Significance
Mosquitoes use neuronal-expressed odorant receptors in their antennae to locate blood meal sources via chemical cues emitted by hosts. Although their expression in nonsensory tissues is known, the potential for odorant receptors to also mediate endogenous signaling events in insects has remained unexplored. In this study, we have identified a subset of odorant receptors showing transcript expression in the testes of the malaria mosquito, Anopheles gambiae. In addition, we provide functional evidence that the broadly conserved insect coreceptor, Orco, mediates flagellar activation in mosquito spermatozoa. These results are reminiscent of odorant receptor function in human sperm and may represent an intriguing example of convergent evolution.
Keywords: transcriptomics, cell signaling, gamete, chemical ecology, Culicidae
Abstract
Insects, such as the malaria vector mosquito, Anopheles gambiae, depend upon chemoreceptors to respond to volatiles emitted from a range of environmental sources, most notably blood meal hosts and oviposition sites. A subset of peripheral signaling pathways involved in these insect chemosensory-dependent behaviors requires the activity of heteromeric odorant receptor (OR) ion channel complexes and ligands for numerous A. gambiae ORs (AgOrs) have been identified. Although AgOrs are expressed in nonhead appendages, studies characterizing potential AgOr function in nonolfactory tissues have not been conducted. In the present study, we explore the possibility that AgOrs mediate responses of spermatozoa to endogenous signaling molecules in A. gambiae. In addition to finding AgOr transcript expression in testes, we show that the OR coreceptor, AgOrco, is localized to the flagella of A. gambiae spermatozoa where Orco-specific agonists, antagonists, and other odorant ligands robustly activate flagella beating in an Orco-dependent process. We also demonstrate Orco expression and Orco-mediated activation of spermatozoa in the yellow fever mosquito, Aedes aegypti. Moreover, we find Orco localization in testes across distinct insect taxa and posit that OR-mediated responses in spermatozoa may represent a general characteristic of insect reproduction and an example of convergent evolution.
To date, studies of odorant receptor (OR) expression and function in mosquitoes and other insects have been limited to adult and larval appendages where the fundamental properties of insect chemosensation continue to be elucidated (1–6). Unlike their mammalian counterparts, which function strictly as G protein-coupled receptors (GPCRs), insect ORs generally act as heteromeric ion channels of at least two subunits: a highly conserved coreceptor (Orco) and a ligand-recognizing receptor (ORx) (7–11), although evidence for second messenger signaling has also been observed (12, 13), especially in sex pheromone signaling (14). Although their exact stoichiometry remains unresolved, OR channels serve as nonspecific channels of monovalent and divalent cations, including calcium, whose relative permeabilities depend upon ORx (9). Within this paradigm, ligands for numerous members of the Anopheles gambiae OR family (AgOrs) have been identified (4, 15–17). Although AgOrs are expressed in tissues beyond adult head appendages, studies regarding AgOr function in nonolfactory tissues have not, until now, been conducted. One intriguing possibility is that AgOrs act to mediate spermatozoa responses to endogenous signaling molecules. Indeed, several studies have suggested the existence of signaling pathways in insect sperm, including proteomics analyses in Aedes aegypti (18) and Drosophila melanogaster (19), although ORs were not identified in those studies. Importantly, OR expression in male germ cells has been reported for numerous mammalian species (20–22) and evidence for functional expression of ORs in human and mouse sperm have been described (23–27), although the requirement for human ORs in ligand recognition and fertilization has been seriously challenged (28). In a potentially striking example of convergent evolution, we describe the expression of a subset of ORs in male germ cells of A. gambiae where they act to modulate activation and perhaps orientation of spermatozoa, which are critical to male reproductive fitness.
Results and Discussion
Nonolfactory Expression of A. gambiae OR Transcripts.
A previous RNA sequencing (RNAseq) study in A. gambiae adults revealed that a subset of AgOrs is enhanced in whole male bodies (5). One interpretation of those data is that AgOrs are functional in nonhead tissues in males where they are used in noncanonical chemosensory roles. Given the previous characterizations of functional OR expression in mammalian sperm (23, 26, 27), we speculated that AgOrs may also contribute functionally to male reproductive tissues in A. gambiae. To address this hypothesis, RNAseq was used to examine relative transcript abundances in A. gambiae testes (Table 1) where more than 30 AgOrs were detected, nine of which had reads per kilobase per million (RPKM) values greater than 1 (Table1) and their percentile ranks ranged between 20 and 45. Interestingly, seven of the 10 most abundant transcripts, AgOrs 3, 4, 5, 6, 8, 34, and 37, are predominantly expressed in tissues other than antennae (Table 1) including the maxillary palps, proboscises, and larval antennae (3, 4, 29). Highly correlated results were obtained from age-matched, mated versus unmated testes samples (Fig. S1, Dataset S1), suggesting that mating itself does not alter Or abundance in male testes (Fig. S1B). In these studies, AgOrco was present at a very low level in one sample, but absent in the other (Dataset S1). The expression of the most abundant AgOrs in testes was confirmed by reverse-transcription PCR, whereas attempts to amplify AgOrco were marginally successful in two of five biological replicates (Fig. S2). Independently, expression of AgOrs in testes, including AgOrco and three other AgOrs identified in our RNAseq studies (Table 1), was found in a previous microarray study by Baker et al., where AgOr37 was specifically recognized as being enhanced in male testes (30). These multiple lines of evidence support the hypothesis that AgOr transcripts are expressed in testes, and in some cases, their expression is similar in amplitude to antennal-expressed AgOr transcripts where RPKMs were often observed to be less than 10 (5). Additionally, several testes/sperm-specific transcripts were found in high abundance in our RNAseq samples, indicating both tissue/RNA integrity and the comparatively low abundances of AgOrs (Dataset S1).
Table 1.
OR transcript expression in A. gambiae tissues
| Transcript | Testes | Mbod | Mant | Fbod | Fant |
| AgOr37** | 12.4 | 0.2 | 0.1 | 0.1 | 0.0 |
| AgOr6 | 8.1 | 0.3 | 0.7 | 0.4 | 3.1 |
| AgOr34 | 7.3 | 0.3 | 0.0 | 0.0 | 0.0 |
| AgOr8 | 4.8 | 0.2 | 0.1 | 0.1 | 0.5 |
| AgOr70nd | 3.6 | 0.6 | 2.7 | 0.1 | 10.1 |
| AgOr5* | 2.7 | 0.7 | 0.8 | 0.3 | 0.1 |
| AgOr22* | 2.6 | 0.3 | 1.4 | 0.0 | 11.1 |
| AgOr47 | 1.2 | 1.2 | 5.2 | 0.3 | 22.5 |
| AgOr3 | 1.0 | 0.3 | 0.1 | 0.0 | 0.0 |
| AgOrco* | 0.0 | 19.7 | 185.5 | 3.1 | 916.2 |
Abundances of selected OR transcripts expressed as RPKMs from various A. gambiae adult tissues: m, male; f, female; bod, bodies; ant, antennae. RPKM values for bodies and antennae are from ref. 5. Asterisks indicate transcripts that were present in at least one of four testes samples as determined by an independent microarray analysis (30); double asterisks indicate transcripts that displayed enhanced expression in testes relative to at least one other tissue; nd, not determined; probe for these transcripts not included on microarray (30). See Dataset S1 for a more detailed listing of RNAseq results.
Expression of AgOrco Protein in Male Reproductive Tissues.
Detection of AgOr transcripts in testes raised the possibility that some Ors are expressed as functional proteins in spermatozoa. However, the lack of apparent AgOrco transcript might also indicate that AgOrs in testes function in a unique manner, one that does not rely on AgOrco. Alternatively, AgOrco protein may be present, despite the near absence of detectable transcript, and is stable throughout spermatogenesis. To examine this possibility, we first used Multidimensional Protein Identification Technology (MudPIT) to investigate the A. gambiae testis proteome and were unable to identify any AgOr proteins. By comparison, only two AgOrs (AgOrco and AgOr39) were detected in a parallel MudPIT analysis of the proteome of A. gambiae antennae, where AgOrs were expected to be highly enriched. These results strongly suggest that more sensitive techniques are needed to identify AgOr protein expression in A. gambiae testes. Therefore, a previously characterized Orco antibody (11) that specifically labels the Orco protein in adult antennae of A. gambiae and D. melanogaster (Fig. S3) was used in immunohistochemical (IHC) examinations of AgOrco protein expression in testes. In these studies, AgOrco was detected in testes sections, including multiple developmental zones where the most robust fluorescence was detected in immature regions (Fig. 1). AgOrco expression appeared as puncta along the flagella of mature spermatozoa, coincident with α-tubulin that did not extend into the midpiece or head region (Fig. 1B, Inset and Fig. 2A). Antibody labeling was effectively blocked by an Orco antigen-specific peptide (Fig. 2B, Fig. S3), but not by a nonspecific peptide (Fig. 2C, Fig. S3). These results indicate that the AgOrco protein is expressed in male sperm where it may colocalize with testes-expressed tuning AgOrs that were identified in our RNAseq analyses to form functional ligand-gated ion channels. One potential explanation for the apparent absence of AgOrco transcript in testes is that its expression occurs at an earlier life stage, perhaps during larval gonad development, and that the translated protein is highly stable, such that it is active throughout the adult male life. The apparent persistence of AgOrco across multiple stages of sperm development in the adult testis is an indication of this stability (Fig. 1). Altogether, our data raise the possibility that AgOrs perform previously unknown functions in A. gambiae spermatozoa where they may mediate responses to chemical signals.
Fig. 1.
AgOrco protein expression in testes and spermatozoa. (A) Differential interference contrast (DIC) image of A. gambiae testis showing zones of sperm development. (B) Immunolabeling of AgOrco (green) in whole testis counterstained with the nucleic acid dye, propidium iodide (magenta). Germ cell/spermatogonia regions demarcated with dotted line. (Inset) Higher magnification of single spermatozoa; h, head; m, midpiece; f, flagellum. (C) AgOrco (green) in germ cell/spermatogonia region of A. gambiae testis; a, anterior; p, posterior.
Fig. 2.
AgOrco protein expression in spermatozoa. IHC labeling of spermatozoa with an Orco antibody. (A) Left, anti-Orco (green); Center, anti–α-tubulin (blue); Right, overlay of green and blue signals (cyan) plus propidium iodide (magenta). (B) Left, anti-Orco preincubated with AgOr18 peptide (green); Center, anti–α-tubulin (blue); Right, overlay of green and blue signals (cyan) plus propidium iodide (magenta). (C) Left, anti-Orco preincubated with Orco-specific peptide (green); Center, anti–α-tubulin (blue); Right, overlay of green and blue signals (cyan) plus propidium iodide (magenta). Scale bar in C applies to all images.
Activation of Spermatozoa.
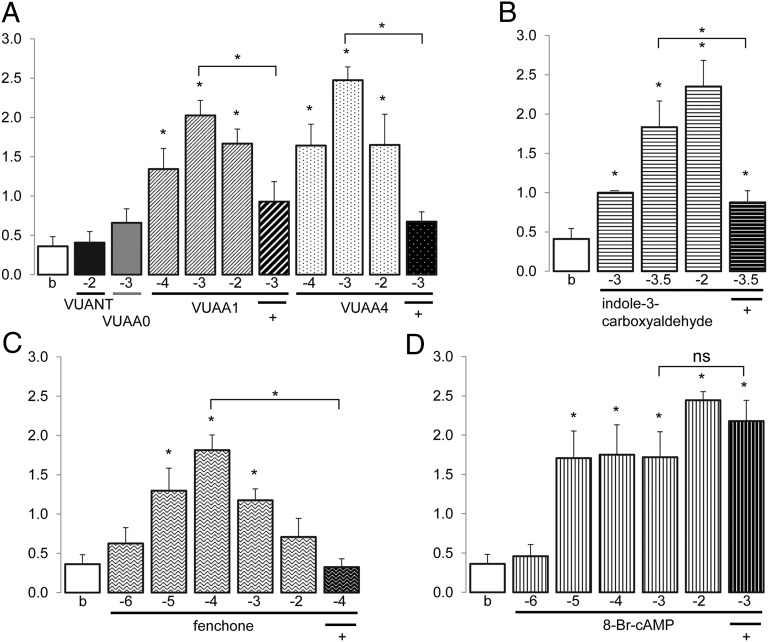
To explore the possible biological function of AgOrs in A. gambiae testes, and in light of the known responses of vertebrate spermatozoa to exogenous chemical stimuli (23, 26, 27), we have developed a simple video-based bioassay (Fig. S4) to examine the amplitudes of flagellar movements of bulk spermatozoa nascent to ruptured testes in response to a range of natural and synthetic compounds. Responses were scored by post hoc examination of video clips in a double-blinded fashion (Movie S1). The stimuli used in these assays were comprised of a range of unitary odorants as well as highly specific Orco modulators that have been recently characterized (8, 31, 32). Flagellar beating responses were significantly elevated in the presence of two Orco agonists, VUAA1 and VUAA4, but not in the presence of identical concentrations of a nonpotent structural analog, VUAA0 (32) (Fig. 3A). Moreover, the Orco antagonist, VU0183254 (hereafter VUANT) (31), did not activate spermatozoa flagella on its own, whereas VUAA1 and VUAA4 responses were significantly reduced when VUANT was coapplied (Fig. 3A).
Fig. 3.
Activation of A. gambiae sperm by applied compounds. Activation indices (y axes; + SEM) at given log10[M] (x axes) for (A) Orco modulators: VUANT (black bar, n = 8), VUAA0 (gray bar, n = 11), VUAA1 (pink bars, n = 8, 10, and 9, respectively), VUAA4 (aqua bars, n = 7, 10, and 5, respectively) or buffer (white bar, n = 27), VUAA1 plus VUANT [1 × 10−2 M] (pink/black striped bar, n = 7), and VUAA4 plus VUANT (aqua/black striped bar, n = 10) activation. (B) Indole-3-carboxyaldehyde (blue bars, n = 4, 3, and 5, respectively), plus VUANT (blue/black striped bar, n = 8) or buffer (white bar, n = 14). (C) Fenchone (green bars, n = 8, 11, 12, 10, and 6, respectively), plus VUANT (green/black striped bar, n = 10) or buffer (white bar, n = 27). (D) 8-Br-cAMP (orange bars, n = 6, 6, 6, 8, and 9, respectively), plus VUANT (orange/black striped bar, n = 7) or buffer (white bar, n = 27). Asterisks indicate significant differences between compound and control buffer samples or between compound with or without VUANT (Mann–Whitney U, P < 0.01).
The presence of other AgOr transcripts in testes suggests the presence of heteromeric Or complexes in spermatozoa. We therefore speculated that a subset of the known AgOr ligands (15, 16) would also activate flagella, mimicking the effect of Orco agonists. To examine this, we used a panel of odorant ligands in the spermatozoa flagella bioassay, revealing that fenchone, which can activate several AgOrs, including the testes-expressed AgOr11 (15, 16), induced significant spermatozoa movements in a concentration-dependent manner (Fig. 3C). The fenchone responses increased from 10−6 to a peak activity at 10−4 molar and then decreased at 10−3 and 10−2 molar, becoming insignificant compared with buffer alone (Fig. 3C). A newly identified AgOr6 ligand, indole-3-carboxyaldehyde, also activated A. gambiae spermatozoa in a concentration-dependent manner with highest activity at 10−2 molar (Fig. 3B). Importantly, both the fenchone and indole-3-carboxyaldehyde responses were inhibited by the coapplication of the Orco antagonist VUANT (Fig. 3 B and C), indicating that flagellar responses to both compounds require a functional Orco subunit. These results support the hypothesis that flagellar beating responses of sperm can be modulated by heteromeric AgOr complexes and constitutes evidence for their function outside of sensory neurons in A. gambiae.
Interestingly, a membrane-permeable form of cyclic adenosine monophosphate (8-Br-cAMP) also induced a significant increase in flagellar beating at several concentrations (Fig. 3D). Both cAMP and cyclic guanosine monophosphate (cGMP) are important second messengers that regulate flagellar beating in response to activators and chemoattractants of mammalian and marine invertebrate sperm (33, 34). The cAMP activation of A. gambiae sperm was unaffected by VUANT, suggesting the presence of a second messenger-mediated activation pathway that is either independent of Orco or performs downstream of Orco in A. gambiae (Fig. 3D). Furthermore, the lack of VUANT antagonism of the cAMP activation response also demonstrates that the VUANT reagent is not inherently toxic to A. gambiae spermatozoa and that the reductions in VUAA1-, VUAA4-, fenchone-, and indole-3-carboxyaldehyde–evoked flagellar beating responses in the presence of VUANT are specific to their Orco and tuning Or targets, respectively. Numerous other AgOr-activating compounds were tested in our bioassay but failed to elicit flagellar responses. These compounds included geranyl acetone, 1-octen-3-ol, 2-acetophenone, butylamine, and 4-methyl cyclohexanol. The lack of responses to these compounds could indicate technical impediments to their delivery to receptors in our assay or real differences in their contextual recognition in this tissue. Further studies will be needed to clarify these issues.
Although the concentrations of compounds that elicited spermatozoa activation are arguably high and likely to be outside the range of physiological relevance, we propose that several factors may be responsible for these high response thresholds. First, the effective dose that is experienced by receptors on individual spermatozoa may not correlate exactly with the compound dilutions because of the potential effects of the numerous other testes-derived cells and the compounds that they may release into the preparation. Second, other factors may be impacting the threshold concentrations required to elicit flagellum activation in these assays. For example, unknown factors released by male accessory glands during mating might prime the sperm for subsequent activation in the female reproductive tract. Such factors could conceivably lower the threshold responses of AgOr complexes in vivo by impacting their localization or activity, but were not explored in our bioassay. Third, the concentrations of VUAA1 and VUAA4 that activate spermatozoa in our bioassays are actually comparable to concentrations that have been shown to elicit activity in AgOrco channels expressed in heterologous systems and in endogenous olfactory neurons (8). The bioassay data suggest that heteromeric complexes of AgOrs represent one of potentially several signaling pathways that participate in the activation of spermatozoa in A. gambiae.
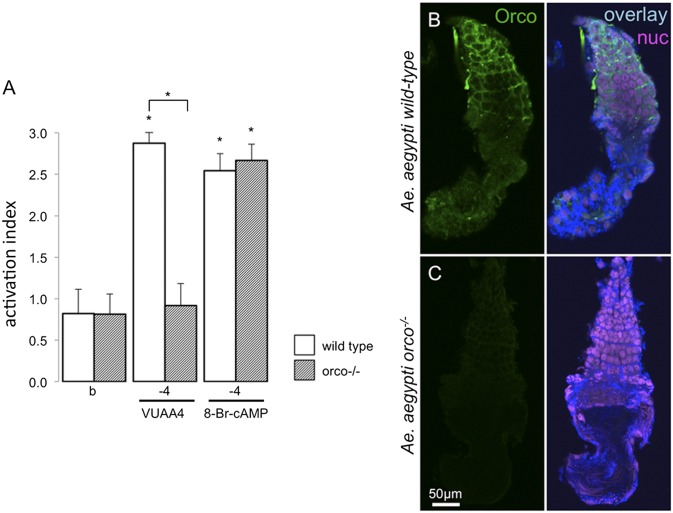
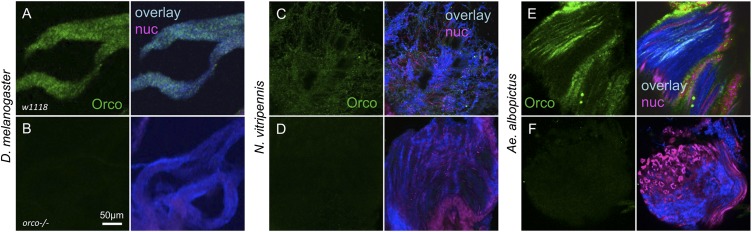
In light of the A. gambiae bioassay results, we used a recently described orco−/− mutant strain of A. aegypti (35) to examine the specificity of spermatozoa flagellar responses to the Orco agonist, VUAA4 (Fig. 4A). Strikingly, spermatozoa from a wild-type strain of A. aegypti responded robustly to the application of VUAA4, whereas spermatozoa from the orco−/− strain were unresponsive (Fig. 4A). Similar to A. gambiae, A. aegypti sperm flagellar beating was also stimulated by the application of 8-Br-cAMP in both the wild-type and orco−/− mutant strains (Fig. 4A). These results further implicate Orco in the VUAA spermatozoa response and strongly support the hypothesis that the cAMP response is independent of Orco. Importantly, IHC labeling also confirmed the presence of Orco protein in wild-type A. aegypti testes as well as the absence of Orco in orco−/− mutant testes (Fig. 4 B and C). IHC labeling was evident throughout A. aegypti testes developmental zones, but strongest in the immature zones (Fig. 4B), mirroring the observations of Orco expression in A. gambiae testes (Fig. 1). We also attempted to perform bioassays on spermatozoa in wild-type and orco−/− mutant D. melanogaster (36). However, D. melanogaster sperm exhibited a very high background flagellar beating in our experimental conditions, thus precluding discrimination of activating responses to compounds. Nonetheless, IHCs in D. melanogaster demonstrated Orco antibody labeling in wild-type but not in orco−/− mutant testes (Fig. 5 A and B).
Fig. 4.
Orco function and expression in A. aegypti sperm/testes. (A) Activation indices (y axis; + SEM) at given log10[M] (x axis) for wild-type (white bars) or orco−/− mutant spermatozoa (striped bars) in response to buffer (Left), VUAA4 (Center), or cAMP (Right). n = 6 for all compound treatments and n = 7 for buffer control. (B and C) IHC labeling of A. aegypti testes. (B) Wild-type and (C) orco−/−. Left, anti-Orco (green); Right, overlay of anti-Orco (green), anti–α-tubulin (blue), and propidium iodide (magenta) signals.
Fig. 5.
Orco protein expression in spermatozoa. IHC labeling of spermatozoa with an Orco antibody. Left, anti-Orco (green); Right, overlay of anti-Orco (green), anti–α-tubulin (blue), and propidium iodide (magenta) signals. (A) Anti-Orco labeling in D. melanogaster w1118 testes. (B) Anti-Orco labeling in D. melanogaster orco−/− testes. (C and D) Anti-Orco labeling in N. vitripennis testes without (C) or with (D) preincubated Orco-specific peptide. (E and F) Anti-Orco labeling in A. albopictus testes without (E) or with (F) preincubated Orco-specific peptide. Scale bar in B applies to all images.
Additional IHC studies revealed the potential presence of highly conserved Orco protein orthologs within spermatozoa of other holometabolaous insects, including the parasitic wasp, Nasonia vitripennis (Fig. 5 C and D) and the mosquito Aedes albopictus (Fig. 5 E and F). These results raise the possibility that Orco expression in testes/spermatozoa is broadly conserved across insect lineages. If so, the functioning of OR complexes in sperm activation that are suggested by our bioassay data may be a general feature of insect reproduction.
Although we recognize that overt viability and fecundity defects have not been reported for laboratory-reared orco mutants in D. melanogaster (35) and A. aegypti (36), such conditions do not preclude the presence and biological importance of a subtle yet significant OR-based reproductive fitness advantage being active in natural insect populations. Furthermore, it is also possible and indeed likely that other ion channel and chemosensory receptor gene families may also facilitate parallel signaling functions in spermatozoa. Our RNAseq transcriptome profiling studies revealed that transcripts for multiple members of A. gambiae variant ionotropic receptor (AgIr), gustatory receptor (AgGr), and odorant-binding protein (AgObp) gene families are present in the testes of A. gambiae males (Dataset S1). In total, we found 14 AgGrs, 17 AgIrs, and six AgObps with RPKMs greater than 1, among which two AgGrs, four AgIrs, and five AgObps had transcript abundances above the median of the entire testis transcriptome (Dataset S1). These highly expressed chemosensory genes include AgGr22, which encodes a carbon dioxide receptor, and several conserved AgIrs with significant antennal expression (5).
A. gambiae females are generally monandrous, and remating is rare in wild populations (37, 38). This necessitates the long-term storage of sperm in the spermatheca as well as mechanisms for their efficient use over the reproductive life of each female. Few studies have explored the pathways used to identify bioactive substances that elicit responses from conspecific insect sperm, and it is unlikely that the volatile AgOr ligands used here comprise the endogenous signals involved in A. gambiae spermatozoa activation. Indeed, endogenous ligands for human sperm ORs have recently been characterized, which are distinct from their previously identified volatile ligands (39). Examples of directed movement of sperm have been extensively characterized in marine invertebrates and mammals (reviewed in ref. 40) as well as several insect species. For example, in the beetle, Drusilla canaliculata, sperm migrate into the spermathecae (41), whereas the spermathecal gland in the boll weevil, Anthonomus grandis, is required for sperm activation, storage, clearance, and fertility (42, 43) In D. melanogaster, sperm swim backward upon entering the female reproductive tract, and genetic ablation of the spermatheca secretory cells (SSCs) before mating leads to sperm storage defects: sperm fail to migrate into spermathcae and become inactive within the seminal receptacle (44–46). Moreover, SSC-ablated females display reduced fertility over time and ovovivipary (47). These experiments suggest that substances in spermathecae, SSCs, or perhaps other tissues are involved in the activation and chemoattraction of insect sperm (48).
Reproductive fitness is an important component in establishing and maintaining insect populations, and accordingly, the vectorial capacity of malaria vectors. Despite ongoing efforts to characterize the functions of accessory gland proteins and sperm in the formation of the A. gambiae mating plug and fertilization (49–55), the potential signals that induce sperm activation, spermatozoa localization, retention, or fertilization, within the female reproductive tract remain unknown. An intriguing possibility is that females produce and release chemicals that activate male sperm before fertilization that also act as chemotactic cues to orient or otherwise direct sperm motility. Importantly, the overall reproductive success of A. gambiae males correlates positively with the presence of motile spermatozoa in mated female spermathecae and negatively with sperm length (56, 57). In this context, an enhanced understanding of A. gambiae sperm activation/motility and the molecular processes that impinge upon them will be significant in terms of both basic biology and as a potential means to develop new vector and, more broadly, insect control methods.
The activation of insect sperm via ionotropic Ors is reminiscent of capacitation of mammalian sperm, which has been linked to signaling pathways mediated by metabotropic ORs (23, 26, 27) as well as the activity of several ionotropic channels (58, 59). These include calcium channels, most notably the sperm cation channels, CatSpers (60–63), potassium channels such as Kcnu1 (64, 65) as well as sodium, proton, bicarbonate, and chloride channels that are localized along the sperm flagella and speculated to act downstream of receptors for diverse extracellular ligands (33, 59). The modulation of insect and mammalian sperm via proteins of distinct evolutionary origins yet that encompass conserved modes of signal transduction represents a potent example of convergent evolution impacting upon a singularly essential biological process.
Materials and Methods
RNAseq.
Testes were dissected from sexually mature, unmated or mated males at 4–6 days posteclosion into TRIzol reagent for subsequent total RNA isolation. Messenger RNA was isolated, and sample libraries were prepared for RNAseq on the Illumina HiSeq platform by the Hudson Alpha Institute for Biotechnology. Approximately 20 million, 50 bp paired-end reads were generated for each sample. Quality filtered reads were mapped to the A. gambiae genome using the TopHat2 short read mapper (66) and quantified using generalized fold change (GFOLD) differential expression analysis program (67). Transcript abundance values were calculated for unmated and mated samples separately.
Immunolocalization of AgOrco.
Cryosections of paraformaldehyde-fixed A. gambiae testes were collected on gelatin-coated glass slides and dried. Slides were processed according to a previously published protocol (2) and used as substrates for immunohistochemistry with an Orco-specific antibody (11).
Spermatozoa Bioassay.
We developed a bioassay to examine sperm flagellum activation in response to a range of chemical cues. We took advantage of previous AgOr deorphanization studies that uncovered ligands and modulators for both AgOrco and tuning AgOrs. Briefly, a single testis was isolated from a sexually mature, 4–6-day-old A. gambiae male and placed in 2 µL assay buffer [145 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, 5 mM d-glucose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes), pH 7.4] containing 10% (vol/vol) DMSO and test chemicals on a clean glass microscope slide (24 × 50 mm GOLD SEAL, LOT# 121311–9) using a pair of blunt-end forceps to prevent tissue damage. A coverslip (22 × 22 mm VWR, 040912–9) was placed on top of the preparation and gently pressed four times to squeeze open the testis wall and release spermatozoa into the assay buffer (Fig. S4). The slide was placed under an inverted microscope equipped with a digital video camera (Ikegami Digital/Zeiss Axiovert 35 at 200× magnification). Videos were recorded for ∼2 min using Ethovision software (Noldus), while the microscope slide was slowly manipulated in the X/Y and focal planes every 10 s to scan around the entire testis area (Fig. S4). Each compound and vehicle treatment was repeated 5–21 times with spermatozoa isolated from different individuals. 8-Bromo-cAMP was obtained from Sigma-Aldrich, Inc. (Cat# B5386). VUAA-class compounds were prepared as previously described (8, 31, 32). All other compounds were obtained from Sigma-Aldrich at the highest purity available. Video-recorded bioassays were arranged in randomized orders and processed using premier pro software (Adobe Inc.) to remove unnecessary focal adjustment as well as stage moving so that a minimum of four fields of view were obtained for subsequent scoring. Each video clip was viewed by four independent observers who were blinded to the treatment conditions and trained to provide a general assessment on the activation level of the spermatozoa by assigning an “activation index” (AI) (Fig. S4). The qualitative AI scale ranges from 0, no flagella moving, to 3, nearly all flagella moving. All spermatozoa within the field of view were considered. This assay has proven to be very robust and allowed us to rapidly assess sperm responses to chemical treatments. The JMP10 statistical software package (SAS Institute, Inc.) was used to identify statistically significant differences between mean AIs of test compounds and vehicle, via the nonparametric Mann–Whitney U test (P < 0.01).
Supplementary Material
Acknowledgments
We thank Drs. Rob Brucker and Seth Bordenstein (Vanderbilt University) for providing N. vitripennis, Ms. Zhen Li for mosquito rearing, and the Hudson Alpha Institute for Biotechnology for RNA sample preparation and Illumina sequencing. We also thank members of the L.J.Z. laboratory for scoring sperm bioassays and critical reading of the manuscript. The following reagents were obtained through the Malaria Research and Reference Reagent Resource Center (MR4) as part of the Biodefense and Emerging Infections Research (BEI) Resources Repository, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health: A. aegypti LVP-IB12, MRA-735, deposited by Dr. Mark Q. Benedict (Università degli Studi di Perugia), and A. aegypti orco−/− (Orlando), deposited by Dr. Leslie B. Vosshall (Rockefeller University), who also generated the D. melanogaster orco−/− line (Bloomington Stock Center #23129) and generously provided the anti-Orco IC3 antisera (11) used in this study. This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University and was supported by grants from the Innovation and Discovery in Engineering and Science program of Vanderbilt University, the National Institutes of Health (NIAID, AI056402), and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (VCTR121) (to L.J.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322923111/-/DCSupplemental.
References
- 1.Hill CA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298(5591):176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 2.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101(14):5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17(18):1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia Y, et al. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci USA. 2008;105(17):6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 2011;12:271. doi: 10.1186/1471-2164-12-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinker DC, et al. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proc Natl Acad Sci USA. 2013;110(20):8260–8265. doi: 10.1073/pnas.1302562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452(7190):1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 8.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011;108(21):8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pask GM, Jones PL, Rützler M, Rinker DC, Zwiebel LJ. Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS ONE. 2011;6(12):e28774. doi: 10.1371/journal.pone.0028774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pask GM, Bobkov YV, Corey EA, Ache BW, Zwiebel LJ. Blockade of insect odorant receptor currents by amiloride derivatives. Chem Senses. 2013;38(3):221–229. doi: 10.1093/chemse/bjs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4(2):e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452(7190):1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 13.Wicher D, et al. dOr83b—Receptor or ion channel? Ann N Y Acad Sci. 2009;1170:164–167. doi: 10.1111/j.1749-6632.2009.04101.x. [DOI] [PubMed] [Google Scholar]
- 14.Nolte A, et al. In situ tip-recordings found no evidence for an Orco-based ionotropic mechanism of pheromone-transduction in Manduca sexta. PLoS ONE. 2013;8(5):e62648. doi: 10.1371/journal.pone.0062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107(9):4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464(7285):66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallem EA, Nicole Fox A, Zwiebel LJ, Carlson JR. Olfaction: Mosquito receptor for human-sweat odorant. Nature. 2004;427(6971):212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- 18.Sirot LK, et al. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. PLoS Negl Trop Dis. 2011;5(3):e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorus S, et al. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat Genet. 2006;38(12):1440–1445. doi: 10.1038/ng1915. [DOI] [PubMed] [Google Scholar]
- 20.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J Cell Biol. 1993;123(6 Pt 1):1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang N, Koo J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012;45(11):612–622. doi: 10.5483/BMBRep.2012.45.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderhaeghen P, Schurmans S, Vassart G, Parmentier M. Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species. Genomics. 1997;39(3):239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 23.Spehr M, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299(5615):2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 24.Spehr M, et al. Dual capacity of a human olfactory receptor. Curr Biol. 2004;14(19):R832–R833. doi: 10.1016/j.cub.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Spehr M, Schwane K, Riffell JA, Zimmer RK, Hatt H. Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm. Mol Cell Endocrinol. 2006;250(1-2):128–136. doi: 10.1016/j.mce.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda N, Yomogida K, Okabe M, Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci. 2004;117(Pt 24):5835–5845. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- 27.Veitinger T, et al. Chemosensory Ca2+ dynamics correlate with diverse behavioral phenotypes in human sperm. J Biol Chem. 2011;286(19):17311–17325. doi: 10.1074/jbc.M110.211524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenker C, et al. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012;31(7):1654–1665. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon HW, Lu T, Rützler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2006;103(36):13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker DA, et al. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics. 2011;12:296. doi: 10.1186/1471-2164-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PL, et al. Allosteric antagonism of insect odorant receptor ion channels. PLoS ONE. 2012;7(1):e30304. doi: 10.1371/journal.pone.0030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RW, et al. Structure-activity relationship of a broad-spectrum insect odorant receptor agonist. ACS Chem Biol. 2012;7(10):1647–1652. doi: 10.1021/cb300331z. [DOI] [PubMed] [Google Scholar]
- 33.Buffone MG, et al. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev. 2012;79(1):4–18. doi: 10.1002/mrd.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaupp UB, Hildebrand E, Weyand I. Sperm chemotaxis in marine invertebrates—Molecules and mechanisms. J Cell Physiol. 2006;208(3):487–494. doi: 10.1002/jcp.20669. [DOI] [PubMed] [Google Scholar]
- 35.DeGennaro M, et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498(7455):487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Baimai V, Green CA. Monandry (monogamy) in natural populations of anopheline mosquitoes. J Am Mosq Control Assoc. 1987;3(3):481–484. [PubMed] [Google Scholar]
- 38.Tripet F, Touré YT, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68(1):1–5. [PubMed] [Google Scholar]
- 39.Hartmann C, et al. Sperm-activating odorous substances in human follicular fluid and vaginal secretion: Identification by gas chromatography-olfactometry and Ca2+ imaging. Chempluschem. 2013;78(7):695–702. doi: 10.1002/cplu.201300008. [DOI] [PubMed] [Google Scholar]
- 40.Kaupp UB. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat Rev Neurosci. 2010;11(3):188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- 41.Werner M, Gack C, Speck T, Peschke K. Queue up, please! Spermathecal filling in the rove beetle Drusilla canaliculata (Coleoptera, Staphylinidae) Naturwissenschaften. 2007;94(10):837–841. doi: 10.1007/s00114-007-0257-8. [DOI] [PubMed] [Google Scholar]
- 42.Villavaso EJ. Role of spermathecal gland of Boll-Weevil, Anthonomus-grandis. J Insect Physiol. 1975;21(8):1457–1462. [Google Scholar]
- 43.Grodner ML, Steffens WL. Evidence of a chemotactic substance in the spermathecal gland of the female boll weevil (Coleoptera: curculionidae) Trans Am Microsc Soc. 1978;97(1):116–120. [PubMed] [Google Scholar]
- 44.Yang Y, Lu X. Drosophila sperm motility in the reproductive tract. Biol Reprod. 2011;84(5):1005–1015. doi: 10.1095/biolreprod.110.088773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner M, Simmons LW. Insect sperm motility. Biol Rev Camb Philos Soc. 2008;83(2):191–208. doi: 10.1111/j.1469-185X.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- 46.Köttgen M, et al. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS ONE. 2011;6(5):e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9(11):e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfner MF. Precious essences: Female secretions promote sperm storage in Drosophila. PLoS Biol. 2011;9(11):e1001191. doi: 10.1371/journal.pbio.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhoek BA, Takken W. Age effects on the insemination rate of Anopheles gambiae sl in the laboratory. Entomol Exp Appl. 1994;72(2):167–172. [Google Scholar]
- 50.Tripet F, Thiemann T, Lanzaro GC. Effect of seminal fluids in mating between M and S forms of Anopheles gambiae. J Med Entomol. 2005;42(4):596–603. doi: 10.1093/jmedent/42.4.596. [DOI] [PubMed] [Google Scholar]
- 51.Dottorini T, et al. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104(41):16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers DW, et al. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA. 2008;105(49):19390–19395. doi: 10.1073/pnas.0809723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers DW, et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7(12):e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Med Vet Entomol. 2010;24(1):91–94. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 55.Thailayil J, Magnusson K, Godfray HC, Crisanti A, Catteruccia F. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108(33):13677–13681. doi: 10.1073/pnas.1104738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voordouw MJ, Koella JC, Hurd H. Comparison of male reproductive success in malaria-refractory and susceptible strains of Anopheles gambiae. Malar J. 2008;7:103. doi: 10.1186/1475-2875-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voordouw MJ, Koella JC, Hurd H. Intra-specific variation of sperm length in the malaria vector Anopheles gambiae: Males with shorter sperm have higher reproductive success. Malar J. 2008;7:214. doi: 10.1186/1475-2875-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13(3):395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaupp UB. 100 years of sperm chemotaxis. J Gen Physiol. 2012;140(6):583–586. doi: 10.1085/jgp.201210902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren D, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi H, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104(4):1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirichok Y, Lishko PV. Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod. 2011;17(8):478–499. doi: 10.1093/molehr/gar044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 64.Martínez-López P, et al. Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun. 2009;381(2):204–209. doi: 10.1016/j.bbrc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santi CM, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584(5):1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng J, et al. GFOLD: A generalized fold change for ranking differentially expressed genes from RNA-seq data. Bioinformatics. 2012;28(21):2782–2788. doi: 10.1093/bioinformatics/bts515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.