Significance
We aimed to solve a longstanding conundrum about the light response of primate cones. Unlike those of lower vertebrates, the primate cones’ response to light has long been reported as being biphasic. This surprise has also raised a yet-unanswered question about the requisite signal processing in the retina. More recently, human paired-flash electroretinographic data have challenged the biphasic waveform of the primate cone response. Our suction-pipette recordings from single primate cones now show directly that the light responses of primate and other mammalian cones are in fact very predominantly monophasic, much like those in invertebrates.
Keywords: retina, mammal, monkey, phototransduction
Abstract
Retinal cones are photoreceptors for daylight vision. For lower vertebrates, cones are known to give monophasic, hyperpolarizing responses to light flashes. For primate cones, however, they have been reported to give strongly biphasic flash responses, with an initial hyperpolarization followed by a depolarization beyond the dark level, now a textbook dogma. We have reexamined this primate-cone observation and, surprisingly, found predominantly monophasic cone responses. Correspondingly, we found that primate cones began to adapt to steady light at much lower intensities than previously reported, explainable by a larger steady response to background light for a monophasic than for a biphasic response. Similarly, we have found a monophasic cone response for several other mammalian species. Thus, a monophasic flash response may in fact be the norm for primate and other mammalian cones as for lower-vertebrate cones. This revised information is important for ultimately understanding human retinal signal processing and correlating with psychophysical data.
Previous suction-pipette recordings have demonstrated that, unlike the typically monophasic flash responses of lower-vertebrate cones (1–8), those of monkey and human cones are distinctly biphasic (9–13). This surprising finding has raised an unanswered question of how retinal circuitry would process such biphasic responses (14). In contrast, rods of mammals and nonmammals alike show monophasic flash responses. More recently, human data extracted from paired-flash electroretinographic (ERG) recordings in conjunction with modeling have suggested, albeit indirectly, that in situ primate-cone responses may actually be monophasic (15, 16). Accordingly, we have reexamined this important question directly with single-cell, suction-pipette recordings, which is the same experimental method as used in previous work (9–13).
Results
Flash-Response Sensitivity and Kinetics.
We recorded from a total of 112 cones from 12 macaque monkeys (Materials and Methods), and found 97 of them (∼90%) to give monophasic flash responses regardless of spectral type (Fig. 1 A–C, Left). Fig. 1D (Left) shows biphasic responses from a minority of the cones, consisting of an initial light-induced reduction in the inward dark current with respect to the outer-segment membrane that, upon recovery from light, is followed by an undershoot, i.e., an enhanced inward dark current. The amplitude of the response undershoot first increased with flash intensity, then decreased with further flash-intensity increase beyond saturation of the inward-current reduction, as found previously (12). Separately, we performed whole-cell voltage-clamp recordings from the inner segment of five macaque cones (Materials and Methods), and likewise found only one cell to show a (rather mild) response undershoot (Fig. S1) (refs. 17–19, but cf. ref. 20).
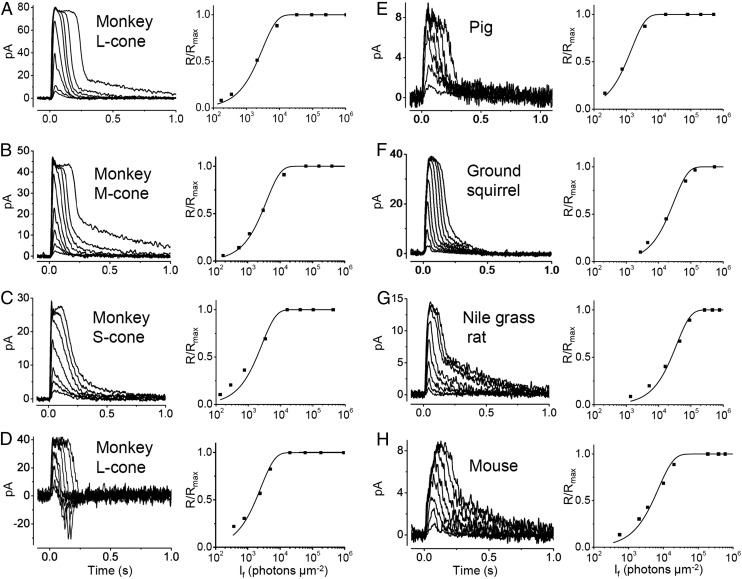
Fig. 1.
Flash-response families of monkey and other mammalian cones. (A–C) Flash-response families of a monkey L-, M-, and S-cone (Left), with corresponding intensity–response relations at transient peak of response (normalized by the saturated response, Rmax; Right). Curve fits are with a saturating-exponential function (Materials and Methods). The half-saturating flash intensity, σ, is 2,114, 2,634, and 1,831 photons⋅μm−2, respectively, at near corresponding λmax values (Materials and Methods). (D) An L-cone showing biphasic responses, with σ being 1,765 photons⋅μm−2 at near λmax. (A) Outer-segment recording; (B–D) inner-segment recordings. (E–H) Flash responses of pig, ground squirrel, Nile grass rat, and mouse cones (Left), with corresponding normalized intensity–response relations (Right) having σ values of 1,006, 20,700, 24,900, and 5,020 photons⋅μm−2, respectively, at near corresponding λmax values (Materials and Methods). In all cases, flash is at time 0, and traces are averages of 2 to 15 responses.
We have also examined, with suction-pipette recording, cones from pig, ground squirrel, Nile grass rat, and mouse, and found the norm to be an absence of the flash-response undershoot (5 of 5, 27 of 27, 9 of 9, and 30 of 34 cells, respectively; Fig. 1 E–H). Previously, others have found ground-squirrel cones to show monophasic flash responses, but approximately one third of them develop over time a small response undershoot during recordings (21); a substantial fraction of chipmunk cones also gave biphasic responses (22) (Discussion). For mouse cones, no response undershoot has been reported (23).
In lower vertebrates, different spectral cone types of a given animal species show quite dissimilar flash sensitivities, with blue cones being the most sensitive (4–6, 24). In contrast, monkey L-cones (red), M-cones (green), and S-cones (blue) were found to have similar sensitivities (11, 12). We confirmed the latter observation, obtaining half-saturating flash intensities (σ) of 1,845 ± 740, 1,665 ± 920 and 1,640 ± 800 photons⋅μm−2 (mean ± SD; n = 10, n = 8, and n = 5), respectively, for macaque L-, M-, and S-cones at near their respective wavelengths of maximal sensitivity (λmax) (Fig. 1 A–C, Right, Table 1, and Materials and Methods), matching previous measurements (12). Thus, the monophasic or biphasic nature of the response does not affect flash sensitivity, which is inversely proportional to σ. Pig was similar to monkey in cone sensitivity (Table 1). The M- and S-cones of ground squirrel likewise were similar to each other in sensitivity (see also ref. 21), but both were ∼10-fold less sensitive than monkey cones (Table 1). Nile grass rat was broadly similar to ground squirrel, and mouse was in between monkey and ground squirrel (see also ref. 23) (Table 1). Overall, rodents showed substantially lower cone sensitivity than primate and pig, although the associated functional significance and underlying mechanism remain unclear. This difference does not appear to be related to nocturnal vs. diurnal habitat because macaque monkey (diurnal) and pig (arguably diurnal) cones are much more photosensitive than ground squirrel (diurnal) and Nile grass rat (arguably diurnal; ref. 25) cones, whereas mouse (nocturnal) cones are in between.
Table 1.
Cone flash response parameters for several mammals
| Type | Rmax, pA | σ, photons⋅μm−2 | tpeak, ms | tint, ms | SF, pA photon−1⋅μm2 | a, pA⋅photon−1 | AL-P, s−2 | n |
| Monkey | ||||||||
| L | 34 ± 17 | 1,845 ± 740 | 43 ± 2 | 72 ± 3 | 0.015 ± 0.006 | 0.04 ± 0.01 | 4.2 ± 1.0 | 10 |
| M | 26 ± 7 | 1,665 ± 920 | 44 ± 2 | 80 ± 12 | 0.020 ± 0.005 | 0.04 ± 0.01 | 3.4 ± 0.5 | 8 |
| S | 24 ± 10 | 1,640 ± 800 | 40 ± 4 | 73 ± 20 | 0.013 ± 0.007 | 0.03 ± 0.02 | 3.4 ± 1.3 | 5 |
| US* | 28 ± 14* | 1,630 ± 970* | 39 ± 1* | 27 ± 16* | 0.010 ± 0.009* | 0.02 ± 0.02* | 4.4 ± 1.9* | 2* |
| Pig | ||||||||
| L | 10 ± 1 | 1,400 ± 280 | 62 ± 3 | 86 ± 10 | 0.009 ± 0.002 | 0.03 ± 0.01 | 3.2 ± 0.6 | 5 |
| Ground squirrel | ||||||||
| M | 29 ± 9 | 16,300 ± 6,800 | 28 ± 2 | 38 ± 12 | 0.0033 ± 0.002 | 0.005 ± 0.003 | 1.7 ± 0.1 | 22 |
| S | 25 ± 7 | 15,300 ± 8,300 | 31 ± 2 | 31 ± 7 | 0.0023 ± 0.0005 | 0.004 ± 0.001 | 1.1 ± 0.3 | 5 |
| Nile grass rat | ||||||||
| M | 25 ± 3 | 20,800 ± 4,200 | 43 ± 1 | 70 ± 8 | 0.002 ± 0.0005 | 0.01 ± 0.003 | 2.1 ± 0.4 | 9 |
| Mouse | ||||||||
| M | 8 ± 2 | 4,250 ± 1,700 | 75 ± 8 | 81 ± 16 | 0.003 ± 0.0014 | 0.016 ± 0.007 | 1.9 ± 1.2 | 6 |
| S | 9 ± 3 | 5,600 ± 2,100 | 84 ± 12 | 89 ± 17 | 0.003 ± 0.001 | 0.015 ± 0.005 | 1.5 ± 1.3 | 24 |
tint, integration time of dim-flash response, given by ∫ f(t)dt/fp, where f(t) is the response waveform and fp is the waveform’s transient-peak amplitude. All data, given as mean ± SD, are derived from responses low-passed-filtered at DC-200 Hz (eight-pole Bessel). Monkey cones indicated by “US” and marked by an asterisk gave biphasic responses, i.e., with an undershoot (note the correspondingly low tint). Eight of the monkey cones listed are from outer-segment recordings (four L-, two M-, and one S- cone, together with one L-cone that showed undershoot), but their results were similar to those obtained with inner-segment recordings. For the parameter AL-P, see Data Analysis in Materials and Methods. The table lists only those cells that were stable enough to provide all of the indicated response parameters, which is why their total number (e.g., monkey cones) do not necessarily match the total number of cells recorded as stated in the text.
The single-photon response amplitude, a, is calculated as SF/Ac, where SF is dim-flash sensitivity in picoamperes per photon × micrometer square (pA⋅photons−1⋅µm2) and Ac is the effective collecting area of the cone outer segment, both at λmax (Table 1 and Materials and Methods). Across the animal species studied here, a covaries qualitatively with 1/σ (Table 1).
The dim-flash response’s time to peak (tpeak), which reflects to some degree the speed of response termination, also broadly covaries with σ across species (Table 1), such as would happen if the Ca2+ feedback that regulates the cone light response (26) somehow differed in degree across species. It is currently unclear whether this is the case, and, if so, why. Whether for monophasic or biphasic responses, the tpeak of primate cones as found by us (∼40 ms, with low-pass filtering at DC to 200 Hz) is generally faster than previously reported (with low-pass filtering at DC to between 20 and 150 Hz; see refs. 9–13), although our value is still slower than that extracted from human ERG recordings (∼20 ms) (15, 16). In the latter case, the cone response is extracted from the ERG recordings by using a rod-saturating background (15), so its tpeak is likely shortened by light adaptation. Table 1 lists the dim-flash response’s integration time, tint (see legend to Table 1), which, except for the biphasic cells, broadly covaries with tpeak (Adaptation to Background Light).
The saturated cone-response amplitude ranged mostly from 20 to 40 pA across species, but was distinctly lower for pig and mouse cones (Table 1). For mouse cones, this difference at least partly reflects the recording method. Mouse cones, being buried among the rods, cannot be individually identified; at the same time, their outer segment is quite fragile (see also ref. 23). Thus, instead of recording from a single targeted outer segment with a suction pipette as conventionally done, we drew several inner segments/somata in the distal-most two rows of cell bodies of the outer nuclear layer (where cone somata are situated) of the Gnat1−/− (i.e., rod-transducin KO) mouse into a recording suction pipette with a tip inner diameter intentionally large enough to fit several cells so as to increase the chance of including a cone cell (Materials and Methods) (23). By trial and error, a cone cell could be recorded from along with several nonphotoresponsive rods. As such, a fraction of the cone’s dark current was probably not recorded. Also, considering the low density of mouse cones (∼3% of all photoreceptors; ref. 27), the chance of more than one cone being recorded was very low. As for pig, a single cone was recorded in the standard “outer-segment-in” configuration, but they are very fragile and could have sustained some injury.
Effect of External Ca2+.
The response undershoot is most likely a result of negative feedback (e.g., refs. 12, 28). Its emergence may reflect a change from the normal balance in amplitude and/or time course between the cGMP-phosphodiesterase (PDE) and guanylyl cyclase (GC) activities underlying phototransduction, controlled in part via their negative-feedback modulations by Ca2+ (reviewed in ref. 29). For several of the recorded monkey cones that happened to show biphasic responses, we lowered the extracellular Ca2+ concentration and were indeed able to remove the undershoot (four of four cells; Fig. 2A); this change was reversible upon restoring normal Ca2+ concentration. Because the same extracellular Ca2+ concentration (1.2 mM) was used in the past (e.g., ref. 12) and the present work, this cannot account for the different response behaviors in the two cases. Nonetheless, this observation does reinforce the notion that the Ca2+ feedback is somehow involved. In our experiments, we also found that several macaque cones with a response undershoot (four of 15 cells) showed the phenomenon of spontaneously losing this undershoot in an all-or-none and reversible manner, at least at near-saturating flash intensities (Fig. 2B).
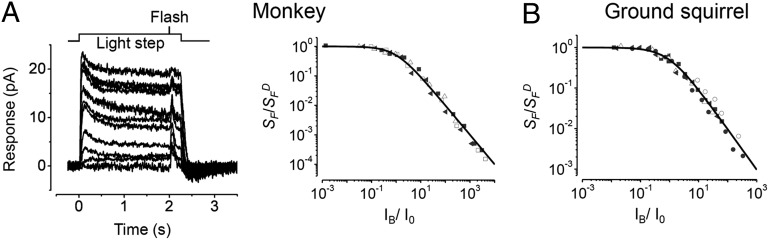
Fig. 2.
Appearance/disappearance of undershoot of monkey cone flash response. (A) When (occasionally) observed, the response undershoot of a monkey L-cone could be removed by lowering extracellular Ca2+ concentration. Flash (marked by arrow) had an intensity of 21,000 photons⋅μm−2 at 560 nm. (B) A monkey L-cone showed spontaneous, all-or-none, appearance/disappearance of the response undershoot at near-saturating flash intensities. Flash intensities were marked as log10 units of attenuation, with the unattenuated, 560-nm flash intensity being 8.9 × 106 photons⋅μm−2. The responses shown at each intensity were consecutive. A and B were inner-segment recordings.
Adaptation to Background Light.
Because the predominantly monophasic flash responses we observed in macaque cones were unlike previous findings (9, 10, 12), we reexamined their adaptation to steady light. In the incremental-flash-on-background experiment on L-cones (Fig. 3A), the reduction in flash sensitivity by steady light followed the Weber–Fechner relation:  , where SF is flash sensitivity in the presence of steady light of intensity IB,
, where SF is flash sensitivity in the presence of steady light of intensity IB, 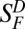 is dark-adapted flash sensitivity (i.e., no background light), and Io is the intensity of IB at which
is dark-adapted flash sensitivity (i.e., no background light), and Io is the intensity of IB at which 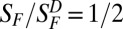 . The Io value, which we found to be 9,000 ± 5,300 photons⋅μm−2⋅s−1 at λmax (n = 4), is considerably lower than previous measurements [20,000–140,000 photons⋅μm−2⋅s−1, with a mean of 71,000 photons⋅μm−2⋅s−1 (12)]. In these previous measurements, because the flash responses were biphasic, they had a very small integration time (tint; Table 1 legend). Consequently, the cone’s steady-state response to a given steady-light intensity would also be small—much smaller than would be the case for a monophasic flash response. It is therefore not surprising that a cone with biphasic responses begins to adapt to background light only at much higher intensities, i.e., has a much larger Io value (see ref. 30 for a discussion on rods that should apply to cones here). For ground squirrel (Fig. 3B), which is the only other mammal examined here for background adaptation by cones, the Io value in comparison with that of monkey is almost 20-fold higher (142,000 ± 90,000 photons⋅μm−2⋅s−1 at λmax; n = 5; note that the data in Fig. 3A, Right, and Fig. 3B are plotted on normalized coordinates). Based on the same reasoning, this finding can be explained (although this should not be pushed too quantitatively) by the ∼10-fold lower cone flash sensitivity and approximately twofold shorter flash-response tint of ground-squirrel cones compared with monkey cones. Incidentally, others have reported an Io value of 30,000 photons⋅µm−2⋅s−1 for mouse M-cones and 70,000 photons⋅µm−2⋅s−1 for mouse S-cones at their respective λmax values (23), which are several-fold higher than the Io value we found for monkey L-cones. Again, this is qualitatively in line with the several-fold lower flash sensitivity, but a comparable flash-response tint, of mouse cones compared with monkey cones.
. The Io value, which we found to be 9,000 ± 5,300 photons⋅μm−2⋅s−1 at λmax (n = 4), is considerably lower than previous measurements [20,000–140,000 photons⋅μm−2⋅s−1, with a mean of 71,000 photons⋅μm−2⋅s−1 (12)]. In these previous measurements, because the flash responses were biphasic, they had a very small integration time (tint; Table 1 legend). Consequently, the cone’s steady-state response to a given steady-light intensity would also be small—much smaller than would be the case for a monophasic flash response. It is therefore not surprising that a cone with biphasic responses begins to adapt to background light only at much higher intensities, i.e., has a much larger Io value (see ref. 30 for a discussion on rods that should apply to cones here). For ground squirrel (Fig. 3B), which is the only other mammal examined here for background adaptation by cones, the Io value in comparison with that of monkey is almost 20-fold higher (142,000 ± 90,000 photons⋅μm−2⋅s−1 at λmax; n = 5; note that the data in Fig. 3A, Right, and Fig. 3B are plotted on normalized coordinates). Based on the same reasoning, this finding can be explained (although this should not be pushed too quantitatively) by the ∼10-fold lower cone flash sensitivity and approximately twofold shorter flash-response tint of ground-squirrel cones compared with monkey cones. Incidentally, others have reported an Io value of 30,000 photons⋅µm−2⋅s−1 for mouse M-cones and 70,000 photons⋅µm−2⋅s−1 for mouse S-cones at their respective λmax values (23), which are several-fold higher than the Io value we found for monkey L-cones. Again, this is qualitatively in line with the several-fold lower flash sensitivity, but a comparable flash-response tint, of mouse cones compared with monkey cones.
Fig. 3.
Background-light adaptation of monkey and ground-squirrel cones. (A) Incremental-flash-on-background experiment on a monkey L-cone. (Left) Representative traces (not averages) and (Right) collected data from four monkey L-cones, with normalized flash sensitivity (SF/SFD; see text) plotted against normalized background-light intensity (IB/Io; see text) on log-log scales. For each cell, shown by a different symbol, the measurement at each background intensity is the average of multiple trials of the sort shown at Left. The curve is the Weber–Fechner function (see text) with Io of 9,000 ± 5,300 photons⋅μm−2⋅s−1 at 560 nm. (B) Collected data from five ground-squirrel cones, with Io of 142,000 ± 90,000 photons⋅μm−2⋅s−1 at 560 nm. All inner-segment recordings, and all cells gave monophasic flash responses in the absence of background.
Discussion
At least in our hands, monophasic rather than biphasic flash responses appear to be the norm for primate and other mammalian cones, hence similar to what is long known for lower-vertebrate cones. This finding is also in agreement with the conclusion drawn indirectly from human ERG experiments (15, 16).
By using low (0.1 mM) extracellular Ca2+ concentration, we were able to convert the occasionally observed biphasic response into a monophasic response in monkey cones, reinforcing the notion that the Ca2+ feedback on phototransduction is involved. The biphasic response is perhaps triggered by an abnormal increase in intracellular Ca2+ concentration. Our (also occasional) observation of a spontaneous, all-or-none emergence of the response undershoot and its reversibility, as well as the observation by others that some ground-squirrel cones developed an undershoot during recordings (21), suggests that the balance between the PDE and GC activities underlying the light response may be delicate and labile, so that a response undershoot may appear when this balance is off and favors a domination by GC activity. At least for goldfish cones, we have sometimes noticed an emergence of biphasicity in their response in parallel with a progressive morphological deterioration of the recorded outer segment viewed under the microscope, consequently leading possibly to an accumulation of internal Ca2+ caused by an excessive inward Ca2+ leak and/or a defective Ca2+ extrusion. Incidentally, although rods of primates and other mammals almost invariably give monophasic responses (28, 30, 31), we did notice that primate rod responses sometimes showed an initial biphasicity during recording, but became monophasic over time (28). This opposite time sequence in response behavior between primate rods and cones, when it happens, may arise from quantitative differences between the rod/cone phototransduction processes with respect to the underlying reaction kinetics and the strength of the Ca2+ feedback. It further underscores a subtle balance in strength and/or time between the PDE and GC activities during the light response.
From reviewing published data on mammalian, including primate, cones (Acknowledgments), we have noticed a possible correlation between the adopted experimental procedure and the cone-response waveform, in that a response undershoot was typically observed when the experimental preparation was made by chopping/mincing an isolated retina into tiny fragments (9–13, 21, 22), but not observed when the retina was sliced or kept intact as one piece (present study and refs. 17–20, 32, although not ref. 23). The former procedure is presumably more prone to cause cell injury. After collecting and analyzing all of the data reported in Results, this thought prompted us to compare the two procedures directly with a pair of monkey eyes. Although we failed to find any increase in the occurrence of biphasic monkey cone responses with the chopping/mincing procedure (only 1 of 15 cones showed it; these data are not included in Table 1), this may be inconclusive because chopping/mincing is not exactly a well-defined or quantifiable procedure in different hands.
A monophasic cone response to light should make it easier to understand how the observed photopic visual signals in retinal ganglion cells come out, by not requiring a signal operation to convert a biphasic cone response into a monophasic response in retinal ganglion cells (see ref. 14). Also, our background-adaptation measurements gave a half-desensitizing background intensity (Io) of 9,000 photons⋅µm−2⋅s−1 at 560 nm, or approximately 4,000 photoisomerizations per second per cone after being multiplied by an effective collecting area (Ac) of 0.43 µm2 (Materials and Methods). This Io value essentially matches that obtained by others with patch-clamp recording from L-cones in retinal slices (calculated by multiplying the Io value of ∼5,700 absorbed photons per second per cone estimated from figure 1b in ref. 19 with the factor of 0.67, which is the quantum efficiency of photoisomerization, Qisom; Materials and Methods), which happened also not to give biphasic cone flash responses. As described in Results, we attribute the 10-fold higher Io value reported by Schnapf et al. (12) to the biphasic flash response they observed.
Materials and Methods
Animals.
Monkey (Macaca fascicularis) eyes were obtained from the laboratories of Stephen Hsiao, Rudiger von der Heydt, and Matthias Ringkamp (The Johns Hopkins University, Baltimore). Eyes from normal-sized pigs (i.e., not miniswines; Archer Farms) were obtained from Sue Eller (The Johns Hopkins University School of Medicine, Baltimore). Thirteen-lined ground squirrels were purchased from TLS Research. Nile grass rats were provided by Laura Smale (Michigan State University, East Lansing, MI). Gnat1−/− mice were provided by Ching-Kang Chen (Virginia Commonwealth University, Richmond, VA).
Monkey Retina Preparation and Recording.
Under light-adapted conditions (at the end of the donor laboratory’s experiments unrelated to ours), an animal was anesthetized and euthanized, and the eyes removed (for one animal, the eyes were removed from the anesthetized animal before euthanasia). During transfer to our laboratory, the eyes were dark-adapted in a light-proof container (with a slit cut on the posterior eye capsule to promote O2 penetration, but not cut open further to keep the pigment epithelium attached to the retina and thus promote visual-pigment regeneration) at room temperature (23 °C) in 95% O2/5% CO2-bubbled HCO3–Locke solution (in mM): 120 NaCl, 3.6 KCl, 1.2 CaCl2, 2.4 MgCl2, 10 glucose, 10 Hepes, 20 NaHCO3, 3 Na2-succinate, pH 7.4 with NaOH. Upon arrival at the laboratory (within an hour after enucleation), the anterior part of both eyes was removed, leaving the eyecups, and stored as such in the dark at room temperature under 95% O2/5% CO2-bubbled HCO3–Locke solution for as long as 24 h until use. When needed, a retina in an eyecup was peeled from the pigment epithelium under Locke solution, and a small piece was removed by cutting (with the rest returned to storage as before), put on nitrocellulose filter paper, cut into slices of 200-µm thickness, transferred into the recording chamber, and anchored with silicone grease.
For two retinae, we also compared the responses of cones under the condition of storing the retina in Locke solution at room temperature (n = 6) vs. in L-15 medium at 4 °C (n = 18), with the latter having been reported to make the stored retina less healthy and to reduce the rod single-photon-response amplitude/accelerate the rod response kinetics (33). Although we found no obvious difference between the two conditions with respect to the cone-response parameters (including the absence/presence of the undershoot), we adhered to Locke solution at room temperature just to be on the safe side.
Light was from a 75-W xenon arc lamp, with most IR removed with a water filter and the wavelength and intensity controlled by 10-nm interference filters and neutral-density filters, respectively. The beam went through an electronic shutter and was delivered to the microscope via a liquid light-guide. Flashes were 12.1 ms (by measurement) in duration and 2.5 to 10.0 s in interflash interval depending on the intensity, sufficient for full recovery after each stimulus. The tpeak of the flash response was measured as the duration between the middle of the flash and the transient peak of the response. A second light beam was used for background light in adaptation experiments. The light intensity was periodically calibrated with a radiometer. The stimulation wavelengths were 560 nm, 530 nm, and 440 nm for monkey L-, M-, and S-cones, respectively.
Suction-pipette recording was used for almost all experiments, either from a single monkey cone outer segment (for approximately one third of the cones recorded) or from its inner segment (for the rest). The purpose of recording from the inner segment was to allow changes in extracellular Ca2+ surrounding the outer segment in the event that the response was biphasic (which turned out to be very infrequent; Results). The suction pipette’s tip opening was ∼1.2 µm for outer-segment recordings and approximately 4.5 to 5.5 μm for inner-segment recordings (the diameter of the monkey cone inner segment varies from central to peripheral retina). The suction pipette contained Hepes–Locke solution, which is essentially the same as HCO3–Locke solution except that the 20 mM NaHCO3 in the latter was replaced by 20 mM NaCl (adjusted to 7.4 with NaOH). A limited amount of whole-cell recording was also carried out from the inner segment of cones. The patch pipette had a resistance of 2 to 5 MΩ when empty and was coated with Sylgard to reduce the capacitance. The pipette solution contained (in mM): 110 KCl, 13 NaCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 Hepes, adjusted to pH 7.2 with KOH and to 280 mOsm/L in osmolarity with glucose. All recordings were carried out at 35 to 37 °C in HCO3–Locke solution.
In each experiment, the spectral type of the recorded cone (L-, M-, or S-, with λmax of 561, 531, and 430 nm, respectively; ref. 10) was first identified based on its spectral sensitivity as follows: the ratio of sensitivities at 660 and 560 nm should be <0.1 (1/16) for L-cones, <0.01 (1/189) for M-cones, and ∼0 (1/3,227) for S-cones. S-cones can be further confirmed by their higher sensitivity at 440 nm than at 500 nm. All signals were filtered at DC to 200 Hz (eight-pole Bessel) and sampled at 10 kHz. The low-pass filter was deliberately set at a relatively high frequency to minimize its effect on the mammalian cone response waveform, which is much faster than that for mammalian rods and lower-vertebrate cones. Data are given in means ± SD when applicable.
Pig, Ground Squirrel, Nile Grass Rat, and Mouse Cone Recordings.
For pig, the eyes were removed immediately after euthanasia of the animal upon completion of surgical experiments by The Johns Hopkins Surgery Department. For all rodents, the animal was dark-adapted overnight, euthanized under anesthesia, and the eyes removed immediately. Otherwise, tissue preparation was quite similar to that for monkey. HCO3–Locke solution was used for storing tissue and recording. For ground squirrel, to compare with a previous report (21), a Locke solution containing 1.2 mM MgCl2, 2.4 mM CaCl2 (instead of our standard 2.4 mM MgCl2, 1.2 mM CaCl2) was also tried; from six M-cones, we still found no sign of a response undershoot or a change in the other response parameters. Ground squirrel cones were easy to identify and to record. Pig cone outer segments were extremely fragile, making recording difficult. Nile grass rat cone outer segments are short, narrow, tapered, and buried within rods, making it difficult to record. To fit the respective outer segments, the pipette tip openings for ground squirrel, pig, and Nile grass rat were 2.5, 1.2, and 1.0 µm, respectively. Mouse cones are likewise buried among the ∼97% rod photoreceptors, making outer-segment recordings difficult. Accordingly, for mouse cones, we recorded exclusively from the cone inner segment with suction-pipette recording (following ref. 23, but using retinal slices and slightly smaller suction pipettes with tip openings of 3.5–4.5 µm). Several cell bodies and adjoining inner segments near the outer boundary of the outer nuclear layer were drawn blindly into the suction pipette to increase the chance of including a cone cell. The use of Gnat1−/− mouse, with nonphotoresponsive rods, facilitated the recording.
Pig has two cone types, L- and S-cones, with λmax at 556 nm and 439 nm, respectively (34); the cones we recorded from turned out to be all L-cones, but we did not determine their exact λmax. The stimulating wavelength was 560 nm. Ground squirrel has M- and S-cones, with λmax reported to be 520 nm and 435 nm, respectively (21); from just a few measurements, we found broadly similar values of 524 nm and 440 nm, respectively. We used stimulation wavelengths of 520 nm and 440 nm, respectively. Nile grass rat also has M- and S-cones (35), but we encountered only M-cones, with λmax measured at 522 nm. We used a stimulating wavelength of 520 nm. Finally, mouse has M- and S-cones, with λmax at 508 nm and 360 nm, respectively (23). Because the great majority of mouse cones coexpress M- and S-opsins, we designated those cones with the spectral-sensitivity ratio S360/S508 <1 as M-cones, and those with S360/S508 >1 as S-cones. We used stimulating wavelengths of 500 nm and 380 nm, respectively.
The saturated flash responses of some cones showed an early transient that subsequently relaxed to a slightly lower “plateau.” This transient is presumed to reflect a hyperpolarization-activated membrane current (Ih) (23). Thus, for these cells, the nonsaturated cone responses were normalized to the plateau level.
Data Analysis.
Intensity–response relations were fit with the saturating-exponential function R/Rmax = 1 − exp(−If/ρ), where R is the flash-response amplitude, Rmax is the maximum response amplitude, If is flash intensity, and ρ is a constant. The half-saturating flash intensity, σ, is given by σ = ρ ln2.
The effective collecting area, Ac, of the cone outer segment for incident light transverse to the outer segment’s long axis is given by Ac = 2.303fQisomVα, where f is a factor that depends on the polarization of the incident light relative to the plane of the disk membrane; it equals 0.5 for unpolarized light (12, 36). Qisom is the quantum efficiency of photoisomerization (0.67), and α is the axial pigment density (0.016 μm−1; ref. 12). V is the volume of the outer segment, calculated to be 35 ± 7 µm3 for monkey cones (based on the measured length as well as tip and base diameters of 26 randomly chosen cone outer segments). This gave an Ac of 0.43 ± 0.1 µm2 for monkey cones, similar to the 0.37 µm2 used by Schnapf et al. (12). Likewise, Ac for pig, ground squirrel, and Nile grass rat was calculated as 0.3 ± 0.06 μm2 (n = 8), 0.63 ± 0.09 μm2 (n = 16), and 0.2 ± 0.05 μm2 (n = 9), respectively. Our Ac value for ground squirrel is similar to that used by Kraft (21). For mouse, we simply adopted the value of 0.2 μm2 used by Nikonov et al. (23).
The activation phase of the normalized flash response, R/Rmax, was fitted with the Lamb–Pugh model (37) of phototransduction, given by 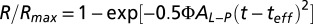 , where Φ is the number of photoisomerizations (given by If × Ac), AL-P is the “amplification constant,” and teff is an effective time delay contributed by all short phototransduction steps. By fitting this function to the response’s activation phase with AL-P and teff as free parameters, we obtained an AL-P value of 3.4 to 4.2 s−1 and teff of 9.4 ± 5.5 ms (n = 23) for monkey. In this paper, we did not make use of the AL-P and teff values, but the AL-P values are included in Table 1 for reference.
, where Φ is the number of photoisomerizations (given by If × Ac), AL-P is the “amplification constant,” and teff is an effective time delay contributed by all short phototransduction steps. By fitting this function to the response’s activation phase with AL-P and teff as free parameters, we obtained an AL-P value of 3.4 to 4.2 s−1 and teff of 9.4 ± 5.5 ms (n = 23) for monkey. In this paper, we did not make use of the AL-P and teff values, but the AL-P values are included in Table 1 for reference.
Supplementary Material
Acknowledgments
We thank Denis A. Baylor, Marie E. Burns, Timothy W. Kraft, Jeremy Nathans, Julie Schnapf, Robert M. Shapley, and members of the K.-W.Y. laboratory for comments on the manuscript; and Fred Rieke for an e-mail discussion of our findings. Dr. Timothy W. Kraft (University of Alabama at Birmingham) has communicated to us his independently performed suction-pipette recordings from pig cones, 60% to 70% of which showed a response undershoot; he used the retina-chopping method (see text). Separately, Dr. Dennis M. Dacey (University of Washington) has communicated to us his independently performed recordings from the flat-mount, isolated monkey retina (without retinal pigment epithelium) with whole-cell (current- and voltage-clamp) recordings, and found that most cones gave responses without an undershoot. The present work was supported by National Institutes of Health Grant EY06837 and the António Champalimaud Vision Award (Lisbon).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400268111/-/DCSupplemental.
References
- 1.Schnapf JL, McBurney RN. Light-induced changes in membrane current in cone outer segments of tiger salamander and turtle. Nature. 1980;287(5779):239–241. doi: 10.1038/287239a0. [DOI] [PubMed] [Google Scholar]
- 2.Nakatani K, Yau K-W. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- 3.Matthews HR, Fain GL, Murphy RL, Lamb TD. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol. 1990;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller JL, Korenbrot JI. Phototransduction and adaptation in rods, single cones, and twin cones of the striped bass retina: A comparative study. Vis Neurosci. 1993;10(4):653–667. doi: 10.1017/s0952523800005356. [DOI] [PubMed] [Google Scholar]
- 6.Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26(1):181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 7.Tachibanaki S, Tsushima S, Kawamura S. Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc Natl Acad Sci USA. 2001;98(24):14044–14049. doi: 10.1073/pnas.241396898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo D-G, Yue WWS, Ala-Laurila P, Yau K-W. Activation of visual pigments by light and heat. Science. 2011;332(6035):1307–1312. doi: 10.1126/science.1200172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunn BJ, Schnapf JL, Baylor DA. Spectral sensitivity of single cones in the retina of Macaca fascicularis. Nature. 1984;309(5965):264–266. doi: 10.1038/309264a0. [DOI] [PubMed] [Google Scholar]
- 10.Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnapf JL, Kraft TW, Baylor DA. Spectral sensitivity of human cone photoreceptors. Nature. 1987;325(6103):439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- 12.Schnapf JL, Nunn BJ, Meister M, Baylor DA. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraft TW, Neitz J, Neitz M. Spectra of human L cones. Vision Res. 1998;38(23):3663–3670. doi: 10.1016/s0042-6989(97)00371-4. [DOI] [PubMed] [Google Scholar]
- 14.Shapley R, Kaplan E, Purpura K. Contrast sensitivity and light adaptation in photoreceptors or in the retinal network. In: Shapley R, Lam DMK, editors. Contrast Sensitivity. Cambridge, MA: MIT Press; 1993. pp. 103–116. [Google Scholar]
- 15.Friedburg C, Allen CP, Mason PJ, Lamb TD. Contribution of cone photoreceptors and post-receptoral mechanisms to the human photopic electroretinogram. J Physiol. 2004;556(Pt 3):819–834. doi: 10.1113/jphysiol.2004.061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hateren JH, Lamb TD. The photocurrent response of human cones is fast and monophasic. BMC Neurosci. 2006;7:34. doi: 10.1186/1471-2202-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat Neurosci. 2004;7(7):745–750. doi: 10.1038/nn1274. [DOI] [PubMed] [Google Scholar]
- 18.Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25(48):11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn FA, Lankheet MJ, Rieke F. Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature. 2007;449(7162):603–606. doi: 10.1038/nature06150. [DOI] [PubMed] [Google Scholar]
- 20.Schneeweis DM, Schnapf JL. The photovoltage of macaque cone photoreceptors: Adaptation, noise, and kinetics. J Neurosci. 1999;19(4):1203–1216. doi: 10.1523/JNEUROSCI.19-04-01203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft TW. Photocurrents of cone photoreceptors of the golden-mantled ground squirrel. J Physiol. 1988;404:199–213. doi: 10.1113/jphysiol.1988.sp017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Wensel TG, Kraft TW. GTPase regulators and photoresponses in cones of the eastern chipmunk. J Neurosci. 2003;23(4):1287–1297. doi: 10.1523/JNEUROSCI.23-04-01287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127(4):359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, et al. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32(3):451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 25.Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14(5):364–377. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- 26.Nakatani K, Yau K-W. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol. 1989;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188(2):245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- 28.Tamura T, Nakatani K, Yau K-W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo D-G, Xue T, Yau K-W. How vision begins: An odyssey. Proc Natl Acad Sci USA. 2008;105(29):9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatani K, Tamura T, Yau K-W. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991;97(3):413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol. 2009;19(19):1665–1669. doi: 10.1016/j.cub.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azevedo AW, Rieke F. Experimental protocols alter phototransduction: The implications for retinal processing at visual threshold. J Neurosci. 2011;31(10):3670–3682. doi: 10.1523/JNEUROSCI.4750-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neitz J, Jacobs GH. Spectral sensitivity of cones in an ungulate. Vis Neurosci. 1989;2(2):97–100. doi: 10.1017/s0952523800011949. [DOI] [PubMed] [Google Scholar]
- 35.Gaillard F, Kuny S, Sauvé Y. Topographic arrangement of S-cone photoreceptors in the retina of the diurnal Nile grass rat (Arvicanthis niloticus) Invest Ophthalmol Vis Sci. 2009;50(11):5426–5434. doi: 10.1167/iovs.09-3896. [DOI] [PubMed] [Google Scholar]
- 36.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 37.Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.