPase activity and are constitutively in the active GTP-bound state. Constitutively active Rab5 promotes the formation of enlarged endosomes, in line with its function in homotypic endosome fusion (23). Likewise, microinjection of Rab5Q79L-PEG-CC or Rab5Q79L-(GGS)n-PEG-CC protein into the cell leads to the formation of enlarged vesicles (Fig. 3H). Moreover, both of them colocalized with the Rab5Q79L mutant on the enlarged early endosomes when they were introduced into cells expressing Cherry-Rab5Q79L (Fig. 3 C and D). These results suggest that PEGylated Rab1 and Rab5 proteins are correctly targeted and functional, indicating that the hypervariable domain of Rab1 and Rab5 is not essential for their membrane targeting and function.
Fig. 3.
Subcellular localization of EGFP-Rab-PEG-CC and EGFP-Rab-(GGS)n-PEG-CC proteins. EGFP-Rab1-PEG-CC (A) and EGFP-Rab1-(GGS)n-PEG-CC (B) colocalize with mCherry-Rab1 wild-type protein at the Golgi apparatus. EGFP-Rab5Q79L-PEG-CC (C) and EGFP-Rab5Q79L-(GGS)n-PEG-CC (D) colocalize with mCherry-Rab5Q79L on enlarged endosomes. EGFP-Rab7-PEG-CC (E) colocalize with mCherry-Rab7 wild-type protein at the late endosome/lysosome. EGFP-Rab7-(GGS)n-PEG-CC does not colocalize with mCherry-Rab7 wild-type protein (F) but colocalizes with mKate2-Giantin at the Golgi apparatus (I). (G) EGFP-Rab35-PEG-CC localizes in the perinuclear region but not the plasma membrane as shown for the ECFP-Rab35 wild-type protein in MDCK cells. (J) EGFP-Rab35-PEG-CC colocalizes with mKate2-Giantin at the Golgi apparatus. (H) EGFP-Rab5Q79L-PEG-CC and EGFP-Rab5Q79L-(GGS)n-PEG-CC induce formation of enlarged vesicles. (K) Pearson’s colocalization coefficient analysis of chimeric Rab proteins with wild-type Rab proteins or the Golgi marker. Measurements were performed in HeLa cells except where otherwise indicated. (Scale bars: 10 µm.) In the measurements shown in A, B, I, and J, cells were fixed and subject to extraction of cytosolic proteins by saponin.
To further confirm our conclusions, we substituted the hypervariable domain of Rab1 and Rab5 with a (GGS)n-VKL-(GGS)n-CSC or (GGS)n-VKL-(GGS)n-CC fragment, referred to as RabΔ-CSC or RabΔ-CC, respectively (SI Appendix, Fig. S7). In these chimeric proteins only the CIM and the prenylation motif were retained while the rest of the HVD was replaced with flexible GGS repeats. To examine the effects of carboxyl methylation on Rab protein localization, two kinds of prenylation motifs, CXC and CC, were included. After prenylation, Rab proteins ending in CXC undergo carboxyl methylation by isoprenylcysteine carboxymethyltransferase (Icmt) at the ER membrane, whereas those having CC and CCXX sequences in their C termini are not carboxymethylated (24–26). Analogous scenarios were observed in the cells expressing Rab1 and Rab5 chimeric proteins, i.e., correct membrane localization and induction of the enlarged early endosomes by Rab5Q79LΔ35-CSC or Rab5Q79LΔ35-CC (SI Appendix, Fig. S7).
Interestingly, Rab protein conjugates (Rab1, Rab7, Rab5, and Rab5Q79L) with the replacement of the C-terminal residues downstream from the CIM by a PEG-SH moiety are largely cytosolic and not functional in the cell (Fig. 1 and SI Appendix, Fig. S8), although they can be prenylated in vitro (SI Appendix, Fig. S5C). These results suggest that digeranylgeranylation rather than the sequence of the prenylation motif is essential for the correct subcellular localization and function of Rab proteins, in keeping with a previous report on Rab5 membrane localization (27).
Binding to Rab-Interacting Lysosomal Protein Is Essential for Rab7 Membrane Targeting.
Rab7-PEG-CC with the C-terminal sequence downstream from the CIM replaced by a PEG-CC moiety colocalizes with wild-type Rab7 at late endosomes or lysosomes in the perinuclear region (Fig. 3 E and K). However, Rab7-(GGS)n-PEG-CC with the substitution of the whole hypervariable domain is located differently from wild-type Rab7 protein (Fig. 3 F and K). We found that Rab7-(GGS)n-PEG-CC colocalizes with the Golgi marker, Giantin (Fig. 3 I and K). To confirm its Golgi localization, cells were treated with nocodazole, which disrupts the microtubule network, leading to the formation of Golgi fragments that are distributed throughout the cell (28). Rab7-(GGS)n-PEG-CC was found to colocalize extensively with Giantin on the Golgi fragments in the nocodazole-treated cells (SI Appendix, Fig. S9 A–C). These results suggest that the HVD of Rab7 upstream from the CIM is essential for membrane targeting, and mutation in this region leads to mistargeting to the Golgi apparatus.
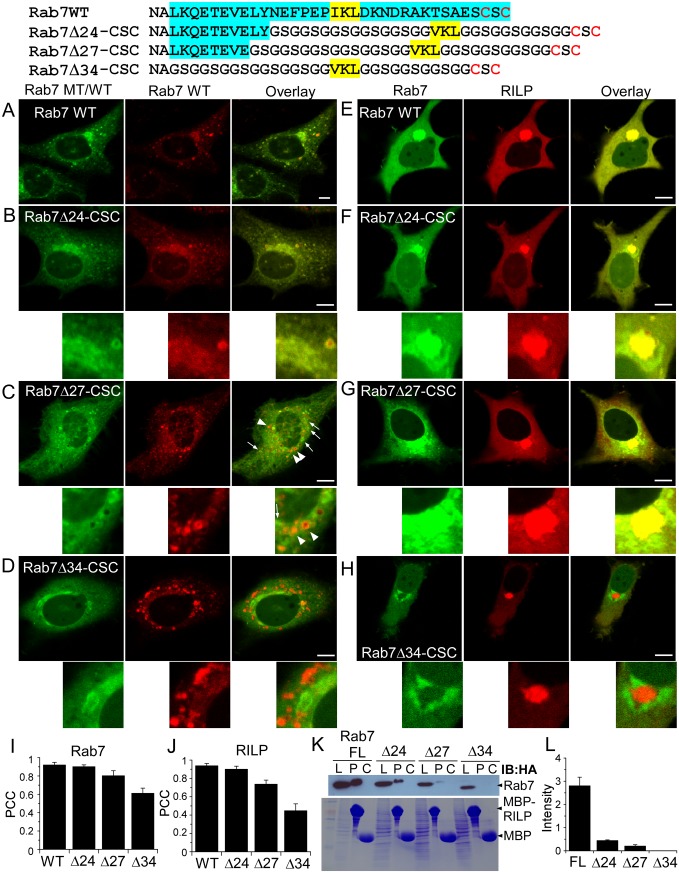
To map the region that is essential for Rab7 targeting, we substituted the Rab7 HVD partially or completely with (GGS)n-VKL-(GGS)n-CSC or (GGS)n-VKL-(GGS)n-CC fragments. The chimeric Rab7 proteins were expressed in HeLa cells, and their subcellular localization was examined. Rab7Δ24-CSC colocalizes with the wild-type protein, whereas partial colocalization and no significant colocalization but rather mislocalization to the Golgi apparatus were observed in Rab7Δ27-CSC and Rab7Δ34-CSC/CC proteins, respectively (Fig. 4 A–C and SI Appendix, Fig. S10), suggesting that residues (174–183) of the HVD are involved in Rab7 membrane targeting. These residues are known to be involved in binding to the Rab7 effector, Rab-interacting lysosomal protein (RILP). In particular, V180, L182, and Y183 are involved in hydrophobic interactions with RILP (29). Binding of Rab7 full-length, Rab7Δ24-CSC, Rab7Δ27-CSC, and Rab7Δ34-CSC to RILP declines sequentially as shown in the pull-down assay (Fig. 4 K–L). No detectable binding is observed between Rab7Δ34-CSC and RILP. To exclude the possibility that C-terminal truncation might affect nucleotide exchange, we examined the GTP/GDP ratio of Rab7 and Rab7Q67L (GTPase deficient) proteins in HeLa cells. We found that the truncated Rab7 chimera proteins undergo normal nucleotide exchange in the cell. (SI Appendix, Fig. S11). Taken together, these results suggest that residues of Rab7 HVD, particularly amino acids 174–183, which are indispensable for the Rab7–RILP interaction, are also essential for correct targeting. Therefore, the interaction with RILP appears to be essential for Rab7 membrane targeting. Indeed, colocalization of RILP with Rab7 wild-type, Rab7Δ24-CSC, Rab7Δ27-CSC, and Rab7Δ34-CSC/CC proteins in the clustered late endosomes/lysosomes decreases sequentially (Fig. 4 E–G and J), in line with the Rab7WT colocalization and the RILP pull-down results (Fig. 4I).
Fig. 4.
Subcellular localization of EGFP-Rab7Δ-CSC proteins. The HVD of Rab7 is partly or completely substituted. The HVD is highlighted in cyan, the CIM is highlighted in yellow, and prenylatable cysteines are in red. EGFP-Rab7 wild-type protein and EGFP-Rab7Δ-CSC proteins are coexpressed with mCherry-Rab7 (A–D) or mCherry-RILP (E–H) in HeLa cells. The arrowheads indicate Rab7 vesicles that do not contain Rab7Δ27-CSC, whereas the arrows indicate Rab7Δ27-CSC vesicles that do not contain Rab7. (Scale bars: 10 µm.) Pearson’s correlation coefficients (PCC) for colocalization with Rab7 (I) and RILP (J). (K) Binding of Rab7Δ-CSC proteins with RILP. HeLa cells were transfected with full-length and truncated (C-terminally replaced) HA-Rab7Q67L (constitutively active) proteins. Proteins were pulled down from cell lysate with MBP-RILP or MBP (control)-coated beads. Beads were subjected to SDS/PAGE and Western blotting with anti-HA antibody (Upper). The protein level of cell lysate, MBP-RILP and MBP on the beads was examined by Coomassie blue staining (Lower). C, control; L, cell lysate; P, pull-down. (L) Quantitation of Rab7 proteins bound to RILP as shown in K. Measurements were performed in triplicate.
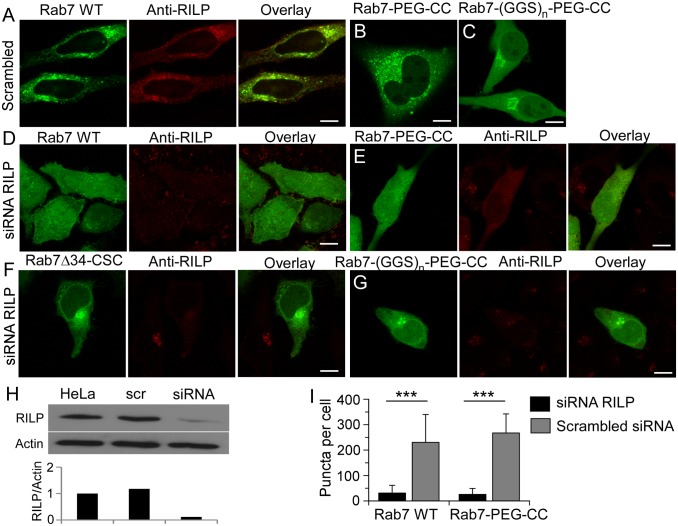
To further investigate the role of RILP in Rab7 membrane targeting, we knocked down RILP in HeLa cells by siRNA (Fig. 5H). Rab7 wild type and Rab7-PEG-CC are largely cytosolic in RILP knock-down cells (Fig. 5 D, E, and I and SI Appendix, Fig. S12). The subcellular localization of these proteins is normal in control siRNA cells (Fig. 5 A, B, and I). In contrast, the membrane localization of Rab7-(GGS)n-PEG-CC and Rab7Δ34-CSC, which do not bind to RILP and are mistargeted to the Golgi apparatus, is not affected by RILP knock-down (Fig. 5 F and G). These results demonstrate that RILP plays an essential role in Rab7 membrane targeting.
Fig. 5.
Effect of RILP RNAi on Rab7 localization. (A–C) Subcellular localization of EGFP-Rab7 WT (A), EGFP-Rab7-PEG-CC (B), and EGFP-Rab7-(GGS)n-PEG-CC (C) in control siRNA cells. (D–G) Subcellular localization of EGFP-Rab7 WT (D), EGFP-Rab7-PEG-CC (E), EGFP-Rab7Δ34-CSC (F), and EGFP-Rab7-(GGS)n-PEG-CC (G) in RILP siRNA cells. Endogens RILP was detected by immunofluorescence. (H) Knockdown of endogenous RILP in HeLa cells, detected by Western blot using anti-RILP antibody. Scrambled siRNA (scr) cells were used as a control. (I) Quantification of membrane localization of Rab7 WT and Rab7-PEG-CC in siRNA or control cells by counting Rab7-positive vesicles in the cell. ***P < 0.001. (Scale bars: 10 µm.)
The C-Terminal Polybasic Cluster Is Important for Rab35 Localization to the Plasma Membrane.
Rab35-PEG-CC, with its C-terminal polybasic cluster substituted by the PEG linker, does not localize to the plasma membrane (PM) but mistargets to the Golgi apparatus (Fig. 3 G and J and SI Appendix, Fig. S6). Replacement of the Rab35 HVD with a (GGS)n-VKL-(GGS)n-CSC or (GGS)n-VKL-(GGS)n-CC fragment also leads to mistargeting to the Golgi apparatus (SI Appendix, Fig. S7 E and F). To confirm its Golgi localization, cells were treated with nocodazole and Rab35-PEG-CC was found to colocalize extensively with Giantin on the Golgi fragments in the nocodazole-treated cells (SI Appendix, Fig. S7 D–F). These results demonstrate that the polybasic region is essential for the PM targeting and/or PM membrane affinity, presumably because of interaction with negatively charged phosphatidylinositol phosphate lipids (30). It is not clear whether Rab35 initially targets to the Golgi apparatus and subsequently redistributes to the PM.
Discussion
In the present study, we have combined synthetic chemistry, bioorthogonal chemistry, and protein engineering to introduce unnatural C-terminal fragments into Rab proteins. This method resolves inherent problems of the traditional HVD swapping approach in the analysis of Rab membrane targeting. Because the HVD may be only a partial determinant for membrane targeting in some Rabs, investigation of chimeric Rab proteins may lead to ambiguous results (11–13).
In vitro prenylation analysis of the PEGylated Rab proteins probes provides insights into the mechanism of Rab protein prenylation. This study together with previous reports suggests that the specificity of the Rab prenylation machinery is conferred by three binding interfaces, involving the GTPase domain, the CIM, REP, and RabGGTase (5, 6). The HVD of Rabs, with the exception of the CIM, does not contribute specificity to the assembly of the ternary protein complex. Once the ternary protein complex is established, the Rab C terminus is concentrated within the microenvironment of the complex, thus enhancing the probability of C-terminal cysteines reaching the active site of RabGGTase. As a consequence, the protein substrate specificity of RabGGTase does not need to be encoded in the Rab C terminus, in contrast to that of CaaX protein prenyltransferases. Rab proteins therefore outsource the specificity to REP. The model is consistent with the fact that RabGGTase has essentially no sequence preference for the context of the prenylatable cysteines, and the C-terminal sequences occurring in Rab GTPases include CC, CXC, CCX, CCXX, CCXXX, and CXXX. Hence, any cysteine- or thiol-containing fragment that can be properly presented to the active site of RabGGTase is able to undergo prenylation. This property allows RabGGTase to process all members of the family of more than 60 Rab proteins with hypervariable C-terminal sequences, a feature that is uncommon in protein-modifying enzymes. This unique property of the Rab prenylation machinery also enables it to process Rab proteins with unnatural C-terminal moieties.
Analysis of subcellular localization and function of the PEGylated Rab proteins probes facilitates elucidation of Rab membrane targeting and the role of the hypervariable domain in this process. Our findings suggest that the HVDs of individual Rab proteins play distinct roles in membrane targeting. In the case of Rab1 and Rab5, the HVD is not required for membrane targeting, probably because it is not involved in binding with potential targeting factors, including effectors and GEFs (12, 31–33). It serves rather as an anchoring chain that physically connects the functional GTPase domain with the membrane. The HVD can be substituted by a nonnative PEG linker of sufficient length. The digeranylgeranyl lipid anchor is important for membrane association, whereas the sequence of the prenylation motif is not essential for correct membrane targeting. However, natively monogeranylgeranylated Rabs (e.g., Rab8, Rab13, Rab18, Rab23, and Rab28) obviously do not require diprenylation for appropriate targeting to subcellular membranes.
In contrast, some of the N-terminal residues of the Rab7 HVD are involved in binding with the Rab7 effector RILP, and the results presented here suggest that this interaction stabilizes Rab7 association with late endosomes/lysosomes. Because residues of the Rab7 HVD are one of the elements involved in the interaction with RILP (binding to the GTPase domain is also required), a Rab chimeric protein with the Rab7 HVD would bind to RILP less efficiently (29). In keeping with this result, replacing the hypervariable domain of Rab5 with that of Rab7 only led to partial mislocalization from early endosomes (13). Moreover, knockdown of RILP renders Rab7 largely cytosolic in cells. Therefore, RILP appears to be a targeting factor for Rab7 and determines the steady-state distribution of Rab7 on subcellular compartments. Evidence has been presented that the Rab9 effector TIP47 might interact with the Rab9 HVD, and this interaction has been implicated in Rab9 localization (12).
The C-terminal polybasic cluster of Rab35 HVD appears to play a key role for PM targeting, probably due to the electrostatic interaction with negatively charged lipids on the PM (30). However, we cannot rule out the possibility that other unidentified Rab35 effectors that associate with the C terminus of Rab35 may play a role in enhancing its interaction with the PM.
It is not clear how and why those Rabs lacking in affinity to effectors or lipids are mistargeted to the Golgi apparatus. There might be two hypotheses for the role of the Golgi membranes as the initial site for Rab attachment or as the default localization of mislocalized Rabs. In the former case, newly synthesized prenylated Rabs would be delivered to the Golgi membranes and be subsequently sorted to the designated compartments. Loss of targeting elements would lead to accumulation of Rabs in the Golgi apparatus. In the latter case, Rabs would initially target to their subcellular compartments and be transported to the Golgi apparatus via GDI recycling and/or vesicular transport, because Rabs could not stably associate with their cognate membranes. However, the question then arises as to the origin of the specificity of Golgi localization. Further work is required to elaborate this question.
From the results presented here together with evidence from earlier studies, it appears that Rab membrane targeting is governed by complex mechanisms, with the involvement of Rab regulators and binding partners including GEFs, GAPs, and effectors. GEF-mediated nucleotide exchange provides the thermodynamic driving force for Rab membrane insertion, which is indispensable for the stable attachment of Rabs to membranes (15, 34, 35). GEFs and GAPs appear to regulate Rab localization, because the nucleotide-bound state defines Rab membrane association (15). Indeed, emerging evidence suggests that Rabs are relayed on the trafficking pathway through GEF and GAP cascades, which determine the boundary between Rab proteins (36–40). In such cascades, Rab A recruits the GEF that targets Rab B along the pathway to the membrane, and Rab A is subsequently inactivated by a GAP recruited through Rab B and, hence, is detached from the membrane. As a consequence, conversion of a Rab A to a Rab B membrane is achieved. Once Rab proteins are activated and stabilized on the membrane (GTP-bound), the steady-state Rab localization might be dictated by binding partners, including effectors and lipids (Fig. 6). Such a stabilization of Rabs on their cognate membranes by effectors appears to play an essential role in Rab membrane targeting, because depletion of effectors (RILP knockdown) or loss of binding to effectors (Rab HVD replacement) leads to mislocalization of Rab proteins. Some effectors associate with a specific membrane independently of Rabs (41). For those effectors that are recruited to membranes in a Rab-dependent manner (2), there must be a synergy between Rabs and effectors in membrane localization. For example, Rabenosyn-5 has two distinct binding sites for Rab4 and Rab5, suggesting its coordinative role in Rab4 and Rab5 localization and function (42).
Fig. 6.
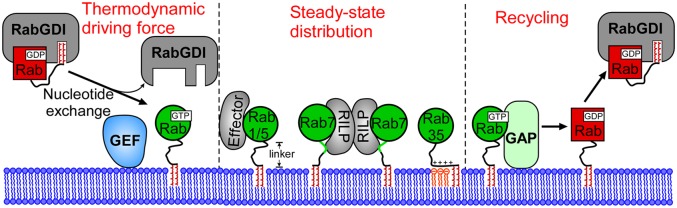
Model for Rab membrane targeting. Initial insertion of Rab proteins into membranes is driven by GEF-mediated nucleotide exchange. Binding of activated Rab proteins (GTP-bound) with effectors or other binding partners determines the steady-state distribution of Rab proteins on membranes. GAPs deactivate Rab proteins at specific sites and trigger the recycling of Rab proteins from membranes. The GTPase cycle controlled by GEFs and GAPs dictates the thermodynamic equilibrium of Rab membrane localization and defines the boundary of a Rab realm.
Methods
Preparation of PEGylated Rab Proteins.
Keto-PEG-C(StBu)C(StBu) and (Keto-PEG-SH)2 were synthesized on solid phase and liquid phase, respectively. Purification of protein thioesters, subsequent oxime ligation with PEG moieties, quality analysis by LC-ESI-MS, and in vitro prenylation assay were performed as described (18, 22) (for details, see SI Appendix).
Cell Culture and Transfection.
HeLa or MDCK cells were maintained at 37 °C and 5% CO2 in high glucose minimum essential medium (MEM) (21969-035; Invitrogen) supplemented with 10% (vol/vol) FBS, 1% sodium pyruvate, 1% non-essential amino acids (NEAA), and 1% L-Glutamine, and MDCK cells in MEM were supplemented with 10% FBS. For confocal microscopy, 2.0 × 105 cells were cultured on 35-mm glass bottom dishes (MatTek) for 12 h before transfection. Transient plasmid expression was achieved by overnight transfection with X-treme GENE HP DNA transfection reagent (06366244001; Roche). For nocodazole treatment, cells were treated with 20 μM nocodazole for 1.5 h. For the details of cell fixation and immunofluorescence, RNAi, pull down assay, and determination of GTP/GDP ratio, see SI Appendix.
Quantitative Analysis of Colocalization.
Confocal micrographs of various proteins and microinjected proteins were selected randomly. After the background subtraction using the BG Subtraction from ROI plugin of ImageJ software (version 1.44; National Institutes of Health), Pearson's correlation coefficient (PCC) values for the relation between the EGFP signals and Cherry signals were then calculated with the Intensity Correlation Analysis plug-in of ImageJ software.
Supplementary Material
Acknowledgments
This work was supported in part by Deutsche Forschungsgemeinschaft Grants SPP 1623 (to Y.-W.W.) and SFB 642 (to R.S.G. and Y.-W.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313655111/-/DCSupplemental.
References
- 1.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 3.Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313(4):889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 4.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271(10):5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 5.Pylypenko O, et al. Structure of Rab escort protein-1 in complex with Rab geranylgeranyltransferase. Mol Cell. 2003;11(2):483–494. doi: 10.1016/s1097-2765(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Rak A, et al. Structure of the Rab7:REP-1 complex: Insights into the mechanism of Rab prenylation and choroideremia disease. Cell. 2004;117(6):749–760. doi: 10.1016/j.cell.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Wu YW, Goody RS, Abagyan R, Alexandrov K. Structure of the disordered C terminus of Rab7 GTPase induced by binding to the Rab geranylgeranyl transferase catalytic complex reveals the mechanism of Rab prenylation. J Biol Chem. 2009;284(19):13185–13192. doi: 10.1074/jbc.M900579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YW, Tan KT, Waldmann H, Goody RS, Alexandrov K. Interaction analysis of prenylated Rab GTPase with Rab escort protein and GDP dissociation inhibitor explains the need for both regulators. Proc Natl Acad Sci USA. 2007;104(30):12294–12299. doi: 10.1073/pnas.0701817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirac-Svejstrup AB, Sumizawa T, Pfeffer SR. Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J. 1997;16(3):465–472. doi: 10.1093/emboj/16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425(6960):856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 11.Chavrier P, et al. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991;353(6346):769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- 12.Aivazian D, Serrano RL, Pfeffer S. TIP47 is a key effector for Rab9 localization. J Cell Biol. 2006;173(6):917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali BR, Wasmeier C, Lamoreux L, Strom M, Seabra MC. Multiple regions contribute to membrane targeting of Rab GTPases. J Cell Sci. 2004;117(Pt 26):6401–6412. doi: 10.1242/jcs.01542. [DOI] [PubMed] [Google Scholar]
- 14.Beranger F, Paterson H, Powers S, de Gunzburg J, Hancock JF. The effector domain of Rab6, plus a highly hydrophobic C terminus, is required for Golgi apparatus localization. Mol Cell Biol. 1994;14(1):744–758. doi: 10.1128/mcb.14.1.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YW, et al. Membrane targeting mechanism of Rab GTPases elucidated by semisynthetic protein probes. Nat Chem Biol. 2010;6(7):534–540. doi: 10.1038/nchembio.386. [DOI] [PubMed] [Google Scholar]
- 16.Hackenberger CP, Schwarzer D. Chemoselective ligation and modification strategies for peptides and proteins. Angew Chem Int Ed Engl. 2008;47(52):10030–10074. doi: 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- 17.Vila-Perelló M, Muir TW. Biological applications of protein splicing. Cell. 2010;143(2):191–200. doi: 10.1016/j.cell.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi L, et al. One-pot dual-labeling of a protein by two chemoselective reactions. Angew Chem Int Ed Engl. 2011;50(36):8287–8290. doi: 10.1002/anie.201100840. [DOI] [PubMed] [Google Scholar]
- 19.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301(4):1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 20.Rak A, et al. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 2003;302(5645):646–650. doi: 10.1126/science.1087761. [DOI] [PubMed] [Google Scholar]
- 21.Evers TH, van Dongen EM, Faesen AC, Meijer EW, Merkx M. Quantitative understanding of the energy transfer between fluorescent proteins connected via flexible peptide linkers. Biochemistry. 2006;45(44):13183–13192. doi: 10.1021/bi061288t. [DOI] [PubMed] [Google Scholar]
- 22.Wu YW, et al. A protein fluorescence amplifier: Continuous fluorometric assay for rab geranylgeranyltransferase. ChemBioChem. 2006;7(12):1859–1861. doi: 10.1002/cbic.200600377. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark H, et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13(6):1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergo MO, et al. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276(8):5841–5845. doi: 10.1074/jbc.C000831200. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Stahl PD. Post-translational processing and membrane association of the two early endosome-associated rab GTP-binding proteins (rab4 and rab5) Arch Biochem Biophys. 1993;304(2):471–478. doi: 10.1006/abbi.1993.1377. [DOI] [PubMed] [Google Scholar]
- 26.Smeland TE, Seabra MC, Goldstein JL, Brown MS. Geranylgeranylated Rab proteins terminating in Cys-Ala-Cys, but not Cys-Cys, are carboxyl-methylated by bovine brain membranes in vitro. Proc Natl Acad Sci USA. 1994;91(22):10712–10716. doi: 10.1073/pnas.91.22.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes AQ, et al. Membrane targeting of Rab GTPases is influenced by the prenylation motif. Mol Biol Cell. 2003;14(5):1882–1899. doi: 10.1091/mbc.E02-10-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lippincott-Schwartz J. Cytoskeletal proteins and Golgi dynamics. Curr Opin Cell Biol. 1998;10(1):52–59. doi: 10.1016/s0955-0674(98)80086-0. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Wang T, Loh E, Hong W, Song H. Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J. 2005;24(8):1491–1501. doi: 10.1038/sj.emboj.7600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heo WD, et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314(5804):1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y, et al. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133(7):1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delprato A, Lambright DG. Structural basis for Rab GTPase activation by VPS9 domain exchange factors. Nat Struct Mol Biol. 2007;14(5):406–412. doi: 10.1038/nsmb1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra A, Eathiraj S, Corvera S, Lambright DG. Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of Early Endosomal Autoantigen 1 (EEA1) Proc Natl Acad Sci USA. 2010;107(24):10866–10871. doi: 10.1073/pnas.1000843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarafder AK, et al. Rab27a targeting to melanosomes requires nucleotide exchange but not effector binding. Traffic. 2011;12(8):1056–1066. doi: 10.1111/j.1600-0854.2011.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blümer J, et al. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200(3):287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464(7289):778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordmann M, et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20(18):1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141(3):497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106(34):14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H, Liang Z, Li G. Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol Biol Cell. 2009;20(22):4720–4729. doi: 10.1091/mbc.E09-06-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houghton FJ, Chew PL, Lodeho S, Goud B, Gleeson PA. The localization of the Golgin GCC185 is independent of Rab6A/A’ and Arl1. Cell. 2009;138(4):787–794. doi: 10.1016/j.cell.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 42.Eathiraj S, Pan X, Ritacco C, Lambright DG. Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature. 2005;436(7049):415–419. doi: 10.1038/nature03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.