Significance
Type I and type II natural killer T (NKT) cells are unique T-cell types with potent immunomodulatory functions. Because of lack of markers available to track type II NKT cells, this subset has not been as extensively studied as type I NKT cells. In this study we used a unique mouse model as a tool to decipher the developmental requirements, antigen specificity, and functional potential of type II NKT cells. We found that even though type I and type II NKT cells exhibited similar developmental needs, they were functionally distinct. In addition, we demonstrated a means of harnessing the therapeutic potential of these cells in cancer immunity by transforming their tumor-suppressive functions into a protective response.
Keywords: T-cell development, tumor immunity
Abstract
CD1d-restricted natural killer T (NKT) cells are innate-like T cells with potent immunomodulatory function via rapid production of both Th1 and Th2 cytokines. NKT cells comprise well-characterized type I NKT cells, which can be detected by α-galactosylceramide-loaded CD1d tetramers, and less-studied type II NKT cells, which do not recognize α-galactosylceramide. Here we characterized type II NKT cells on a polyclonal level by using a Jα18-deficient IL-4 reporter mouse model. This model allows us to track type II NTK cells by the GFP+TCRβ+ phenotype in the thymus and liver. We found type II NKT cells, like type I NKT cells, exhibit an activated phenotype and are dependent on the transcriptional regulator promyelocytic leukemia zinc finger (PLZF) and the adaptor molecule signaling lymphocyte activation molecule-associated protein (SAP) for their development. Type II NKT cells are potently activated by β-D-glucopyranosylceramide (β-GlcCer) but not sulfatide or phospholipids in a CD1d-dependent manner, with the stimulatory capacity of β-GlcCer influenced by acyl chain length. Compared with type I NKT cells, type II NKT cells produce lower levels of IFN-γ but comparable amounts of IL-13 in response to polyclonal T-cell receptor stimulation, suggesting they may play different roles in regulating immune responses. Furthermore, type II NKT cells can be activated by CpG oligodeoxynucletides to produce IFN-γ, but not IL-4 or IL-13. Importantly, CpG-activated type II NKT cells contribute to the antitumor effect of CpG in the B16 melanoma model. Taken together, our data reveal the characteristics of polyclonal type II NKT cells and their potential role in antitumor immunotherapy.
Natural killer T (NKT) cells are a distinct subset of T cells that recognize lipid antigens presented by CD1d (1). NKT cells are divided into two subsets: type I and type II. Type I NKT cells express an invariant T-cell receptor (TCR)α chain (Vα24Jα18 in humans and Vα14Jα18 in mice) and can be detected by CD1d/α-GalCer tetramers. Notably, a small population of CD1d/α-GalCer tetramer-positive cells in mice was reported to use canonical Vα10Jα50 rearrangement (2). The availability of CD1d/α-GalCer tetramers allows for the tracking of type I NKT cells and has been instrumental in defining the developmental program and biological functions of type I NKT cells. In contrast, type II NKT cells express a more diverse TCR repertoire and do not bind to CD1d/α-GalCer tetramers. A fraction of type II NKT cells has been reported to recognize sulfatide (3). However, CD1d/sulfatide tetramers have not been widely used to characterize type II NKT cells because of high background staining and low stability. Because of the lack of a direct method to identify type II NKT cells in vivo, the overall knowledge of type II NKT cells remains very limited. However, type II NKT cells are more prevalent than type I NKT cells in humans and are known to accumulate and play a role in several diseases, such as ulcerative colitis, hepatitis, and multiple myeloma (4–9). Therefore, a more in-depth characterization of type II NKT cells is warranted to better understand their regulation and function in health and disease.
The distinct phenotypic and functional properties of type I NKT cells are mostly attributed to their unique developmental pathway (10, 11). Type I NKT cells are positively selected by CD1d-expressing thymocytes that provide unique costimulatory signals to NKT cell precursors through homotypic interactions with signaling lymphocytic activation molecule family receptors. These interactions lead to the recruitment of signaling lymphocyte activation molecule-associated protein (SAP), the Src-family kinase Fyn, and downstream activation of NF-κB (12). After positive selection, TCR signaling by agonist self-ligands leads to the induction of promyelocytic leukemia zinc finger (PLZF), which directs acquisition of the activated phenotype and effector program of type I NKT cells (13, 14). Studies with TCR transgenic mice expressing a clonotypic type II NKT TCR have shown that the majority of the transgene-positive T cells express NK-lineage markers, have an activated phenotype, and secrete large amounts of IL-4 and IFN-γ upon activation, similar to type I NKT cells (15). However, it is still unknown whether type II NKT cells follow the unique developmental pathway of type I NKT cells.
Type I NKT cells are activated early during a variety of infections and contribute to the subsequent development of adaptive immune responses. During infection, type I NKT cells can be activated directly by microbial glycolipids or indirectly by inflammatory cytokines produced through Toll-like receptor (TLR)-mediated signaling and/or self-lipids presented by CD1d (16, 17). The respective roles of each TLR pathway in dendritic cells (DCs) on the activation of type I NKT cells have been reported. TLR9-activated DCs have the most potent effect on IFN-γ production by type I NKT cells, followed by TLR7 and TLR4 (18). Recent studies showed that CpG oligodeoxynucleotides (ODNs), TLR9 agonists, activate type I NKT cells through IL-12 or type I IFN produced by DCs and glycosphingolipids presented by CD1d-expressing myeloid DCs (18, 19). CpG ODNs have a strong antitumor effect in a variety of tumor models. Notably, in the B16 metastatic melanoma model, the CpG-mediated antitumor effect is in part through type I NKT cells (18). In contrast to type I NKT cells, the roles of type II NKT cells in tumor models are typically associated with suppression of tumor immunosurveillance through secretion of IL-13 (20-22). However, it is not clear whether CpG can skew type II NKT cells toward a Th1 immune response and the consequences of such a skewing on tumor immunity.
In this study we demonstrated that we could faithfully track polyclonal type II NKT cells in Jα18-deficient IL-4 reporter mice by their spontaneous GFP expression. We showed that type II NKT cells share similar surface phenotype and developmental requirements with type I NKT cells. Despite their differences in TCR usage, both NKT cell subsets can be potently activated by β-D-glucopyranosylceramide (β-GlcCer) containing C12:0 and C24:1 acyl chains. Interestingly, type II NKT cells produced more IL-13 relative to IFN-γ upon TCR stimulation compared with type I NKT cells. However, CpG treatment induced type II NKT cells to secrete IFN-γ but not IL-13, which in turn contributed to the antitumor effect of CpG in B16 melanoma. Hence, our findings not only unravel the characteristics of type II NKT cells at a polyclonal level but also demonstrate a means by which the immunosuppressive effects of type II NKT cells can be transformed into a protective response in the context of tumor immunity.
Results
The Majority of GFP+TCRβ+ Population in Thymus and Liver of Jα18−/−4get Mice Are Type II NKT Cells.
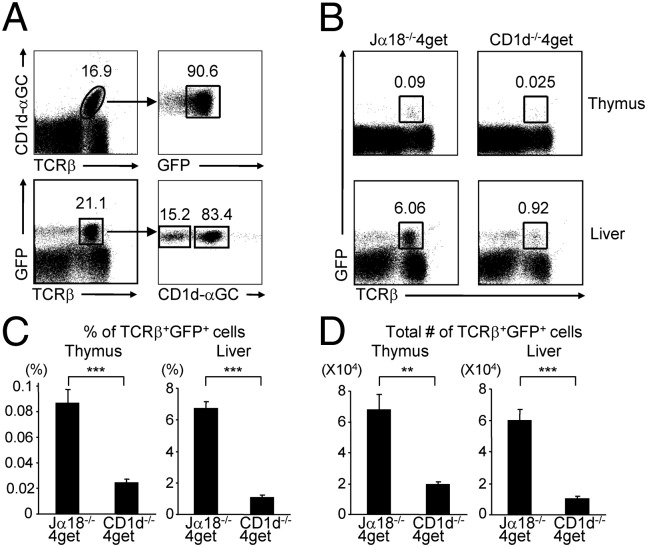
Type I NKT cells have been shown to constitutively contain IL-4 mRNA and are spontaneously fluorescent in naïve IL-4 GFP enhanced transcript (4get) mice (23). Consistent with previous studies, we found that approximately 90% of hepatic type I NKT cells were GFP-positive in naïve 4get mice (Fig. 1A). Because type II NKT cells contribute to the early burst of IL-4 induced by anti-CD3 (24), it is conceivable that they also constitutively express IL-4 transcripts and spontaneously express GFP in 4get mice. In fact, 4get mice had a population of GFP-expressing T cells that was CD1d/α-GalCer tetramer negative (Fig. 1A). To determine whether this population was type II NKT cells and therefore CD1d restricted, we compared GFP+ TCRβ+ cells in Jα18−/−4get mice (lacking type I NKT cells) and CD1d−/−4get mice (lacking type I and II NKT cells). We found that the percentage and absolute number of GFP+ TCRβ+ cells were decreased by 80–85% in the thymus and liver of CD1d−/−4get mice compared with Jα18−/−4get mice (Fig.1 B–D). Although Vα10+ type I NKT cells were present in Jα18−/−4get mice, they only represented approximately 1–2% of GFP+ TCRβ+ cells in these mice (Fig. S1). This indicated that the vast majority of GFP+ T cells in the thymus and liver of Jα18−/−4get mice were CD1d-restricted type II NKT cells. A small population of GFP+ T cells was also found in the spleen, lymph node, and intestine of Jα18−/−4get mice. However, the difference between Jα18−/−4get and CD1d−/−4get mice was less profound in these organs, suggesting that like type I NKT cells, type II NKT cells are also enriched in the liver. We also compared the frequency of the NK1.1+TCRβ+ population in Jα18−/−4get and CD1d−/−4get mice. NK1.1+TCRβ+ cells in CD1d−/−4get mice decreased by 70% in the thymus but only by 50–60% in the liver compared with Jα18−/−4get mice (Fig. S2). Collectively, our data support the use of the GFP+ TCRβ+ phenotype as a reliable way to identify most polyclonal type II NKT cells in the thymus and liver of Jα18−/−4get mice.
Fig. 1.
GFP-expressing T cells in the thymus and liver of Jα18−/−4get mice are CD1d-restricted type II NKT cells. (A) Flow cytometry analysis on liver mononuclear cells (MNCs) of 4get mice for the expression of GFP on CD1d/α-GalCer tetramers+ cells (Upper) and the reactivity to CD1d/α-GalCer tetramers on the GFP+ T cells (Lower). (B) Cells from Jα18−/−4get and CD1d−/−4get mice were analyzed for GFP expression on TCRβ+ population. (C and D) Bar graphs depict the mean ± SEM of the percentages (in the lymphocyte gate) and absolute numbers of TCRβ+GFP+ cells from Jα18−/−4get and CD1d−/−4get mice (n = 10 per group). ***P < 0.001; **P < 0.01.
Characterization of Surface Phenotype and TCR Repertoire of Type II NKT Cells.
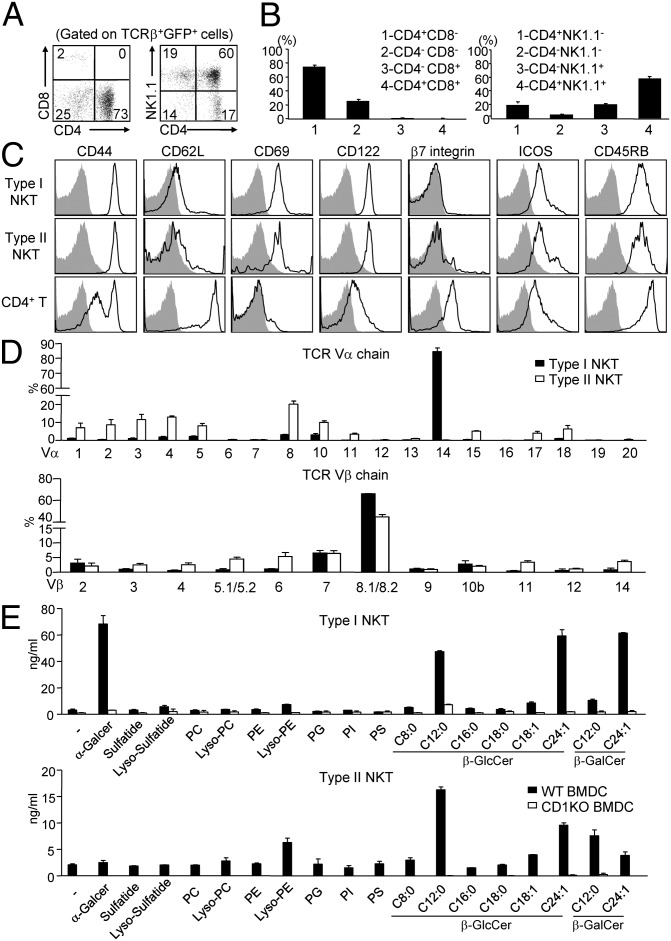
To characterize the surface phenotype of polyclonal type II NKT cells, we examined the coreceptor use and activation marker expression on GFP+ T cells in the liver of Jα18−/−4get mice (hereafter referred to as 4get+ type II NKT cells). Similar to type I NKT cells, 4get+ type II NKT cells were either CD4+ or CD4−CD8− (Fig. 2 A and B) and exhibited an activated phenotype (CD69hiCD44hiCD62Llo) (Fig. 2C). The majority of 4get+ type II NKT cells also expressed NK1.1 and CD122.
Fig. 2.
Characterization of surface phenotype, TCR repertoire, and antigen specificity of type II NKT cells. (A) GFP+TCRβ+ cells in the liver of Jα18−/−4get mice were analyzed for the expression of CD4, CD8, and NK1.1. (B) Bar graphs depict the mean ± SEM for the proportion of the indicated populations. (C) Expression of activation makers (black line) on type I NKT cells (TCRβ+tetramer+) from WT mice, type II NKT cells (TCRβ+GFP+), and conventional CD4+ T cells (TCRβ+GFP−CD4+) from Jα18−/−4get mice, compared with isotype control (gray filled). (D) (Upper) Bar graphs depict the percentage of individual Vα in all analyzed Vα by real-time PCR in sorted type I and type II NKT cells. Relative quantification of each Vα expression was calculated by 2-ΔCt method based on Ct of β-actin. Data are representative of three experiments. (Lower) Flow cytometry analysis of Vβ use by type I and type II NKT cells. Bar graphs depict the mean ± SEM of the percentages of indicated TCR Vβ chains in type I and type II T cells (n = 9). (E) Bar graphs depict the IFN-γ production by short-term type I (Upper) and type II (Lower) NKT cell cultures in response to WT or CD1KO BMDC with indicated antigens. Data are representative of three experiments.
To determine the overall spectrum of TCR use by 4get+ type II NKT cells, we first analyzed their Vβ use by flow cytometry (Fig. 2D). We found that a large percentage (∼50%) of 4get+ type II NKT cells expressed Vβ8.1/8.2, and the remaining 4get+ type II NKT cells expressed a diverse array of Vβ chains. Next we analyzed the Vα chain use of sorted 4get+ type II NKT cells by real-time PCR using a panel of Vα-specific primers (25, 26). Unlike type I NKT cells that predominantly express the Vα14 gene, 4get+ type II NKT cells express a variety of Vα genes, with the Vα8 gene (∼20%) being most prevalent.
β-GlcCer Is a Potent Self-Antigen for 4get+ Type II NKT Cells.
To explore the diversity of lipid antigens involved in the activation of type II NKT cells, we examined the stimulatory capacity of a panel of synthetic lipids on short-term 4get+type II NKT cell cultures. We found that several types of lipids, including β-GlcCer, β-GalCer, and Lyso-PE, can stimulate 4get+ type II NKT cells to secrete significant amounts of IFN-γ when presented by CD1d-expressing bone marrow-derived DCs (BMDCs) (Fig. 2E). This stimulatory effect was abolished when CD1d−/− BMDCs were used as antigen-presenting cells (APCs). Similar to type I NKT cells, the recognition of β-GlcCer by 4get+ type II NKT cells showed a preference for β-GlcCer C12:0 and C24:1 (27). In addition, β-GlcCer more potently activated type II NKT cells than each corresponding β-GalCer, suggesting that carbohydrate head group as well as the N-acyl chain of glycolipids can affect their recognition by type II NKT cells. In contrast, none of the phospholipids tested has stimulatory activity, except Lyso-PE. We also found that 4get+ type II NKT cells did not produce a significant amount of cytokines in response to sulfatide stimulation, suggesting that the frequency of sulfatide-reactive type II NKT cells in 4get+ type II NKT cells was very low.
PLZF and SAP Are Required for the Development of Type II NKT Cells.
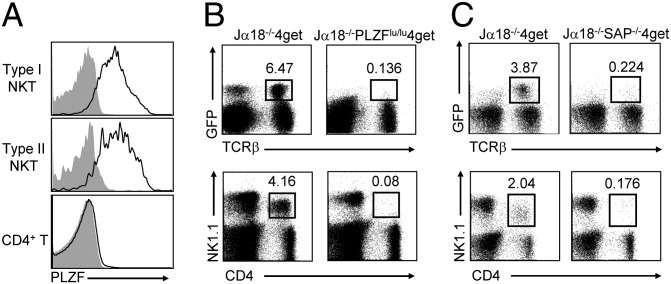
The transcriptional regulator PLZF has been reported as a key factor in establishing the type I NKT cell lineage and effector functions (13, 14). Given the phenotypic similarity between type I and type II NKT cells, it is likely that the development of type II NKT cells might depend on the same transcriptional regulator. Consistent with this notion, we found that PLZF was highly expressed in thymic 4get+ type II NKT cells (Fig. 3A). To further bolster the dependency of type II NKT cell development on PLZF, Jα18−/−4get mice were crossed with PLZF-deficient luxoid (PLZFlu/lu) mice. Indeed, we found that Jα18−/−PLZFlu/lu4get mice exhibited a drastic reduction (>40-fold) of 4get+ type II NKT cells compared with Jα18−/−4get mice (Fig. 3B). Concordantly, the CD4+NK1.1+ T-cell population was also dramatically reduced in Jα18−/−PLZFlu/lu4get mice.
Fig. 3.
PLZF and SAP are required for the development of type II NKT cells. (A) Expression of PLZF (black line) on thymic type I and type II NKT cells and CD4 T cells, compared with isotype control (gray filled). (B and C) Liver MNCs from Jα18−/−4get mice, Jα18−/−PLZFlu/lu4get mice (B), and Jα18−/−SAP−/−4get mice (C) were stained with mAbs to CD4, NK1.1, and TCRβ and then analyzed by flow cytometry. Data are representative of three experiments.
The adaptor molecule SAP plays a crucial role during the development of type I NKT cells, with SAP-deficient humans and mice lacking type I NKT cells (28, 29). We found that SAP deficiency also significantly affected the development of type II NKT cells: the percentages of 4get+ type II NKT cells as well as CD4+NK1.1+ T cells were reduced more than 10-fold in Jα18−/−SAP−/−4get mice compared with Jα18−/−4get mice (Fig. 3C). Together, these data suggest that type II NKT cells may share a similar development program with type I NKT cells.
Type II NKT Cells Have Different Cytokine-Producing Capacity Compared with Type I NKT Cells.
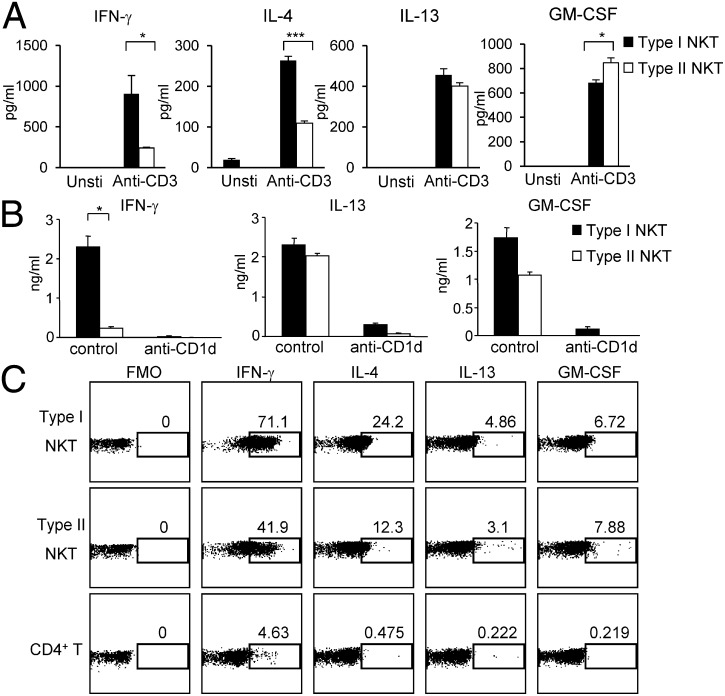
Both type I and type II NKT cells have been implicated in suppressing autoimmunity (3, 30). However, type I and type II NKT cells have been shown to play opposing roles in tumor immunity (31). To better understand the functional potential of type II NKT cells, we compared the cytokine-producing capacity of sorted 4get+ type II NKT cells with that of type I NKT cells. We found that type I and type II NKT cells produced the same major cytokines, such as IFN-γ, IL-4, IL-13, and GM-CSF, upon activation with anti-CD3 (Fig. 4A). Compared with type I NKT cells, type II NKT cells produced lower levels of IFN-γ and IL-4 but similar levels of IL-13 and GM-CSF in response to anti-CD3 stimulation. Additionally, 4get+ type II NKT cells produced substantial amounts of cytokines when stimulated with BMDCs from CD1dTg mice. Similar to anti-CD3 stimulation, less IFN-γ, but comparable amounts of IL-13 and GM-CSF, were detected in cocultures of 4get+ type II NKT cells and CD1dTg BMDC, compared with cocultures containing type I NKT cells (Fig. 4B). This response was inhibited by anti-CD1d, that confirming 4get+ type II NKT cells are indeed CD1d-restricted.
Fig. 4.
Type II NKT cells show different cytokine-producing capacity compared with type I NKT cells. (A and B) Sorted type I or type II NKT cells were stimulated with or without anti-CD3 and anti-CD28 (A) or cocultured with BMDC from CD1dTg mice in the presence of either control IgG or anti-CD1d mAb (B). Forty-eight hours later, cytokines in the supernatant were detected by BD Cytometric Bead Array (CBA) Flex set system. Data are representative of three experiments. ***P < 0.001; *P < 0.05. (C) 4get mice were injected i.v. with anti-CD3 mAb (1.5 μg per mouse). Thirty minutes later, liver MNCs were isolated and intracellularly stained for the indicated cytokines. Data are representative of three experiments.
To evaluate the cytokine-producing ability of type I and type II NKT cells in the same mice under the same stimulatory condition, we injected 4get mice with anti-CD3 mAb. Thirty minutes after injection, cytokine production by type I (GFP+TCRβ+CD1d/α-GalCer+) and type II (GFP+TCRβ+CD1d/α-GalCer−) NKT cells was determined by intracellular cytokine staining. Consistent with the results from in vitro assays, a lower proportion of type II NKT cells produced IFN-γ and IL-4 compared with type I NKT cells (Fig. 4C). In contrast, the percentages of IL-13 and GM-CSF–producing type II NKT cells were similar to that of type I NKT cells. The distinct cytokine-producing capacity of these two NKT cell subsets suggests that they may play distinct roles in the regulation of immune responses.
CpG ODN Induces IFN-γ Production by Type II NKT Cells in Vitro and in Vivo.
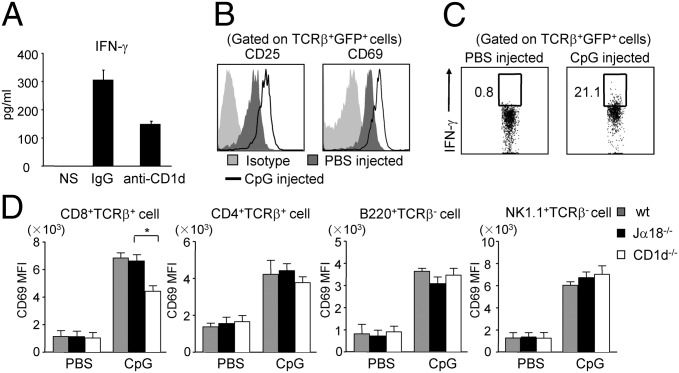
Efforts to modulate NKT cell effector functions have ranged from treatment for autoimmune disease to antitumor immunotherapy (32). Among them, CpG ODN, a potent adjuvant in cancer immunotherapy, promotes the production of IFN-γ, but not IL-4, by type I NKT cells (18). Furthermore, CpG-activated type I NKT cells have been shown to contribute to protection against B16 melanoma (18, 33). However, the effect of CpG on type II NKT cells remains unknown. To address this question, we first examined the response of type II NKT cells to CpG-sensitized DCs in vitro. We found that type II NKT cells produced IFN-γ, but not IL-4 or IL-13, in response to CpG-treated DCs. The production of IFN-γ was partially blocked by anti-CD1d antibody, indicating that full activation of type II NKT cells by CpG was dependent on CD1d (Fig. 5A).
Fig. 5.
CpG ODN induces IFN-γ production by type II NKT cells both in vitro and in vivo. (A) Sorted type II NKT cells were cocultured with CpG-treated BMDCs in the presence of either control IgG or anti-CD1d mAb for 48 h. Cytokines in the supernatant were detected by CBA. (B and C) Jα18−/−4get mice were injected with either PBS or 40 μg of CpG. Six hours later, liver MNCs were isolated and stained with mAbs to TCRβ, CD25, CD69, and IFN-γ. Data shown are gated on type II NKT cells (TCRβ+GFP+). (D) WT, Jα18−/−4get, and CD1d−/−4get mice were injected with either PBS or 40 μg of CpG. Twenty-four hours later, splenocytes were isolated and stained with mAbs to CD69, B220, CD8α, CD4, NK1.1, and TCRβ. Bar graphs show the mean fluorescence intensity (MFI) of CD69 on indicated cell populations (n = 4 per group). Data are representative of three experiments. *P < 0.05.
To study the effect of CpG on type II NKT cells in vivo, Jα18−/−4get mice were injected with CpG. As shown in Fig. 5 B and C, early activation markers like CD25 and CD69 were up-regulated on type II NKT cells, and IFN-γ production was also detected upon CpG stimulation. To investigate whether activation of type II NKT cells leads to transactivation of other cell types, the expression level of CD69 on various cell types and IFN-γ production of NK cells in WT, Jα18−/−, and CD1d−/− mice after CpG injection were compared. The expression level of CD69 on CD8+ T cells was increased in Jα18−/− mice compared with CD1d−/− mice, suggesting that CpG-activated type II NKT cells can transactivate CD8+ T cells but not other cells types (Fig. 5D). Additionally, the percentage of IFN-γ–producing NK cells was significantly higher in WT mice but comparable between Jα18−/− and CD1d−/− mice (Fig. S3). Collectively, these data demonstrate that type II NKT cells can be activated by CpG to produce IFN-γ both in vitro and in vivo. Furthermore, CpG-mediated type II NKT cell activation can enhance the activation of CD8+ T cells.
Type II NKT Cells Enhance the Antitumor Effect of CpG in B16 Melanoma.
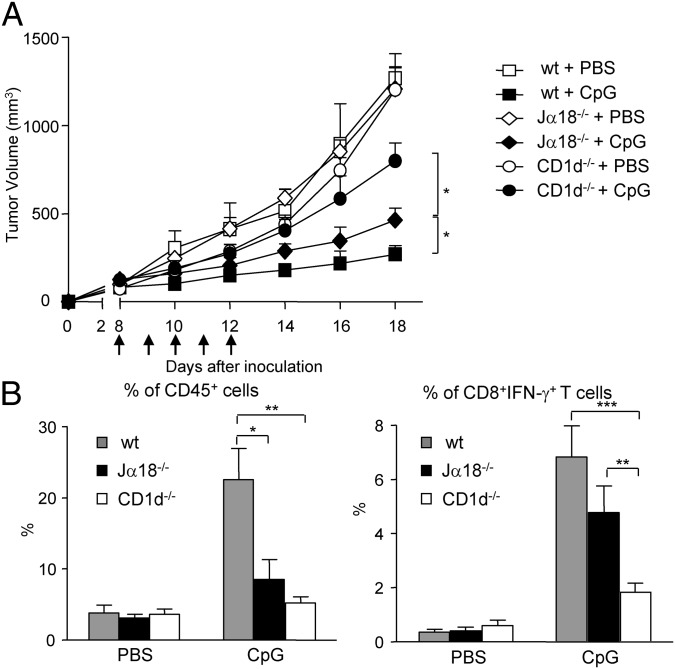
To assess the role of type II NKT cells in CpG-mediated immunotherapy, WT, Jα18−/−, and CD1d−/− mice were inoculated s.c. with B16 melanoma cells, and tumor growth in PBS- or CpG-treated mice was monitored. CpG treatment significantly reduced tumor growth in all mouse strains tested (Fig. 6A). Notably, the tumor volume in CpG-treated Jα18−/− mice was significantly smaller than those in CpG-treated CD1d−/− mice, suggesting that CpG-mediated antitumor effect is in part through type II NKT cells. Moreover, the reduction in tumor growth was even greater in CpG-treated WT mice, suggesting that both NKT cell subsets contribute to the antitumor activity of CpG.
Fig. 6.
Type II NKT cells enhance the antitumor effect of CpG in a B16 melanoma model. (A) WT, Jα18−/−, and CD1d−/− mice were inoculated s.c. with B16 melanoma cells at day 0. Starting from day 8, mice were treated with 40 μg CpG daily for 5 d (arrows) at the tumor site. Tumor volumes are shown as mean ± SEM (n = 6–10 for PBS groups; n = 12 for CpG-treated groups). (B) Mice were killed at day 18, and tumor-infiltrating leukocytes were prepared and stained with mAbs to CD45, CD8α, TCRβ, and IFN-γ. Bar graphs depict the mean ± SEM of the percentages of CD45+ cells in the tumor and IFN-γ+CD8+T cells in the CD45+ cells (n = 4 for PBS groups; n = 6–9 for CpG groups). ***P < 0.001; **P < 0.01; *P < 0.05.
Analysis of tumor-infiltrating cells showed that CpG treatment induced a significantly higher percentage of infiltrating CD45+ cells in WT mice than in Jα18−/− and CD1d−/− mice. However, no significant differences in the number and composition of tumor-infiltrating leukocyte subsets were detected between CpG-treated Jα18−/− and CD1d−/− mice (Fig. 6B and Fig. S4). Nonetheless, CpG-treated Jα18−/− mice had significantly higher proportions of IFN-γ–producing CD8+ T cells compared with CD1d−/− mice (Fig. 6B). Taken together, our data suggests that type II NKT cells can contribute to the antitumor effect of CpG by augmenting CD8+ T-cell responses.
Discussion
Polyclonal type II NKT cells were initially characterized in MHCII−/− mice. They are a mixture of NK1.1+ and NK1.1− T cells with a memory phenotype and express particular TCRα chains, such as Vα3.2 and Vα8 (24, 34). In agreement with previous observations, type II NKT cells as defined by GFP+TCRβ+ cells in Jα18−/−4get mice are a heterogeneous population that contain both NK1.1+ and NK1.1− cells, most of which are CD4+ and exhibit a preactivated phenotype. Additionally, 4get+ type II NKT cells have a diverse TCRα repertoire, with the Vα8 chain being the most highly represented (Fig. 2D). However, very few type II NKT cells in Jα18−/− 4get mice express Vα3.2, whereas more than 20% of their wild-type counterparts (defined as GFP+TCRβ+CD1d/α-GalCer− in 4get mice) are Vα3.2-positive (Fig. S5A). This difference may be due to an unintended effect of gene modification of the Jα18 locus. A recent report has shown that transcription of TCRα gene rearrangements involving J segments upstream of Jα18 is impaired in Jα18−/− mice (35). Despite some differences in their TCRα repertoire, we found that type II NKT cells in Jα18−/−4get mice closely resemble those from WT mice in terms of their surface phenotype and cytokine-producing capacity (Fig. 4 and Fig. S5B). This supports the validity of using GFP+TCRβ+ cells in Jα18−/−4get mice to study the development and function of polyclonal type II NKT cells.
Previous studies with type II NKT cell hybridomas have identified several self-lipid antigens recognized by type II NKT cells, including phospholipids, β-GlcCer, and sulfatide (27, 36–40). Interestingly, we found that 4get+ type II NKT cells are potently activated by β-GlcCer containing C12:0 and C24:1 acyl chains, suggesting that a significant proportion of type II NKT cells recognize β-GlcCer. Therefore, it is conceivable that increased levels of β-GlcCer during infection could also lead to type II NKT cell activation. Consistent with a recent study, we found that Lyso-PE has a stimulatory effect on 4get+type II NKT cells, albeit less potently than β-GlcCer (41). In contrast, sulfatide failed to stimulate detectable cytokine response by 4get+ type II NKT cells. Therefore, type II NKT cells defined by this method likely represent a population distinct from those identified by CD1d/sulfatide tetramers. This notion is further supported by the fact that most CD1d/sulfatide tetramer+ cells are phenotypically distinct from 4get+type II NKT cells: they have been shown to be CD69low and preferentially use the Vα3/Vα1 and Vβ8.1/Vβ3.1 segments (42).
Our data suggest that type II NKT cells might be inherently prone to producing a higher ratio of IL-13 to IFN-γ upon TCR stimulation. Indeed, IL-13 production by type II NKT cells has been indicated as a crucial factor for their pathogenic role in several disease settings. For example, an increased frequency of IL-13–producing type II NKT cells has been found in patients with ulcerative colitis (4) and multiple myeloma (7). Furthermore, in several tumor models, type II NKT cells suppress immunosurveillance through the secretion of IL-13 (20, 21). Previous studies have shown that the strength of TCR signal affects the types of cytokines type I NKT cells produce. GM-CSF and IL-13 require the least stimulation, followed by IFN-γ and IL-4 (43). It is possible that the threshold for type II NKT cells to mount a similar level of activation is higher than that for type I NKT cells. However, different antigens can skew type I NKT cells to either Th1 or Th2 directions. Thus, how type II NKT cells respond to different antigens needs to be further investigated.
Similar to type I NKT cells, we found that type II NKT cells can be activated by CpG, leading to IFN-γ production. Previous studies have shown that the activation of type I NKT cells by CpG can amplify downstream activation of NK cells (33). Although we did not detect a significant effect of type II NKT cells on the activation and function of NK cells, we found that the presence of type II NKT cells enhanced the activation and function of CD8+ T cells and contributed to the antitumor effect of CpG in the B16 melanoma model. It is possible that early production of IFN-γ by type II NKT cells in response to CpG enhances the maturation and function of DCs, thereby promoting tumor-specific CD8+ T-cell responses. Our finding that CpG treatment induces IFN-γ production by type II NKT cells is in agreement with a study by Paget et al. (33) showing that splenocytes from CD1−/− mice produced less IFN-γ compared with Jα18−/− mice in response to either ODN1826 or ODN1668 (Fig. 5 and Fig. S6). However, our data are contradictory with a study by Sfondrini et al. (44), which reported that CpG-dependent tumor growth inhibition was only observed in the absence of type II NKT cells in B16 melanoma model and that splenocytes from CD1d−/− mice produced higher levels of IFN-γ compared with Jα18−/− and WT mice upon CpG stimulation. Recent reports suggest that housing conditions may alter the function of type I NKT cells (45). Therefore, it is possible that the conflicting data are a result of such a variable or genetic heterogeneity of mutant mice used in these studies.
The nature and function of type II NKT cells especially on a polyclonal level are poorly understood. In this study we characterized polyclonal type II NKT cells and compared them head to head with type I NKT cells. Our data indicate that type I and type II NKT cells may function differently upon activation. This warrants the study of type II NKT cells in greater depth, especially with evidence showing type II NKT cells as the dominant subset in humans. We also demonstrated the feasibility of manipulating the function of type II NKT cells by CpG and shifting their role from immunosuppressive to protective in tumor immunity. Our data suggest that CpG-activated type II NKT cells have potential to be therapeutically harnessed for cancer treatment.
Materials and Methods
Mice.
Jα18−/−, CD1d−/−, Kb-CD1dTg, IL-4 GFP enhanced transcript (4get) mice, SAP−/−, and PLZF-deficient luxoid (PLZFlu/lu) mice have been described elsewhere (46–51). 4get mice were crossed with Jα18−/−CD1d−/− mice to obtain Jα18+/−CD1d+/−4get mice, which were then intercrossed to generate Jα18−/−4get and CD1d−/− 4get mice, respectively. Jα18−/−4get mice were further crossed with SAP−/− and PLZFlu/lu mice to generate Jα18−/−SAP−/−4get and Jα18−/−PLZFlu/lu4get mice. All mice used in this study were backcrossed to C57BL/6 at least 10 times. All animal work was approved by the Northwestern University Institutional Animal Care and Use Committee.
Reagents and Antibodies.
CpG ODN 1826 (5′-tccatgacgttcctgacgtt-3′) and ODN 1668 (5′-tccatgacgttcctgatgct-3′) were synthesized at Integrated Device Technology. CD1d/α-GalCer tetramers were provided by the National Institutes of Health tetramer facility. Lipid antigens were obtained from Avanti Polar Lipids. The generation of CD1d-specific mAb 5C6 has been described previously (52). Fluorochrome-labeled mAbs against mouse B220, CD4, CD8α, CD11b, CD11c, CD25, CD45.2, CD44, CD62L, CD69, IL-4, IFN-γ, NK1.1, Ly6G, Foxp3, IL-13, GM-CSF, CD122, ICOS, β7 integrin, CD45RB, TCRβ, Vα3.2, Vα8.3, Vα11.1, Vβ2, Vβ3, Vβ4, Vβ5.1/5.2, Vβ6, Vβ7, Vβ8.1/8.2, Vβ9, Vβ10b, Vβ11, Vβ12, and Vβ14 were purchased from eBioscience, BD Biosciences, or BioLegend.
Flow Cytometry.
For cell surface staining, cells were incubated with anti-CD16/32 mAb before staining with the appropriate combinations of mAbs. PLZF expression was analyzed via intracellular staining using the FoxP3 staining buffer set (eBioscience) with anti-PLZF mAb (Santa Cruz Biotechnology). For intracellular cytokine staining, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and then stained with anti-cytokine mAbs. Flow cytometry was performed with a FACS Canto II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Cell Preparations and NKT Cell Sorting.
Cell suspension of thymus, spleen, and liver were prepared as described previously (53). BMDCs were prepared using GM-CSF and IL-4 as previously described (48). Tumor-infiltrating cells were isolated by digestion with 1 mg/mL collagenase and 100 μg/mL DNase at 37 °C for 30 min. For sorting type I NKT cells, hepatic leukocytes from 4get mice were stained with CD1d/α-GalCer tetramers and anti-B220. For sorting type II NKT cells, hepatic leukocytes from Jα18−/−4get mice were stained with anti-TCRβ. Cells were sorted in a FACSAria (BD Biosciences). Sorted type I NKT cells (Tet+B220−) and type II NKT cells (TCRβ+GFP+) were more than 90% pure.
NKT Cell Stimulation and Cytokine Analysis.
For antigen stimulation, sorted type I and II NKT cell were cultured in vitro for 7 d as described previously (54), except cells were cultured in the medium containing IL-7 (2 U/mL) and IL-15 (50 ng/mL) from day 5 to day 7. NKT cells (0.5–1 × 105 cells) from the short-term culture were stimulated with α-GalCer (10 ng/mL) or other lipid antigens (20 μg/mL) in the presence of either WT or CD1−/− BMDCs for 48 h. To compare cytokine producing capacity, sorted NKT cells (0.5–1 × 105 cells) were stimulated with plate-bound anti-CD3 (10 μg/mL)/CD28 (10 μg/mL) or with BMDCs from Kb-CD1dTg mice in a 96-well plate for 48 h. To determine the effect of CpG, BMDCs from WT mice were treated with 10 μg/mL CpG ODN 1826 overnight. After extensive washing, CpG-treated and untreated BMDCs (1 × 104 cells) were cocultured with sorted NKT cells (5 × 104 cells) for 48 h. Cytokines in the supernatant were detected using Cytometric Bead Array or by ELISA.
RNA Extraction and Quantitative Real-Time PCR.
RNA from sorted NKT cells was extracted using the RNeasy kit (Qiagen). After reverse transcription, equal amounts of cDNA were subjected to PCR using specific Vα and Cα primers (25, 26). Real-time PCR was performed on an i-cycler (BioRad) using SYBR Green Master Mix (BioRad).
B16 Melanoma Model.
Mice were inoculated s.c. with 3 × 105 B16 melanoma cells. Eight days later, mice were treated with either PBS or CpG ODN1826 (40 μg in 100 μL of PBS) daily for 5 d at the tumor site. Two perpendicular diameters of the tumor mass were measured every 2 d, and tumor volumes were calculated as: π/6 × length × width2.
Statistical Analysis.
Mean values were compared using unpaired Student t tests. All statistical analyses were performed using Prism software. Results with a value of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Luc Van Kaer for Jα18−/− mice, Dr. Paul Stein for SAP−/− and 4get mice, Dr. Jonathan Licht for PLZFlu/lu mice, the National Institutes of Health (NIH) tetramer core facility for CD1d tetramers, the Northwestern University flow cytometry core facility for cell sorting, and Sharmila Shanmuganad and Ying-He for technical assistance. This work is supported by NIH Grant R01AI43407 and a Crohn's and Colitis Foundation of America Senior Research Award (to C.-R.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323845111/-/DCSupplemental.
References
- 1.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uldrich AP, et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12(7):616–623. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahng A, et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuss IJ, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exley MA, et al. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168(4):1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 6.Durante-Mangoni E, et al. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173(3):2159–2166. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 7.Chang DH, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112(4):1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exley MA, et al. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167(10):5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 9.Liao CM, et al. dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142(2):326, e1–e2. doi: 10.1053/j.gastro.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 12.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sköld M, Faizunnessa NN, Wang CR, Cardell S. CD1d-specific NK1.1+ T cells with a transgenic variant TCR. J Immunol. 2000;165(1):168–174. doi: 10.4049/jimmunol.165.1.168. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009;21(4):391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. 2010;22(2):79–86. doi: 10.1016/j.smim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27(4):597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Montoya CJ, et al. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol. 2006;177(2):1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 20.Terabe M, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 21.Terabe M, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114(1):80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 23.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu YH, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189(1):103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currier JR, Robinson MA. Spectratype/immunoscope analysis of the expressed TCR repertoire. Curr Protoc Immunol. 2001;Chapter 10:28. doi: 10.1002/0471142735.im1028s38. [DOI] [PubMed] [Google Scholar]
- 26.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: Implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174(6):1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan PJ, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12(12):1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11(3):340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201(5):695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte N, et al. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol. 2004;173(5):3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 31.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Heuvel MJ, Garg N, Van Kaer L, Haeryfar SM. NKT cell costimulation: Experimental progress and therapeutic promise. Trends Mol Med. 2011;17(2):65–77. doi: 10.1016/j.molmed.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paget C, et al. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009;182(4):1846–1853. doi: 10.4049/jimmunol.0802492. [DOI] [PubMed] [Google Scholar]
- 34.Park SH, et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193(8):893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedel R, et al. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13(8):705–706. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhost S, et al. Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012;42(11):2851–2860. doi: 10.1002/eji.201142350. [DOI] [PubMed] [Google Scholar]
- 37.Tatituri RV, et al. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci USA. 2013;110(5):1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. 2012;76(3):246–255. doi: 10.1111/j.1365-3083.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 39.Roy KC, et al. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180(5):2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 40.Blomqvist M, et al. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol. 2009;39(7):1726–1735. doi: 10.1002/eji.200839001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeissig S, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18(7):1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci USA. 2010;107(24):10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112(10):4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sfondrini L, et al. Absence of the CD1 molecule up-regulates antitumor activity induced by CpG oligodeoxynucleotides in mice. J Immunol. 2002;169(1):151–158. doi: 10.4049/jimmunol.169.1.151. [DOI] [PubMed] [Google Scholar]
- 45.Wingender G, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143(2):418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6(4):459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 47.Cannons JL, et al. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21(5):693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Chun T, et al. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197(7):907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36(6):647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 50.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 51.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 52.Mandal M, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35(9):525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 53.Felio K, et al. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206(11):2497–2509. doi: 10.1084/jem.20090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3(1):70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.