Significance
Social interaction deficits in drug users likely impede treatment, increase the burden of the affected families, and consequently contribute to the high costs for society associated with addiction. However, the neural origin contributing to altered social interaction in drug users is unknown thus far. The present study illuminates the nature of basic social interaction deficits as exemplified by social gaze behavior in cocaine users by applying behavioral, psychophysiological, and functional brain-imaging methods. The results suggest that basal social interaction impairments probably arise from blunted social reward processing that was again related to impaired real-life social behavior in cocaine users. These results point to the importance of reinstatement of social reward in the treatment of stimulant addiction.
Keywords: joint attention, reinforcement, social cognition, dopamine, social functioning
Abstract
Social interaction deficits in drug users likely impede treatment, increase the burden of the affected families, and consequently contribute to the high costs for society associated with addiction. Despite its significance, the neural basis of altered social interaction in drug users is currently unknown. Therefore, we investigated basal social gaze behavior in cocaine users by applying behavioral, psychophysiological, and functional brain-imaging methods. In study I, 80 regular cocaine users and 63 healthy controls completed an interactive paradigm in which the participants’ gaze was recorded by an eye-tracking device that controlled the gaze of an anthropomorphic virtual character. Valence ratings of different eye-contact conditions revealed that cocaine users show diminished emotional engagement in social interaction, which was also supported by reduced pupil responses. Study II investigated the neural underpinnings of changes in social reward processing observed in study I. Sixteen cocaine users and 16 controls completed a similar interaction paradigm as used in study I while undergoing functional magnetic resonance imaging. In response to social interaction, cocaine users displayed decreased activation of the medial orbitofrontal cortex, a key region of reward processing. Moreover, blunted activation of the medial orbitofrontal cortex was significantly correlated with a decreased social network size, reflecting problems in real-life social behavior because of reduced social reward. In conclusion, basic social interaction deficits in cocaine users as observed here may arise from altered social reward processing. Consequently, these results point to the importance of reinstatement of social reward in the treatment of stimulant addiction.
Cocaine dependence is a chronically relapsing disorder defined by uncontrolled and compulsive drug use (1). Despite severe negative consequences including disrupted social relationships, loss of employment, and somatic and psychiatric illnesses, an addicted person’s life is often centered around the drug of choice and activities related to it (2). Therefore, drug use is classified as a major social, legal, and public health problem (3). After cannabis, cocaine is the second most prevalent illegal drug in the United States and Europe (4, 5), with a lifetime prevalence among young adults of 6.3% in Europe (15- to 34-y-olds) (4) and 13.3% in the United States (18- to 25-y-olds) (5).
Social cognition and social support for drug users are of great clinical relevance, as they have been reported to influence onset of drug use and development of substance use disorders, and treatment success in patients with substance use disorders (6, 7). Impairments in social cognition may augment the risk of social isolation, aggression, and depression, likely supporting the vicious circle of drug use (8). Additionally, impaired social cognition may contribute to the decay of social relationships in addicted patients (9) with negative consequences for treatment success given that higher social support predicted longer abstinence duration (10). Furthermore, no efficient pharmacological treatment for cocaine addiction is currently available (11), and treatment approaches such as cognitive behavioral therapy rely, at least in part, on the emotional responsiveness and social abilities of drug users (12). Previous results suggest that cocaine users (CUs) show impairments in different facets of social cognition, particularly in emotional empathy, mental perspective taking, and emotion recognition in prosody, which are related to deficits in real-life social behavior such as fewer social contacts and more criminal offenses (13, 14). Furthermore, in money distribution games, CUs act more self-servingly and less altruistically than stimulant-naïve controls (15). Volkow et al. (9) postulated that neuroadaptations in the reward systems of drug users (e.g., ventral striatum and orbitofrontal cortex) alter reward processing such that the value of the abused drug is enhanced and concurrently the value of nondrug rewards, including social interaction, is reduced. Consequently, general social competence might become impaired and promote antisocial and criminal behavior. This may explain why social consequences of drug use (e.g., imprisonment or familial problems) do not prompt drug-addicted people to quit using the drug as well as how they contribute to increased drug use and transition from recreational drug use to addiction (9). However, whereas altered processing of monetary rewards has been reported in CUs (16), social reward processing has not been studied yet, neither on the psychological nor the neural level. Therefore, it remains elusive whether CUs (i) show behavioral differences to reward stemming from social interactions and, if so, (ii) which neural adaptations within reward circuitry underlie these potential changes in social interaction behavior.
An essential part of social interaction is the phenomenon of “social gaze,” which has two aspects: Gaze can be used by the gazing person as a deictic cue to manipulate the attention of others, and can be read out by observers as a hint toward attentional focus of the gazing person (17). Both aspects can converge in joint attention (JA), which is a central element of social interaction (18) and is established when a person follows the direction of another person’s gaze so that both attend to the same object (19). Engagement in JA is considered to reflect our understanding of another person’s point of view (20). The capacity of JA emerges at 8–12 mo of age (21) and is predictive for later language learning (22) and the development of more advanced social skills such as mental perspective taking (e.g., the attribution of intentions and goals to others, also known as theory of mind) (23). Impaired JA is a core symptom of autism spectrum disorders (24).
To test for social gaze differences between CUs and healthy controls (HCs), we applied a paradigm designed to capture the reciprocal and interactive nature of JA (25) (Fig. S1), where participants engage in an online interaction with an anthropomorphic virtual character in real time. Compared with self-initiated nonjoint attention (NJA; i.e., if the counterpart does not follow one’s gaze but rather pays attention to another object), self-initiated JA (i.e., if the counterpart follows one’s own gaze) is perceived as more pleasurable and associated with stronger activation of reward-related brain areas in healthy controls (25). This rewarding nature of JA might underlie the human motivation to engage in the sharing of experiences that emerges in early childhood (22, 25).
It has been suggested that changes in social reward processing might underlie alterations in social behavior and cognition in CUs (9). Here we conducted two studies assessing JA processing, which constitutes an elegant approach to investigate basic social interaction patterns related to social reward processing (25), in CUs and stimulant-naïve HCs by means of behavioral, psychophysiological, and functional brain-imaging methods. In study I, a large sample of relatively pure CUs with few psychiatric comorbidities (n = 80) and stimulant-naïve HCs (n = 63) completed an interactive JA task (25) while valence and arousal ratings, error scores, reaction time, and pupil size were obtained. Pupil dilation provides an objective index of affective processing (26, 27). Based on the observations obtained in study I, we further investigated the neural correlates of the blunted emotional response to social gaze in subsamples of 16 CUs and 16 HCs using functional magnetic resonance imaging (fMRI) during an abridged version of the paradigm (study II). We hypothesized that altered emotional responses to JA are accompanied by less pronounced activation in reward-related brain areas of CUs.
Results
Demographic Characteristics.
In studies I and II, CUs and HCs did not differ with respect to verbal intelligence quotient (IQ), years of education, sex distribution, smoking status, and age (Table 1). As expected, CUs scored significantly higher than HCs in the Beck Depression Inventory (BDI) and Fagerström Test for Nicotine Dependence (FTND) sum score in study I. Furthermore, HCs reported a significantly larger social network than CUs in study I, as shown in this sample before (13). Self-reported drug use, urine toxicology status, and results of 6-mo quantitative hair toxicologies are displayed in Table S1 (study I) and Table S2 (study II).
Table 1.
Demographic data
| Variables | Control group | Cocaine users | Value | df | P value |
| Study I | |||||
| Male/female participants, n, total | 42/21, 63 | 59/21, 80 | χ2 = 0.85* | 1 | 0.36 |
| Age, y | 29.65 (8.95) | 30.06 (9.00) | t = −0.27† | 141 | 0.79 |
| Education, y | 10.51 (1.74) | 10.07 (1.73) | t = 1.50† | 141 | 0.14 |
| Verbal IQ | 105.22 (9.99) | 102.74 (11.22) | t = 1.38† | 141 | 0.17 |
| Smoker/nonsmoker, n | 46/17 | 64/16 | χ2 = 0.97* | 1 | 0.33 |
| FTND sum score, 0–10‡ | 2.46 (2.20) | 3.85 (2.43) | t = −3.10† | 110 | <0.01 |
| BDI sum score, 0–63 | 4.06 (3.89) | 9.36 (7.91) | t = −4.90† | 141 | <0.001 |
| Social network size§ | 26.41 (13.19) (n = 60) | 19.94 (12.05) (n = 72) | t = 2.94† | 130 | <0.01 |
| Study II | |||||
| Male/female participants, n, total | 12/4, 16 | 12/4, 16 | χ2 = 0.00* | 1 | 1.00 |
| Age, y | 32.63 (8.33) | 33.38 (9.12) | t = −0.24† | 30 | 0.81 |
| Education, y | 11.25 (1.61) | 11.00 (1.51) | t = 0.45† | 30 | 0.65 |
| Verbal IQ | 106.63 (11.16) | 104.56 (10.62) | t = 0.54† | 30 | 0.60 |
| Smoker/nonsmoker, n | 9/7 | 11/5 | χ2 = 0.53* | 1 | 0.47 |
| FTND sum score, 0–10‡ | 1.63 (1.85) | 4.40 (3.63) | t = −1.96† | 16 | 0.07 |
| BDI sum score, 0–63 | 2.63 (2.53) | 6.94 (8.63) | t = −1.92† | 30 | 0.07 |
| Social network size§ | 24.88 (14.39) | 20.20 (13.43) (n = 15) | t = 0.93† | 29 | 0.36 |
Significant P values are shown in bold. Means and SD of means are in parentheses.
χ2 test (across all groups) for frequency data.
Independent t test.
FTND was measured in smokers only.
Measured by the Social Network Questionnaire.
Study I.
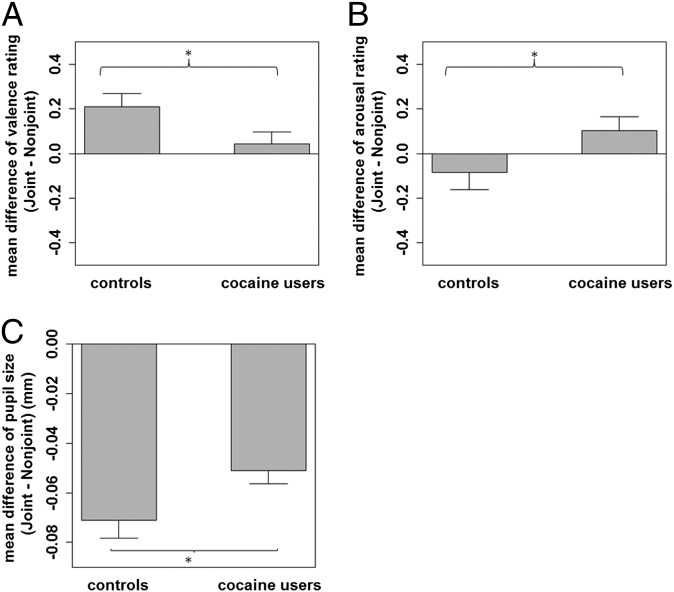
A mixed-effects ANOVA [group(HCs vs. CUs)*initiation(self vs. other vs. object)*joint(JA vs. NJA)] performed for valence ratings yielded a significant main effect for the factor joint [F(1,141) = 10.17, P < 0.01] and a significant group*joint interaction [F(1,141) = 4.35, P < 0.04], indicating that both groups rated JA trials as more pleasant than NJA trials but that CUs differentiated less between JA and NJA trials than HCs (Fig. 1A). The main effects for group and initiation and the group*initiation interaction did not reach statistical significance (all P > 0.09).
Fig. 1.
Mean difference in valence ratings (A) and arousal ratings (B) for joint vs. nonjoint attention trials for controls (n = 60) and cocaine users (n = 83), and mean pupil size difference (C) between joint attention and nonjoint attention trials for controls (n = 60) and cocaine users (n = 72). Error bars refer to SEM. *P < 0.05.
For the arousal ratings, a mixed-effects ANOVA revealed a significant group*joint interaction [F(1,141) = 3.94, P < 0.05] (Fig. 1B), indicating that HCs rated the JA trials as less arousing than NJA trials, whereas CUs showed the reverse pattern. Furthermore, a significant main effect was found for the factor initiation [F(2,282) = 18.06, P < 0.001] (Fig. S2), suggesting that both groups rated self-initiated trials as the least arousing condition, whereas the other-initiated condition was experienced as the most arousing initiation condition. The main effects for group and joint and the group*initiation interaction did not reveal significant differences (all P > 0.38).
A mixed-effects ANOVA performed for pupil size yielded a significant main effect for the factor joint [F(1,130) = 185.02, P < 0.001], suggesting increased pupil dilatation during NJA trials compared with JA trials in both groups. A significant group*joint interaction [F(1,130) = 4.91, P < 0.03] was revealed, indicating that the change in pupil size between JA and NJA trials was larger in HCs than in CUs (Fig. 1C). This corroborates the results obtained for the valence ratings (see above). The main effects for group and initiation and the group*initiation interaction did not reach statistical significance (all P > 0.16). Thus, pupil size data suggest a processing pattern of JA similar to valence ratings: Both measures reveal that CUs process JA differently from HCs. More precisely, CUs do not differentiate to the same degree between JA and NJA as HCs do.
The number of errors (looking at the wrong object in the other and object initiation condition), number of completed trials, and reaction times did not differ between groups (all P > 0.28) and did not reveal a significant group*joint interaction (all P < 0.09) (Table S3), suggesting that HCs and CUs did not show global performance differences in the task.
These results suggest an altered emotional involvement in social gaze interaction in CUs. As social gaze processing has previously been linked to the reward system (25), we further investigated in study II whether the changed social interaction of CUs can be explained by altered activation of reward areas.
Study II.
Valence and arousal ratings obtained during fMRI replicated the results of study I, although the main effects and interactions did not reach significance, most likely due to the small sample size (Fig. S3).
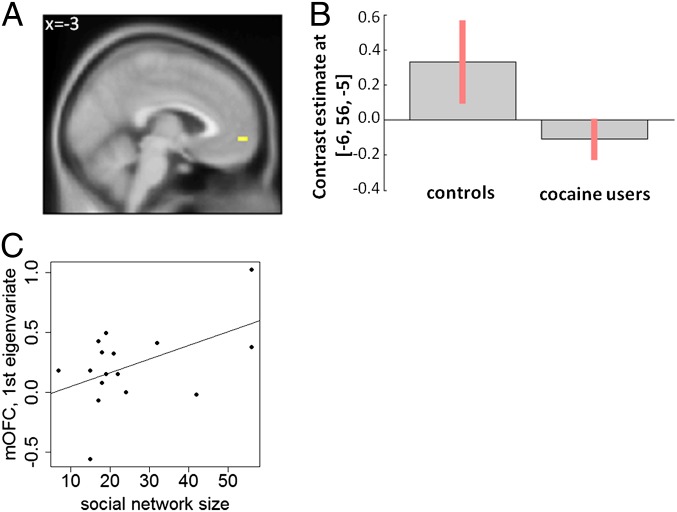
Results of the region-of-interest (ROI) analyses for one-sample (HCs) and between-group (HCs > CUs) tests are displayed in Table S4. We focused on five ROIs [medial orbitofrontal cortex (mOFC), ventral striatum, left posterior hippocampus, medial prefrontal cortex (mPFC), and posterior cingulate cortex (PCC)]. These regions were chosen because they are established core structures of the reward system (for details, see Materials and Methods). Significantly stronger activation for self-initiated JA > self-initiated NJA was found in three of the five ROIs in the control group: mOFC, mPFC, and PCC (Fig. S4 and Table S4). This is in line with the notion that JA is more rewarding than NJA. In the group contrast, CUs displayed significantly less pronounced activations of the mOFC during self-initiated JA > self-initiated NJA compared with HCs (Fig. 2 A and B and Table S4). No significant differences in activation were found for the inverse contrast and any other condition.
Fig. 2.
Between-group activation [controls (n = 16) > cocaine users (n = 16)] for the self-initiated joint attention > self-initiated nonjoint attention contrast (yellow shades represent significant activations displayed at uncorrected P < 0.01) (A) and corresponding contrast estimates in the mOFC (B). Error bars refer to standard error of contrast estimates. Positive association between social network size and mOFC activation for the self-initiated joint attention > self-initiated nonjoint attention contrast in controls (r = 0.49, P < 0.05, first eigenvariate) (C).
A correlation analysis was conducted to investigate the association between blood oxygen level-dependent responses in the mOFC to self-initiated JA > self-initiated NJA and social network size. If the mOFC activation is related to the value of social reward, then there should be a relation to social network size as an indicator of real-life social behavior. To test this prediction, uncontaminated by the already-determined difference in social network size between the two groups, we assessed it only in HCs. A significant positive correlation was found in the mOFC (peak: x = −12, y = 50, z = −5, P < 0.05, familywise error), indicating that lower mOFC activity in response to JA > NJA was associated with the smaller size of the social network a person has. Fig. 2C displays the correlation of social network size and the first eigenvariate of the mOFC ROI for HCs (r = 0.49, P < 0.05). This correlation was also significant in the total sample (r = 0.35, P < 0.05) but not in CUs alone (r = 0.16, P = 0.56). Correlation coefficients did not differ significantly (z = 0.99, P = 0.32) between CUs and HCs. Furthermore, valence ratings of study I were significantly correlated with pupil size of study I (r = −0.39, P < 0.05) and additionally also with mOFC activation in study II (r = 0.36, P < 0.05) in response to JA vs. NJA.
Discussion
The present study demonstrates that CUs process social gaze (joint attention) differently from HCs, and that these impairments are related to a reduced activation of the brain’s reward system in situations of social interactions. To our knowledge, the present study represents a so far unique experimental and multimodal investigation of basic social interaction behavior and its relation to social reward processing in CUs.
The analysis of valence ratings revealed that HCs clearly rated JA as more pleasant than NJA, whereas CUs differentiated less between the two conditions. All participants rated the self-initiated JA condition as the most pleasant, whereas self-initiated NJA was perceived as the least pleasant condition (Fig. S5), which is in accordance with Schilbach et al. (25). Furthermore, arousal ratings showed that CUs feel more aroused by JA trials, whereas HCs rated NJA as more arousing. This demonstrates that emotional involvement and processing of social gaze are altered in CUs. Note that these results cannot be attributed to differences in attention, motivation, or the ability to complete the task, as groups did not differ in the number of errors, number of completed trials, and reaction times.
Providing objective data, reduced reactivity of pupil size also supports the assumption that emotional engagement by social eye contact is altered in CUs: In agreement with valence ratings, the difference in pupil size between JA and NJA was significantly smaller in CUs than in HCs. Pupil size is considered a physiological marker of affective engagement and is highly correlated with other indices of autonomic responsiveness such as skin conductance (28, 29). Pupil changes during the processing of emotionally engaging stimuli are mediated by increased sympathetic activity, which in turn increases activity of the dilator muscle (29). Moreover, pupil responses are sensitive to abnormal social reward and social threat processing in children with autism spectrum disorders (30). The reduced pupil response in CUs may thus reflect reduced sensitivity of CUs to the differential emotional value of JA and NJA and altered autonomous processing of social information. This reduced sensitivity might be a result of blunted social reward processing, as JA processing has been shown to involve reward-related brain areas (25).
It has been hypothesized that impairments in social cognition in drug users arise from an altered reward system by assigning more value to the drug but less value to natural reinforcers (9). The present results test this hypothesis in the social domain and extend previous reports of deficits in social cognition of CUs (13, 31–34). Indeed, basal social mechanisms such as JA abilities had not been investigated in drug users so far. Difficulties at this basic level of social interaction might represent an underlying mechanism that could help explain impairments in more sophisticated social skills (23), as the pupil response to JA was correlated with mentalizing ability (r = 0.29, P < 0.001, n = 132) as measured with the Movie for the Assessment of Social Cognition in the presently studied sample as previously described (13).
Furthermore, JA seems to have a stronger arousing effect than NJA trials in CUs. This might indicate that CUs—unlike controls—perceive gaze contact as socially threatening or as an intrusion of personal space inducing fear or aggression, which is often associated with increased arousal (35, 36). CUs probably display increased sensitivity to social dominance, as previous studies in monkeys showed that cocaine acted as a reinforcer mainly in subordinate but not in dominant monkeys (37). However, this speculation should be tested in future studies.
As the neural basis of altered social interaction in drug users has not been investigated yet, study II was conducted to investigate the involvement of the reward system during social gaze processing in CUs and HCs using fMRI. Corroborating previous findings of studies investigating JA and social cognition (25, 38), we initially demonstrated that self-initiated JA vs. NJA recruited several reward-related areas (mPFC, mOFC, and PCC) in our control participants. These areas have repeatedly been shown to be associated with social cognition (39). Importantly, CUs showed significantly less activation of the mOFC in response to self-initiated JA vs. NJA compared with control participants. This is in accordance with previous studies reporting functional and structural alterations in the OFC in CUs (40, 41). Numerous studies have provided evidence that the mOFC plays a critical role in reward-guided behavior and emotion processing. More specifically, the mOFC has been shown to be associated with encoding and maintenance of stimulus–reward value of primary and secondary reinforcers (42–44), and seems to be critically involved in emotional learning (45). As part of the reward system, the mOFC predominantly projects to the ventral striatum (43, 46), and both areas have been shown to be altered in function in CUs (47–49). Consequently, decreased mOFC activation in CUs in response to JA likely indicates altered social reward processing, and may underlie social deficits in CUs. Significant correlations between mOFC activation, pupil size, and valence ratings further corroborate the assumption of a common underlying process.
Besides reward processing, the mOFC is also involved in other cognitive functions such as impulse control and decision making, considered to be impaired in cocaine users (50, 51). However, behavioral impulsivity measured with the stop-signal task in the current sample (previously described in detail in ref. 52) was not correlated with mOFC activation in controls, CUs, or the complete sample (all parameters P > 0.11). Therefore, differences in impulse control are unlikely to account for the mOFC hypoactivation during JA processing in CUs. However, further studies are necessary to investigate the influence of other factors.
Correlation analyses may provide further evidence for a relationship between mOFC activation and real-life social functioning given that a stronger activation of this region during self-initiated JA vs. NJA was associated with a larger social network size in HCs. Even though correlation coefficients did not differ significantly between groups, the correlation between mOFC activation and social network size was not significant in CUs alone. Future studies are needed to further investigate this relationship. These findings support the assumption that JA could represent a fundamental mechanism of social cognition, which has implications for more complex social abilities (23). Consequently, altered social reward processing in CUs may impact real-life social behavior, such as social withdrawal or social rejection. This can be particularly important, as social environment and behavior are crucial factors in the onset of drug use as well as for the outcome of drug addiction treatment (6, 7). Moreover, enhanced OFC activity in CUs in response to drug-related words has been associated with increased reward valuation for drug cues (53). In conjunction with the current results, this supports the assumption that neuroadaptations in brain reward systems make drug users more sensitive to the abused drug and may reduce responsiveness to the value of nondrug reinforcers such as social interaction (9). Altered reward sensitivity might therefore reduce the motivation to engage in social interaction, decrease the possibilities to learn and apply social skills, and promote antisocial behavior. This might result in impairments in general social competence, and might explain why even substantial negative social consequences such as legal or family problems do not lead the addicted person to give up drug use.
The present study has to be interpreted with the following limitations in mind: Whereas the sample size in study I was large, only a relatively modest subsample could be included in study II. Therefore, due to limited power, we might not have been able to detect differential activations in other reward-related brain areas such as the ventral striatum. Furthermore, to obtain greater experimental control, the participants were interacting with an avatar instead of a real human being. Note, however, that participants who did not believe that the avatar was controlled by another human were excluded from study I and not invited to study II. In addition, previous investigations demonstrate that using avatars is an adequate tool to study the processing of face-based social stimuli (25, 54). Moreover, although JA processing represents an elegant tool to investigate basic social interaction behavior as represented by gaze contact, it cannot cover all facets of social interaction behavior. Even though the correlation of mOFC activation and social network size may indicate that altered processing of social gaze in cocaine users is related to real-life differences in interaction behavior, it is possible that other factors such as personality traits or economic status also contribute to differences in social network size. Finally, due to the cross-sectional design, we cannot exclude that social impairments have preceded cocaine use and possibly represent a vulnerability to start using drugs. Importantly, comprehensive psychiatric diagnostics and objective characterization of drug use by hair toxicology suggest that our CUs showed little psychiatric comorbidities and relatively sparse polytoxic drug use.
In sum, our study provides previously unidentified evidence of impairments of basal social interaction behavior as reflected by changes of social gaze behavior in CUs, and suggests a link between these impairments and altered social reward processing both on the behavioral and neural level. Social cognition and interaction deficits in cocaine users are a relevant problem for the social environment and treatment success of affected individuals and by extension for society at large (3, 6). Understanding the basis of social cognition deficits in stimulant users offers the possibility to develop new targets for prevention and treatment strategies. Training of social reward and social reward processing might be beneficial to abate harm caused by altered social processing in substance use disorders.
Materials and Methods
Participants.
The present data were collected in the context of the Zurich Cocaine Cognition Study (13, 15, 55, 56). For study I, 80 CUs and 63 stimulant drug-naïve control participants were included, whereas a subpopulation of 16 CUs and 16 HCs participated in study II (for recruitment details, see SI Materials and Methods). Inclusion criteria for the cocaine-using group was cocaine use of at least 1 g/mo, cocaine as the primary used illegal drug, and a current abstinence duration of no longer than 6 mo. Exclusion criteria for the CUs were use of opioids, a polytoxic drug use pattern, and axis I DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Ed.) adult psychiatric disorders other than cocaine, nicotine, and alcohol abuse/dependence, history of depression, and attention deficit hyperactivity disorder, due to their high prevalence in CUs. Exclusion criteria for control subjects were any axis I DSM-IV psychiatric disorder with the exception of nicotine dependence, and regular illegal drug use (lifetime use <15 occasions) with the exception of occasional cannabis use. For both groups, exclusion criteria were clinically significant somatic diseases, head injury or neurological disorders, family history of schizophrenia or bipolar disorder, and use of prescription drugs affecting the CNS. Participants were asked to abstain from illegal substances for a minimum of 3 d and from alcohol for at least 24 h. Self-reports were controlled by urine toxicology and 6-mo hair analysis (for details, see refs. 13 and 55). Additionally, for study II, participants had to be right-handed, as confirmed by the Edinburgh Handedness Questionnaire (57), and fulfill MRI safety criteria. All participants had normal or corrected-to-normal vision. The studies were approved by the Cantonal Ethics Committee of Zurich, Department of Health of the Canton of Zurich, Switzerland. All participants provided written informed-consent statements in accordance with the Declaration of Helsinki and were compensated for their participation.
Social Gaze Task.
The interactive social gaze task was based on the test described in previous publications (25, 58). In brief, the participant’s gaze was recorded by an eye-tracking device and used to control the gaze of an anthropomorphic virtual character (avatar). The participants completed three initiation conditions: (i) leading the gaze of the avatar (“self” condition), (ii) following the gaze of the avatar (“other” condition), and (iii) the direction of the gaze was determined by a third object (“object” condition). In every initiation condition, the participant either looked in the same direction as the avatar (JA) or in another direction (NJA). For details of the social gaze task and study procedures, see SI Materials and Methods.
Eye Tracking.
In study I, gaze directions were recorded using the EyeLink 1000 System (SR Research), whereas in study II, an MRI-compliant eye-tracking system including video goggles (Resonance Technology) was used. The systems produced real-time output of gaze positions, which was transferred via a fast network connection to another computer controlling the visual stimulation. For technical details and pupil size analysis, see SI Materials and Methods.
Image Acquisition and Preprocessing.
Magnetic resonance images were acquired on a Philips Achieva 3.0T whole-body scanner (Best) equipped with a 32-channel receive head coil and MultiTransmit parallel radio frequency transmission. Functional MRI data were acquired using a whole-brain gradient-echo planar imaging (EPI) sequence (repetition time, 2,500 ms; echo time, 35 ms; slice thickness, 3 mm; 40 axial slices; no slice gap; field of view, 240 × 240 mm2; in-plane resolution, 3 × 3 mm; sensitivity-encoding reduction factor, 2.0). Additionally, high-resolution anatomical images (voxel size, 1 × 1 × 1 mm) were acquired using a standard T1-weighted 3D magnetization-prepared rapid-acquisition with gradient echo sequence.
Images were analyzed using SPM8 (www.fil.ion.ucl.ac.uk). Preprocessing consisted of realignment, spatial normalization to the standard EPI template of the Montreal Neurological Institute (MNI), and spatial smoothing using a Gaussian kernel of 6-mm FWHM to meet the statistical requirements of the general linear model (GLM).
Statistical Analysis.
Study I.
Frequency data were analyzed by means of Pearson’s χ2 test and quantitative data by mixed-effects ANOVA using PASW 18.0 (IBM). In all ANOVAs, group (CUs vs. HCs) was introduced as a between-subject factor, whereas initiation (self, other, object) and joint (joint vs. nonjoint trials) were introduced as within-subject factors. The confirmatory statistical comparisons of all data were carried out at a significance level set at P < 0.05 (two-tailed).
Study II.
Functional MRI images were analyzed using a GLM as implemented in SPM8. The experimental conditions (self-initiated JA, self-initiated NJA, other-initiated JA, other-initiated NJA, baseline) were modeled as 5-s blocks convolved with a canonical hemodynamic response function in the first-level analysis for each subject. Trials without a successful object fixation were modeled using a separate regressor of no interest. Low-frequency signal drifts were filtered using a 128-s high-pass filter. The following contrasts were computed for each participant: (i) JA > NJA, (ii) self-initiated JA > self-initiated NJA, and (iii) other-initiated JA > other-initiated NJA. The individual contrasts were then entered into a second-level group analysis using a between-group two-sample t test for the comparison between CUs and HCs, and a one-sample t test for the analysis within the control group with a threshold of uncorrected P < 0.005. Group effects were then analyzed using small-volume correction (SVC). Because we were particularly interested in reward-related areas and due to the a priori hypothesis, five ROIs were defined based on previous identification of areas associated with immediate reward processing (59): mOFC, ventral striatum, left posterior hippocampus, mPFC, and PCC. Search volumes were defined as spheres with a 5-mm radius centered on previously reported MNI coordinates (58). These volumes were applied to the one-sample t test in the control group, the two-sample t test to compare CUs and HCs, and the correlation analysis investigating the relationship between brain activation for the self-initiated JA > self-initiated NJA contrast and social network size as an indicator of real-life social behavior. Familywise error corrections were used in all SVC ROI analyses at a threshold of P < 0.05.
Supplementary Material
Acknowledgments
We are grateful to Dr. Philipp Csomor, Dr. Matthias Kirschner, Dr. Rainer Krähenmann, Daniela Jenni, Joelle Barthassat, Kathrin Küpeli, and Franziska Minder for their excellent support. The study was supported by grants from the Swiss National Science Foundation (PP00P1-123516/1 and PP00P1-146326/1) and The Olga Mayenfisch Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 2406.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317090111/-/DCSupplemental.
References
- 1.Hser Y-I, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58(5):503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 3.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 4.European Monitoring Centre for Drugs and Drug Addiction . Annual Report 2012: The State of the Drugs Problem in Europe. Luxembourg: Publications Office of the European Union; 2012. [Google Scholar]
- 5. Substance Abuse and Mental Health Services Administration (2011) Results from the 2010 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Substance Abuse and Mental Health Services Administration, Rockville, MD)
- 6.Ramirez R, Hinman A, Sterling S, Weisner C, Campbell C. Peer influences on adolescent alcohol and other drug use outcomes. J Nurs Scholarsh. 2012;44(1):36–44. doi: 10.1111/j.1547-5069.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortt AL, Hutchinson DM, Chapman R, Toumbourou JW. Family, school, peer and individual influences on early adolescent alcohol use: First-year impact of the Resilient Families programme. Drug Alcohol Rev. 2007;26(6):625–634. doi: 10.1080/09595230701613817. [DOI] [PubMed] [Google Scholar]
- 8.Homer BD, et al. Methamphetamine abuse and impairment of social functioning: A review of the underlying neurophysiological causes and behavioral implications. Psychol Bull. 2008;134(2):301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Baler RD, Goldstein RZ. Addiction: Pulling at the neural threads of social behaviors. Neuron. 2011;69(4):599–602. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutschler J, et al. Functional social support within a medical supervised outpatient treatment program. Am J Drug Alcohol Abuse. 2013;39(1):44–49. doi: 10.3109/00952990.2012.677889. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: Epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30(5):1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 12.Moos RH. Theory-based active ingredients of effective treatments for substance use disorders. Drug Alcohol Depend. 2007;88(2-3):109–121. doi: 10.1016/j.drugalcdep.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preller KH, et al. Impaired emotional empathy and related social network deficits in cocaine users. Addict Biol. 2013 doi: 10.1111/adb.12070. [DOI] [PubMed] [Google Scholar]
- 14.Hulka LM, Preller KH, Vonmoos M, Broicher SD, Quednow BB. Cocaine users manifest impaired prosodic and cross-modal emotion processing. Front Psychiatry. 2013;4:98. doi: 10.3389/fpsyt.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulka LM, et al. Altered social and non-social decision-making in recreational and dependent cocaine users. Psychol Med. 2013 doi: 10.1017/S0033291713001839. [DOI] [PubMed] [Google Scholar]
- 16.Jia Z, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70(6):553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calder AJ, et al. Reading the mind from eye gaze. Neuropsychologia. 2002;40(8):1129–1138. doi: 10.1016/s0028-3932(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci. 2009;13(3):135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Materna S, Dicke PW, Thier P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: A functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20(1):108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd SV. Following gaze: Gaze-following behavior as a window into social cognition. Front Integr Neurosci. 2010;4:5. doi: 10.3389/fnint.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corkum V, Moore C. The origins of joint visual attention in infants. Dev Psychol. 1998;34(1):28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Dev Sci. 2005;8(6):535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charman T, et al. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cogn Dev. 2000;15(4):481–498. [Google Scholar]
- 24.Charman T. Why is joint attention a pivotal skill in autism? Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schilbach L, et al. Minds made for sharing: Initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci. 2010;22(12):2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- 26.Mauri M, Cipresso P, Balgera A, Villamira M, Riva G. Why is Facebook so successful? Psychophysiological measures describe a core flow state while using Facebook. Cyberpsychol Behav Soc Netw. 2011;14(12):723–731. doi: 10.1089/cyber.2010.0377. [DOI] [PubMed] [Google Scholar]
- 27.Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132(3423):349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- 28.Partala T, Surakka V. Pupil size variation as an indication of affective processing. Int J Hum Comput Stud. 2003;59(59):185–198. [Google Scholar]
- 29.Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepeta L, et al. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. J Neurodev Disord. 2012;4(1):17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemmis L, Hall JK, Kingston R, Morgan MJ. Impaired fear recognition in regular recreational cocaine users. Psychopharmacology (Berl) 2007;194(2):151–159. doi: 10.1007/s00213-007-0829-5. [DOI] [PubMed] [Google Scholar]
- 32.Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89(2-3):298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Fox HC, Bergquist KL, Casey J, Hong KA, Sinha R. Selective cocaine-related difficulties in emotional intelligence: Relationship to stress and impulse control. Am J Addict. 2011;20(2):151–160. doi: 10.1111/j.1521-0391.2010.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan MJ, Marshall JP. Deficient fear recognition in regular cocaine users is not attributable to elevated impulsivity or conduct disorder prior to cocaine use. J Psychopharmacol. 2013;27(6):526–532. doi: 10.1177/0269881113477708. [DOI] [PubMed] [Google Scholar]
- 35.Haller J. The neurobiology of abnormal manifestations of aggression—A review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res Bull. 2013;93:97–109. doi: 10.1016/j.brainresbull.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann SG, Ellard KK, Siegle GJ. Neurobiological correlates of cognitions in fear and anxiety: A cognitive-neurobiological information-processing model. Cogn Emotion. 2012;26(2):282–299. doi: 10.1080/02699931.2011.579414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan D, et al. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 38.Saito DN, et al. “Stay tuned”: Inter-individual neural synchronization during mutual gaze and joint attention. Front Integr Neurosci. 2010;4:127. doi: 10.3389/fnint.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16(2):235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15(3):358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein RZ, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30(39):13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Vostroknutov A, Tobler PN, Rustichini A. Causes of social reward differences encoded in human brain. J Neurophysiol. 2012;107(5):1403–1412. doi: 10.1152/jn.00298.2011. [DOI] [PubMed] [Google Scholar]
- 45.Nashiro K, Sakaki M, Nga L, Mather M. Differential brain activity during emotional versus nonemotional reversal learning. J Cogn Neurosci. 2012;24(8):1794–1805. doi: 10.1162/jocn_a_00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gourley SL, et al. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci. 2013;38(3):2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ersche KD, et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134(Pt 7):2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow ND, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 49.Bolla KI, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winstanley CA. The orbitofrontal cortex, impulsivity, and addiction: Probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- 51.Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12(3):405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- 52.Vonmoos M, et al. Differences in self-reported and behavioral measures of impulsivity in recreational and dependent cocaine users. Drug Alcohol Depend. 2013;133(1):61–70. doi: 10.1016/j.drugalcdep.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 53.Smith DG, Simon Jones P, Bullmore ET, Robbins TW, Ersche KD. Enhanced orbitofrontal cortex function and lack of attentional bias to cocaine cues in recreational stimulant users. Biol Psychiatry. 2014;75(2):124–131. doi: 10.1016/j.biopsych.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 54.Mojzisch A, et al. The effects of self-involvement on attention, arousal, and facial expression during social interaction with virtual others: A psychophysiological study. Soc Neurosci. 2006;1(3-4):184–195. doi: 10.1080/17470910600985621. [DOI] [PubMed] [Google Scholar]
- 55.Preller KH, et al. Increased sensorimotor gating in recreational and dependent cocaine users is modulated by craving and attention-deficit/hyperactivity disorder symptoms. Biol Psychiatry. 2013;73(3):225–234. doi: 10.1016/j.biopsych.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Vonmoos M, et al. Cognitive dysfunctions in recreational and dependent cocaine users: Role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203(1):35–43. doi: 10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 57.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 58.Wilms M, et al. It’s in your eyes—Using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Soc Cogn Affect Neurosci. 2010;5(1):98–107. doi: 10.1093/scan/nsq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.