Significance
Antigen-specific therapies are lacking for autoimmunity diseases. The recent discovery of the nature of the IAg7–insulin complex that drives type 1 diabetes in nonobese diabetic (NOD) mice has allowed us to create a monoclonal antibody specific for this complex. This antibody delays diabetes development in NOD mice. Given the similarities between IAg7 and the human diabetes risk alleles, HLA-DQ2 and HLA-DQ8, this work encourages the development of similar monoclonal antibodies for the treatment of the human disease.
Keywords: immunotherapy, antigen processing
Abstract
The primary autoantigen triggering spontaneous type 1 diabetes mellitus in nonobese diabetic (NOD) mice is insulin. The major T-cell insulin epitope lies within the amino acid 9–23 peptide of the β-chain (B:9–23). This peptide can bind within the peptide binding groove of the NOD MHC class II molecule (MHCII), IAg7, in multiple positions or “registers.” However, the majority of pathogenic CD4 T cells recognize this complex only when the insulin peptide is bound in register 3 (R3). We hypothesized that antibodies reacting specifically with R3 insulin–IAg7 complexes would inhibit autoimmune diabetes specifically without interfering with recognition of other IAg7-presented antigens. To test this hypothesis, we generated a monoclonal antibody (mAb287), which selectively binds to B:9–23 and related variants when presented by IAg7 in R3, but not other registers. The monoclonal antibody blocks binding of IAg7-B:10–23 R3 tetramers to cognate T cells and inhibits T-cell responses to soluble B:9–23 peptides and NOD islets. However, mAb287 has no effect on recognition of other peptides bound to IAg7 or other MHCII molecules. Intervention with mAb287, but not irrelevant isotype matched antibody, at either early or late stages of disease development, significantly delayed diabetes onset by inhibiting infiltration by not only insulin-specific CD4 T cells, but also by CD4 and CD8 T cells of other specificities. We propose that peptide–MHC-specific monoclonal antibodies can modulate autoimmune disease without the pleiotropic effects of nonselective reagents and, thus, could be applicable to the treatment of multiple T-cell mediated autoimmune disorders.
In the nonobese diabetic (NOD) mouse, a spontaneous mouse model of type 1 diabetes mellitus (T1DM), autoimmune targeting of (pro)insulin appears essential for development of disease (1–7). For example, 90 percent of CD4+ insulin-reactive T-cell clones isolated from the islets of prediabetic NOD mice target the insulin β-chain 9–23 peptide (B:9–23) (8, 9), and this peptide is likely the primary epitope recognized by T cells that either induce (6, 7, 9) or prevent T1DM (8–14). Similarly, autoimmunity to insulin is essential for the loss of tolerance to the β-cell antigen islet-specific glucose 6 phosphatase catalytic subunit-related protein (IGRP) (11, 15), but the converse is not true. The NOD mouse expresses a single MHC class II (MHCII) molecule, IAg7, whose presence is essential for the development of T1DM (16–18). We previously suggested that a particular version of IAg7–insulin B:9–23 complex is crucial for the initiation of islet autoimmunity in the NOD mouse (19–22) and that a homologous complex involving the structurally related MHCII molecules human leukocyte antigen DQ8 (HLA-DQ8) and HLA-DQ2 may play a similar role in humans (23–25).
As with other MHCII molecules, the selective binding of peptides to the IAg7 peptide binding groove is governed by interactions between the side chains at particular positions in the peptide (p1, p4, p6, and p9) with those MHCII amino acids forming four corresponding binding pockets within the groove (26, 27). Thus, peptides can potentially bind in more than one position or register within the groove. Previous work from this group had shown that the pathogenic T cells in NOD T1DM recognize the insulin B:9–23 peptide in register 3 (R3), placing the amino acids B:14–22 in the core p1 to p9 position (28) and placing an Arg at the p9 position, highly unfavored for the IAg7 p9 pocket. Furthermore, T cells responding to this register can be divided into two groups, one (type A) for which the Glu at p8 is essential for recognition and another (type B) for which this Glu is inhibitory. For the type A group, the peptide is more optimally presented by truncation to remove the p9Arg or, for the type B group, further truncation to remove the p8Glu as well (29). Mimicking these truncations, we have achieved even better presentation of the two epitopes in R3 by using peptide mimotopes in which the p9Arg is substituted with an optimal p9Glu for the type A or, for the type B group of T cells, by removing the offending p8Glu side chain as well by substituting a p8Gly (28). We have used this strategy to prepare IAg7 with covalently linked versions of these two mimotope peptides, which when incorporated into fluorescent tetramers, detect both types of CD4 T cells in islet infiltrates of prediabetic NOD mice (30).
Given the critical role for IAg7 presentation of the B:9–23 peptide in T1DM development in NOD mice and its unusual presentation to pathogenic T cells, we hypothesized that monoclonal antibodies targeting the IAg7–R3 complexes would modulate the autoimmune response. Consistent with this idea, our previous study demonstrated that immunization of NOD mice with soluble IAg7 monomers having the insulin B:9–23 mimotope covalently bound in R3 elicited specific antibodies to the immunogen and, more importantly, significantly delayed the development of insulitis and the onset of diabetes in these animals (22). We now report that a mAb with this specificity, isolated from one immunized mouse, both inhibits pathogenic T cells in vitro and significantly delays the development of diabetes in vivo.
Results
MAb287 Binds Specifically to Insulin B:9–23 Bound to IAg7 in R3.
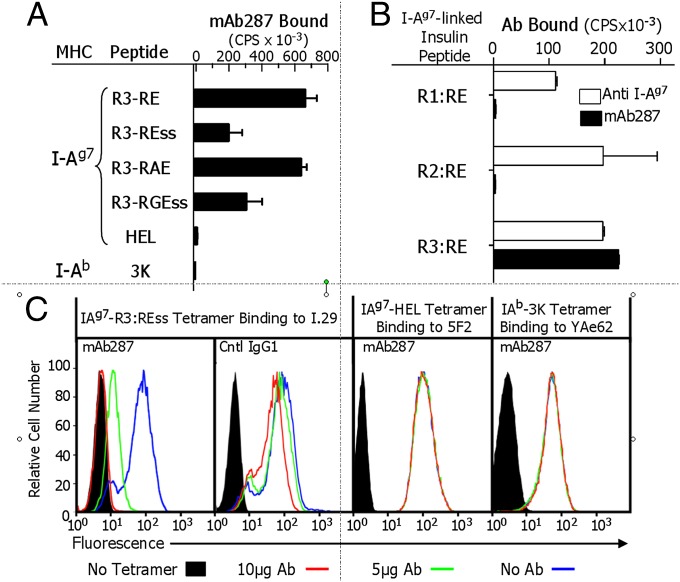
Following immunization with recombinant B:9–23-derived peptides bound to IAg7, all NOD mice developed antibodies recognizing the immunogens. The animal having the highest titer of specific antibodies was selected for hybridoma generation. Of the 1,000 initial wells, a total of 850 showed evidence of growth, with 260 producing detectable IAg7-insulin reactive antibodies. Six of the 32 lines that showed evidence of specificity for IAg7-insulin were cloned by limiting dilution with 3 of the resulting subclones continuing to secrete high levels of specific antibodies. Clone 287 produced the highest levels of antibodies with the desired phenotype, i.e., strong binding to IAg7–R3:RE, but negligible interaction with IAg7–HEL or IAb–3K and was used in all subsequent experiments. Isotype analysis indicated that the monoclonal antibody secreted by this clone (mAb287) is an IgG1.
The binding specificity of mAb287 was retested by using multiple IAg7-insulin variants having the peptide bound in R3, in addition to the two control complexes (peptides used in these studies are listed in Table 1). As shown in Fig. 1A, mAb287 bound to all four IAg7–insulin R3 complexes (RE, REss, RAE, RGEss), albeit with apparently differing affinities, although only two (RAE and REss) were used as immunogens. This cross-reactivity, coupled with the fact that mAb287 did not bind to either the IAg7–HEL or IAb–3K complexes, strongly suggests that the epitope recognized by mAb287 includes the B:9–23 peptide, but is not IAg7 alone or the linker peptide used to tether the insulin peptide to IAg7. Similarly, plate-bound mAb287 selectively captured IAg7–R3:RE, but not IAg7-B:10–23 bound in other registers, R1:RE or R2:RE (Fig. 1B, filled bars), whereas all three complexes were captured by the anti-IAg7,k monoclonal antibody 10–3.62, which recognizes IAg7 regardless of peptide (Fig. 1B, open bars).
Table 1.
Peptides used in these studies
| MHCII | Protein | Peptide | Sequence* Groove position (p) 123456789 | Form (soluble or MHCII-linked) |
| I-Ag7 | Insulin (B:9–23) | Natural | SHLVEALYLVCGERG | Soluble |
| I-Ag7 | Insulin (B:9–23) | R1:RE | HLREALYLVCEERG | Linked |
| I-Ag7 | Insulin (B:9–23) | R2:RE | HLVRALYLVCGERG | Linked |
| I-Ag7 | Insulin (B:9–23) | R3:RE | HLVERLYLVCGEEG | Both |
| I-Ag7 | Insulin (B:9–23) | R3:REss | HLVERLYLVCGEEG-α62† | Linked |
| I-Ag7 | Insulin (B:9–23) | R3:RGE | HLVERLYLVCGGEG | Soluble |
| I-Ag7 | Insulin (B:9–23) | R3:RGEss | HLVERLYLVCGGEG-α62† | Linked |
| I-Ag7 | Insulin (B:9–23) | R3:E | HLVEALYLVCGEEG | Soluble |
| I-Ag7 | Insulin (B:9–23) | R3:RAE | VERLYLVAGEEG | Linked |
| I-Ag7 | Lysozyme | HEL | MKRHGLDNYRGY | Linked |
| I-Ag7 | Chromogranin A | HRPI‡ | HRPIWARMD | Soluble |
| I-Ag7 | Chromogranin A | ChgA‡ | SRLGLWSRMD | Linked |
| I-Ab | MHCII Eα | 3K¶ | ASFEAQKAKANKAVDKA | Linked |
| na | Tetnus toxin | TT | QYIKANSKFIGITE | Soluble |
na, not applicable.
Boldcase indicates differences from the wild type peptide.
Disulfide linked to I-Ag7 (p6Cys to I-Ag7-α62Asn mutated to Cys).
Peptide that mimics the Chromogranin A WE-14 peptide.
Peptide from mouse MHCII Eα mutated at three positions to lysine.
Fig. 1.
mAb287 is specific for IAg7 with insulin peptides bound in R3. (A) ELISA plates were coated with various MHCII–peptide complexes including four different versions of IAg7–R3 complexes and control complexes IAg7–HEL and IAb–3K. Binding of mAb287 to the immobilized complexes was measured as described in Materials and Methods. (B) Binding of biotinylated IAg7–R1:RE, IAg7–R2:RE, or IAg7–R3:RE complexes to immobilized anti-IAg7 monoclonal antibody (clone 10–3.62, open bars) or mAb287 (filled bars) coated plates were measured by ELISA as described in Materials and Methods. (C) The T-cell hybridomas specific for IAg7–R3:REss (I.29), IAg7–HEL (5F2) or IAb–3K (YAe-62.8), were stained with the corresponding fluorescent MHCII-peptide tetramers in the presence or absence of mAb287 or mouse IgG1 and analyzed by flow cytometry. Representative plots of live cells stained in the absence of tetramer (filled profiles), tetramer alone (blue line), or tetramer plus 5 µg (green line) or 10 µg (red line) antibody are shown. Experiments were all repeated at least two more times.
Consistent with these results, mAb287, but not the isotype control antibody, inhibited IAg7–R3:REss tetramer staining of the insulin-specific T-cell hybridoma, I.29, in a dose-dependent fashion, but did not inhibit the binding of a IAg7–HEL or IAb–3K tetramer to T-cell hybridomas specific for these complexes (5F2 and YAe62.8, respectively) (Fig. 1C). In other experiments to confirm specificity, the binding of mAb287 to immobilized soluble IAg7–R3:RE was blocked by preincubation of the mAb with soluble IAg7–R3:RE, but not with IAg7–HEL or IAb–3K (Fig. S1A), and mAb287 was shown to have no affinity for the free unmutated B:9–23 peptide or several of its engineered R3 versions in the absence of IAg7 (Fig. S1B). Finally, in surface plasmon resonance experiments, an Fab version of mAb287 bound to IAg7–R3:RE, but not IAg7–HEL, with a modest affinity (Kd = 130 nM) (Fig. S1C).
MAb287 Inhibits Antigen-Specific T-Cell Responses in Vitro.
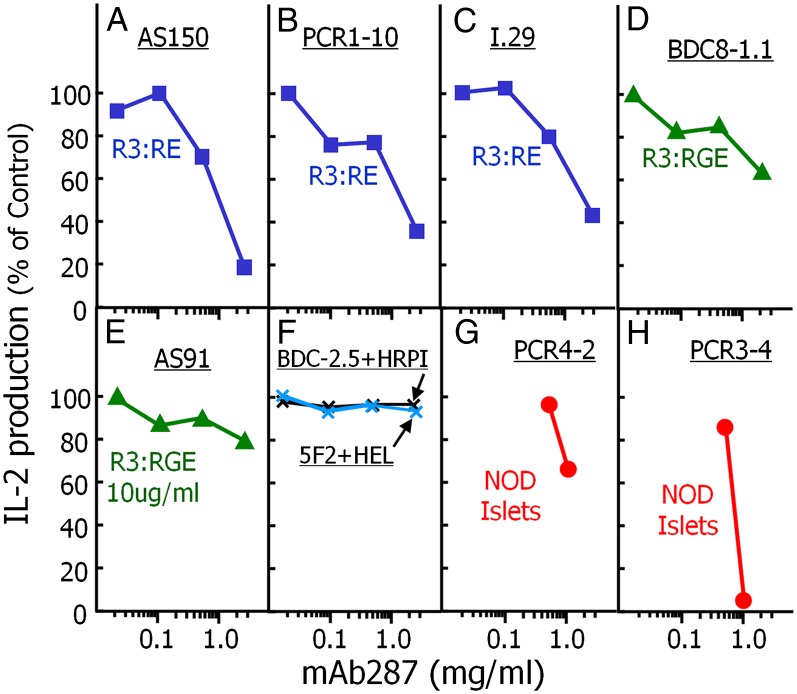
Given the binding specificity of mAb287 described above, we tested whether the antibody could inhibit cytokine production by antigen-specific T cells. Antigen-presenting cells (APCs) were preloaded with either various soluble insulin B:9–23 variants, or a chromogranin A mimotope or HEL peptide, in the presence or absence of various amounts of mAb287 for 2 h. T-cell hybridomas reacting with these peptides were then added, and secreted IL-2 in the supernatant was determined after a subsequent overnight incubation as described in Materials and Methods. As shown in Fig. 2, mAb287 inhibited the IL-2 secretion by both type A (Fig. 2 A–C) and type B (Fig. 2 D and E) insulin B:9–23 reactive T cells (30) in a dose-dependent manner, but had no effect on chromogranin A (ChgA) responsive BDC2.5 T hybridomas and HEL responsive transfectoma, 5F2 (Fig. 2F) (31, 32). We also examined whether mAb287 could inhibit presentation of endogenous B:9–23 by using islets isolated from prediabetic NOD mice as a source of both antigens and APCs (33, 34). Consistent with our expectations, cytokine productions by B:9–23-specific T-cell transfectomas (35) were inhibited by a high dose of mAb287 (Fig. 2 G and H).
Fig. 2.
Specific inhibition of in vitro responses of insulin-specific T-cell hybridomas/transfectomas by mAb287. The ability of mAb287 to inhibit the responses of eight different T cells was assessed in vitro. Type A T cells (56) that prefer Insulin B:21E (blue): AS150 (A); PCR1-10 (B); I.29 (C). Type B T cells (56) that prefer Insulin B:21G (green): AS91 (D); BDC8-1.1 (E). Control T cells: BDC-2.5 specific for ChgA (black) and 5F2 specific for HEL (blue) cells. In A–F, for each T-cell, NOD spleen cells were used as APCs to present a concentration of a peptide known to activate the T-cell sufficient for a response of ∼100 pg/mL of IL-2: R3:RE, 100 µg/mL (A); R3:RE, 5 µg/mL (B and C); R3:RGE, 10 µg/mL (D); R3:RGE, 1 µg/mL (E); ChgA mimotope HRPI and HEL, both 1 µg/mL (E). Other insulin reactive T cells (red): PCR4-2 (type A T cells) (G) and PCR3-4 (type B T cells) (H) were stimulated with 100 NOD islet cells as a source of both natural insulin antigen and APCs. For all 8 T cells, various concentrations of mAb287 were added at the initiation of culture and IL-2 production was assayed at 18 h. Secreted IL-2 was measured as described in Materials and Methods, and the results are shown as the percent of the response remaining compared with the no mAb287 control. The experiment was performed three times with similar results.
MAb287 Delays Spontaneous T1DM.
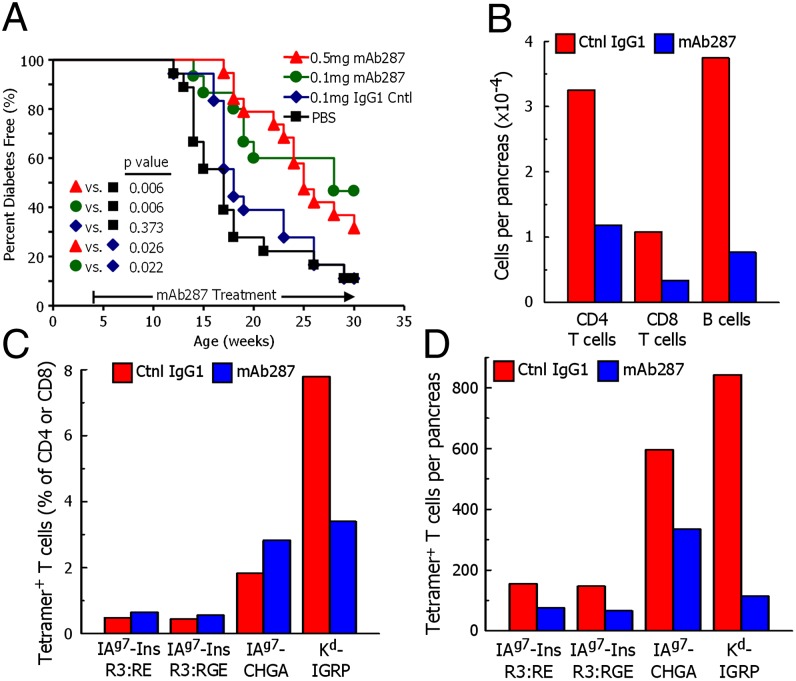
The fact that mAb287 can inhibit the response of B:9–23 reactive T cells suggested that the antibody might also prevent or delay diabetes in vivo. To test this possibility, we treated female NOD mice with mAb287 or control antibodies weekly starting at 4 wk of age. This dosing schedule was determined based on the reported half-life of mouse IgG1 being 6–8 d (36) and, as anticipated, elevated serum levels of mAb287 were maintained over the course of the experiment (Fig. S2). Compared with either mice treated with PBS or the control isotype Ig, the development of diabetes in both low- and high-dose mAb287-treated animals was significantly delayed (Fig. 3A). Both the PBS and isotype-treated groups started to develop diabetes at 12 wk and thereafter progressed at similar rates with 50% being diabetic by ∼17.5 wk of age. In contrast, no mAb287-treated animal developed T1DM before 13 wk of age, 50% of the mice developed diabetes by 25 and 28 wk. Overall there was no statistically significant difference in disease protection between the high- and low-dose mAb287-treated groups, although it should be noted that 7/15 (46.7%) of the animals treated with the low dose remained diabetes-free when the experiment was terminated at 30 wk, compared with only 6/18 (30%) of those treated with the high dose.
Fig. 3.
Monoclonal antibody mAb287 delays diabetes and islet lymphocyte infiltration in NOD mice. (A) Groups of 4-wk-old female NOD mice were treated weekly with PBS (n = 18; black squares), 0.1 mg of mouse IgG1 (n = 18; blue diamonds), 0.1 mg of mAb287 (n = 15; green circles), or 0.5 mg of mAb287 (n = 18; red triangles), and followed up to 30 wk. Diabetes was diagnosed as described in Materials and Methods. The percentages of remaining diabetes free of each group are shown. P values were determined by using χ2 log rank test. (B–D) Groups of eight mice were treated weekly from age 4–11 wk with 0.5 mg of control IgG1 (red bars) or mAb287 (blue bars) at which time pancreatic islets were pooled in each group. A cell suspension was prepared and analyzed by flow cytometry. (B) The average number of live CD4 T cells, CD8 T cells, and 220+ B cells per pancreas were estimated in each sample. (C) The percentages of CD4 T cells specifically binding the IAg7–R3:RE, IAg7-R3:RGE or IAg7-ChgA tetramers, or CD8 T cells specifically binding the Kd-IGRP tetramer were determined as describe in Fig. S6. (D) Average number of islet-infiltrating tetramer-positive cells per pancreas were calculated from the data in B and C. In a second experiment, analysis with the IAg7 tetramers was repeated with similar results by using islets pooled from five mice in each group.
We also looked at the development of serum insulin autoantibody (IAA) in these mice, tested every 2 wk starting at 4 wk of age. Compared with PBS group, both low-dose and high-dose mAb287 inhibited IAAs transiently [at 8, 10, and 12 wk for high dose; 8 and 10 wk for low-dose group (both P < 0.05)] (Fig. S3), but the control antibodies did not.
MAb287 Inhibits the Development of Insulitis by Suppressing T-Cell and B-Cell Infiltration.
Our in vitro experiments demonstrated that mAb287 worked by blocking CD4 T-cell TCR interaction with IAg7-presented insulin, but it was important to determine how the mAb was working in vivo. Were its effects insulin specific or more global in the delay of diabetes? One possibility was that the mAb might be cytotoxic for antigen-presenting cells (B cells, macrophages, DCs) in vivo. This possibility was lessened by the fact that mAb287 is IgG1, a poor IgG isotype in mouse for complement fixation and antibody-dependent cell cytotoxicity, and further diminished by flow cytometric analysis of splenocytes that showed no significant differences in the frequencies of CD19, CD11b, or CD11c-positive cells in the mAb287 versus isotype-treated mice nor was the level of surface IAg7 expression altered in any of these APC classes (Fig. S4). We also looked at other possible global effects. Both the control and mAb287-treated mice exhibited equivalent degrees of autoimmune sialitis (Fig. S5), and no significant changes in weight were observed nor any evidence of inflammation at the injection sites.
The first indication of the mechanism of action of mAb287 was a histological analysis of pancreata from diabetes-protected, 30-wk-old, mAb287-treated mice that showed that the majority of islets were either intact or had only a mild periinsulitis. To examine this change in insulitis more closely, we analyzed the pooled islet infiltrating cells from eight mAb287-treated and eight control antibody-treated mice that had received weekly injection of 0.5-mg antibodies from 4 to 11 wk, a time when control mice were beginning to develop diabetes, but the mAb287-treated mice were not in Fig. 3A. There was a dramatic reduction of lymphocyte islet infiltration in the mAb287-treated mice compared with controls. They contained ∼70% fewer CD4 or CD8 T cells and ∼80% fewer B cells (Fig. 3B). The CD4- and CD8-infiltrating T cells were analyzed with IAg7-insulin and IAg7-chromogranin A tetramers, as well as an Kd-IGRP tetramer (Fig. S6). All of the tetramers identified T cells in the islets of both the mAb287-treated and control mice (Fig. 3C). In the mAb287-treated mice, there was a slight increase in the percentages CD4 T cells binding the IAg7 tetramers and a drop in the number of Kd-IGRP binding CD8 T cells. More significantly, for all four tetramers, there were substantially fewer total tetramer binding T cells per pancreas in the mAb287-treated mice (Fig. 3D). Thus, the mAb treatment resulted in a general loss of all infiltrating cells in the pancreas rather than a specific loss of insulin-reactive T cells.
Administration of MAb287 at a Late Prediabetic Stage Can Delay Progression to Overt Hyperglycemia.
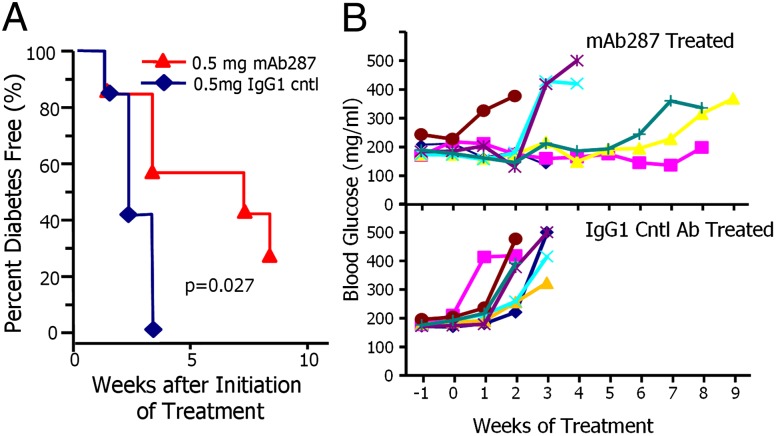
Our data showed that mAb287 could delay diabetes when given early on before any rise in blood glucose levels. To determine whether mAb287 might also be effective at later stages before overt diabetes development, we began administering to mice with a confirmed blood glucose in excess of 170 mg/mL. Beginning at 8 wk of age, blood glucose levels were monitored three times per week with a repeat test the following day if a level ≥170 mg/mL was observed. Animals with two consecutive readings greater than 170 mg/mL were immediately treated with a single injection of 0.5 mg of mAb287 or control antibodies in PBS, and then further injections at weekly intervals until persistent hyperglycemia (blood glucose > 300 mg/dL) was observed or the animals reached 25 wk of age. As shown in Fig. 4A, all mice treated with the isotype control antibodies developed diabetes within 3 wk of the initiation of treatment. In contrast, mAb287 therapy significantly delayed the development of clinical diabetes (P = 0.027), with 4/7 (57.1%) being diabetes free after 3 wk and 2/7 (28.6%) remaining nondiabetic until the termination of the experiment. Individual weekly blood glucose levels for each animal are shown in Fig. 4B. These results show that treatment with mAb287 at this late stage in diabetogenesis was able to halt or delay the progression to frank hyperglycemia in some mice.
Fig. 4.
Treatment of glucose-intolerant prediabetic NOD mice with mAb287 delays progression to clinical diabetes. (A) Groups of 12-wk-old female NOD mice were monitored for glucose intolerance (random blood glucose > 170 mg/mL) then treated weekly with 0.5 mg of mouse IgG1 (n = 7; blue diamonds), or 0.5mg of mAb287 (n = 7; red triangles), until the animals reached 25 wk of age. Diabetes was diagnosed as described in Materials and Methods. (B) Weekly random blood glucose levels of individual mice receiving mAb287 (Upper) or mouse IgG1 (Lower) of mice in A are shown.
Discussion
Our previous work has led us to propose that presentation of the insulin B:9–23 peptide the unfavorable R3 by IAg7 is critical for activating pathogenic T cells and, hence, initiating islet autoimmunity in NOD mice (20, 28, 30). We also have suggested that related complexes may play a similar role in the human disease (23–25, 37, 38). In a previous study, we successfully prevented the development of T1DM in the NOD mice following immunization recombinant with IAg7–R3:RE (22). Here we show that this effect can largely be recapitulated by using a monoclonal antibody selectively recognizing various versions of the IAg7–R3 complex. Only a few other antibodies that selectively recognize defined peptide–MHC class II complexes have been reported (39, 40), but none of these recognize a T-cell ligand involved in an autoimmune disease.
The B:9–23 peptide can bind to IAg7 in at least three distinct registers, with each register characterized by the unique pattern of amino acid side chains that are solvent exposed and, thus, available for T-cell recognition. Our data indicates that the monoclonal antibody mAb287 binds selectively to IAg7-B:9–23 complexes occupying R3, but not in other registers, and not to the free peptide or IAg7 complexes containing noninsulin peptides. Thus, by showing that mAb287 can prevent T1DM in a subset of NOD mice and delay disease onset in others, our results extend two previous seminal observations, namely that insulin B:9–23 is an essential autoantigenic epitope triggering T1DM in NOD mice (11, 15), and secondly, that pathogenic T cells infiltrating islets appear to recognize this peptide when it is presented by IAg7 in R3 (28, 30).
Although treatment with this mAb287 in vivo had no obvious global effects on peripheral lymphoid organs, it caused a diminution in islet lymphocyte infiltration, not only of insulin-specific CD4 T cells, but also of chromogranin A-specific CD4 T cells, IGRP-specific CD8 T cells, and B cells. These results suggest that the mAb works by inhibiting entrance of all types of cells into the islets, consistent with previous studies in which, despite the involvement of pathogenic CD4 and CD8 T cells in the pancreas specific for multiple antigens, a response to insulin B:9–23 is prerequisite for disease in NOD mice (11, 14, 15). There are several possibilities for how this requirement could be imposed. The initial damage by insulin-reactive CD4 T cells in the pancreas may begin the process that attracts these other cells. For example, the reduction in pancreatic B-cell infiltration may indicate an important role for B cells as local APCs for pathogenic CD4 and CD8 T cells in the pancreas. Another possibility is that the pathogenic response to the R3 presented B:9–23 peptide may alter the pathogenic vs. regulatory T-cell balance in the pancreas, allowing T cells of other specificities to break through regulation. In any event, our results offer some encouragement to the idea that suppression of the response to only one major autoimmune epitope may be sufficient to change the course of the disease.
There is increasing appreciation that, in contrast to those of most pathogen-specific T cells, T-cell receptors recognizing autoantigens may often bind unconventional ligands in which the peptides either adopt unfavorable binding registers and/or only partially occupy the peptide binding groove (30, 41). Both mechanisms may be relevant to T1DM, and the R3:RE and R3:RGE mimotopes were designed to mimic the truncated peptides B:9–21 and B:9–20, as previous observations have suggested that these two variants can stimulate distinct subsets of pathogenic B:9–23 T cells (29, 30). Consistent with its expected specificity, mAb287 bound to both and inhibited the response to both of these complexes, but better to R3:RE than the R3:RGE complex, suggesting that the p8E of the R3:RE peptide might be part of the mAb epitope. This differential binding affinity might also, at least in part, explain our observation that even at very high doses mAb287 was unable to fully prevent disease. Improving mAb287’s affinity, in general, and cross-reactivity with the R3:RGE version of the insulin–IAg7 complex by in vitro generation of mAb287 variants could test this idea. We are actively pursuing this approach.
Despite the potential limitations of the current reagent, our results presented here are an encouraging proof of principle that targeting a pathogenic peptide:MHC class II complex is able to interfere with autoimmune disease, even at late stages. Insulin is an autoantigen in human T1DM as well, and there is evidence that T-cell responses to insulin B:9–23 plays a role (42–44). The human MHC class II haplotypes associated with T1DM risk include HLA-DQ8 and HLA-DQ2, which share with IAg7 the polymorphism in the p9 pocket (45–48) that is responsible for the poor binding of the B:9–23 peptide in R3, raising the possibility that this peptide and register might also be involved in the human disease and that monoclonal antibodies with specificities similar to mAb287 may also show benefit in treatment. However, a dominant role for B:9–23 in human disease has not yet been established and the haplotypes also contain HLA-DR4 and HLA-DR3. These alleles can present epitopes from other proteins that have been suggested to be important in T1DM pathogenesis (e.g., the insulin A chain and GAD) (49, 50).
At present, no effective and safe antigen-specific immunologic therapy exists for any autoimmune disease. In contrast to other antibody-based therapies that target molecules common to both pathogenic and protective T cells, our approach is likely to have far fewer side effects because of its inherent specificity and, moreover, if successful, should be applicable to a wide range of autoimmune diseases in addition to T1DM.
Materials and Methods
Animals and Reagents.
Female NOD/LtJ mice were purchased from the Jackson Laboratories and maintained in the University of Colorado Anschutz Medical Campus animal facilities under specific pathogen-free conditions. Animal husbandry and experimental procedures were performed in accordance with protocols approved by the University of Colorado Denver's animal care and use committee.
A series of peptides were used in these studies, either as soluble peptides, obtained at greater than 95% purity from Genemed Synthesis or as peptides covalently linked to IAg7 produced as described (22, 28, 30). They are listed with their sequences in Table 1 with residues that differ from the native protein highlighted in bold. Control unrelated peptides are listed in Table 1 as well. Mouse IgG1 isotype antibodies (catalog no. 554721) and anti-IAg7, k clone 10–3.62 were purchased from BD Biosciences. Multiple T-cell hybridomas and transfectomas, AS91, AS150, I.29 (29), BDC2.5 (51), BDC8-1.1, PCR4-2, PCR3-4, 5F2, and PCR1-10 (35, 52) were maintained as described (35, 53). Tissue culture supplies were from Life Technologies, and all other chemicals from Sigma unless otherwise indicated.
Immunization of NOD Mice.
NOD mice were immunized multiple times with soluble IAg7 bearing various Insulin R3 peptides. Three days after the final immunization, mice showing the highest titer of specific antibodies in its sera as determined by ELISA were euthanized, and a mixture of splenocytes and draining lymph node cells were used for generating B-cell hybridomas (53). Additional details are in SI Materials and Methods.
Hybridoma Generation and Screening.
B-cell hybridomas were produced by fusion of spleen and lymph node cells from the immunized mice to the fusion partner, Sp2/0, followed by growth in 96-well plates and selection in hypoxanthine, aminopterin, thymidine medium (54). Supernatants from the resulting hybridomas were screened by ELISA for antibody specifically binding to IAg7-insulin, but not other MHC peptide combinations mAb287 was selected for further characterization. See SI Materials and Methods for more details.
Binding Assays.
Binding assays were conducted as described (22). Briefly, plates were coated with peptide–MHC complexes, antibodies, or peptides as appropriate and incubated with monoclonal antibodies if necessary. Following extensive washing, biotin-labeled rat anti-(mouse IgG/IgM) or biotinylated peptide–MHC complexes were added, and subsequent binding of Europium-conjugated streptavidin was detected by time-resolved fluorescence.
Flow Cytometry.
I.29 hybridoma T cells (2–10 × 105) (29) were incubated for 2 h at 37 °C in a humidified incubator containing 10% CO2 with IAg7-B:10–23 tetramers (30) (20 μg/mL) and different amounts of mAb287 or mouse IgG1 isotype control antibody (total volume 50 µL). To enhance binding between the TCR and tetramer, 1 μg/mL unlabeled H57-597 Cβ-specific antibodies were also included in the incubation (30). Cells were washed and analyzed by flow cytometry (FACScalibur; BD Biosciences). The staining of 5F2 and YAe-62.8 cells followed the same protocol with IAg7–HEL and IAb–3k tetramers, respectively.
T-Cell Stimulation Assays.
Antigen-presenting cells (NOD splenocytes; 1 × 105) were cultured for 2 h at 37 °C in 100-µL media containing insulin B:9–23 peptides and increasing doses of mAb287 or a mouse IgG1 isotype control. An equal volume of media containing T-cell hybridomas or transfectomas (2 × 106/mL) was then added, and the culture was continued for an additional 16–18 h. Culture supernatants were then harvested, and secreted IL-2 was measured by using a commercial ELISA (BD Biosciences). Alternatively groups of 50 islets from prediabetic NOD mice were cultured for 2 h at 37 °C in 100-µL media containing mAb287 or a mouse IgG1 isotype control without other additions [since islets have sufficient numbers of antigen presenting cells (33)], before addition of T-cell transfectomas.
Antibody Treatment of NOD Mice.
Early intervention.
Female NOD mice (4 wk of age) were randomly assigned to one of four groups: PBS group (n =18), mouse IgG1 group (0.1 mg per injection; n = 18), mAb287 low-dose group (0.1 mg per injection; n = 15) and mAb287 high-dose group (0.5 mg per injection; n = 18). Antibodies dissolved in 100 µL of PBS, or PBS alone, were given weekly to each mouse by i.p. injection. Animal body weights were measured weekly from 4 to 20 wk of age, and every 2 wk thereafter. Serum IAAs were monitored by standard fluid-phased IAA RIA (55) before treatment and every 2 wk thereafter. Blood glucose levels were monitored weekly from 10 wk of age with a ReliOn Ultima blood glucose monitor (Abbott Diabetes Care), and animals were considered diabetic after two consecutive blood glucose values of ≥250 mg/dL. Mice were euthanized either immediately following diagnosis of diabetes or followed to 30 wk of age, and pancreata were harvested for analysis of insulin expression and insulitis by using described methods (22). Nonspecific inflammation was assessed by H&E staining of salivary glands.
Islet infiltrating cells.
Female NOD mice at 4 wk of age received mAb287 (0.5 mg per injection) or equal dose mouse IgG1 (0.5 mg per injection) treatment weekly by i.p. injection. Mice were killed at 11 wk, and islets were hand-picked cultured overnight. All of the cells immigrated from islets were collected for IAg7-insulin tetramer, IAg7-HEL, and IAg7-ChgA tetramer staining with CD8 cells as controls (30), IGRP206–214, and TUM H-2Kd control tetramers staining were also performed following previous description (15). Experiment was repeated with a total of 13 mice in each treatment.
Late intervention.
Blood glucose levels of a group of 14 female NOD mice were tested three times a week starting at 8 wk of age. Once the concentration reached 170 mg/dL, blood glucose was repeated the next day, and those with levels higher than 170 mg/dL in two consecutive tests were randomly assigned to one of two groups that were treated with 0.5 mg of either mAb287 (n = 7) or the mouse IgG1 isotype control (n = 7), respectively. Treatment was initiated immediately and continued weekly as described above. Diagnosis of diabetes used the same criteria previously described, with nondiabetic animals being followed until they had reached 25 wk of age.
Measurement of Binding Affinity.
Fab fragments of mAb287 were generated by papain cleavage and purified by size exclusion and anion exchange chromatography. Biotinylated IAg7–peptide complexes were captured (∼2,000 resonance units) in the flow cells of a BIAcore Streptavidin BIA sensor chip and mAb287 Fab was injected at various concentration for binding affinities determined by surface plasmon resonance (Biacore2000; GE Healthcare Bio-Sciences AB) according to the manufacturer’s instructions.
Statistics.
The values of IAAs were analyzed with the Mann–Whitney test and survival curves with a log-rank test by using PRISM5 software (Graphpad). P values ≤0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. E. Unanue and M. Levisetti (Washington University, St. Louis) for the gifts of monoclonal antibody AIP46.12, and the AS91, AS150, and I.29 T-cell hybridomas, Dr. K. Haskins (National Jewish Health) for the BDC2.5 clone, Dr. P. Santamaria (University of Calgary) for the Kd-IGRP tetramer and Dr. R. Gill for many helpful discussions. K. M. Hutchings, P. Pratt, L. Rook, and P. Londono are thanked for technical assistance. This study was funded by National Institute of Allergy and Infectious Diseases Grant 5U19AI50864, Juvenile Diabetes Research Foundation Grant 10-2011-138, and National Institutes of Health Grants DK032083, DK052068, DK080885, and P30DK057516 in part.
Footnotes
Conflict of interest statement: A patent application has been submitted, but not issued, concerning mAb use in T1D treatment. L.Z., G.S.E., and J.W.K. are listed on this patent.
2Deceased November 13, 2012.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323436111/-/DCSupplemental.
References
- 1.Dardenne M, Lepault F, Bendelac A, Bach JF. Acceleration of the onset of diabetes in NOD mice by thymectomy at weaning. Eur J Immunol. 1989;19(5):889–895. doi: 10.1002/eji.1830190516. [DOI] [PubMed] [Google Scholar]
- 2.Muir A, et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J Clin Invest. 1995;95(2):628–634. doi: 10.1172/JCI117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104(2):189–194. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med. 2003;197(12):1635–1644. doi: 10.1084/jem.20030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thébault-Baumont K, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111(6):851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriyama H, et al. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci USA. 2003;100(18):10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Pagola A, et al. Insulin2 gene (Ins2) transcription by NOD bone marrow-derived cells does not influence autoimmune diabetes development in NOD-Ins2 knockout mice. Scand J Immunol. 2009;70(5):439–446. doi: 10.1111/j.1365-3083.2009.02316.x. [DOI] [PubMed] [Google Scholar]
- 8.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25(4):1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 9.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24(8):1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi M, et al. Conserved T cell receptor alpha-chain induces insulin autoantibodies. Proc Natl Acad Sci USA. 2008;105(29):10090–10094. doi: 10.1073/pnas.0801648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasinski JM, et al. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55(7):1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima K, et al. Combined insulin B:9-23 self-peptide and polyinosinic-polycytidylic acid accelerate insulitis but inhibit development of diabetes by increasing the proportion of CD4+Foxp3+ regulatory T cells in the islets in non-obese diabetic mice. Biochem Biophys Res Commun. 2008;367(4):719–724. doi: 10.1016/j.bbrc.2007.12.191. [DOI] [PubMed] [Google Scholar]
- 14.Prasad S, Kohm AP, McMahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39(4):347–353. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy B, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest. 2006;116(12):3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simone E, et al. T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc Natl Acad Sci USA. 1997;94(6):2518–2521. doi: 10.1073/pnas.94.6.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abiru N, et al. (2001) Peptide and MHC specific breaking of humoral tolerance to native insulin with the B:9-23 peptide in diabetes prone and normal mice. Diabetes 50:1274–1281. [DOI] [PubMed]
- 18.Lieberman SM, DiLorenzo TP. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens. 2003;62(5):359–377. doi: 10.1034/j.1399-0039.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 19.Michels AW, Nakayama M. The anti-insulin trimolecular complex in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):329–334. doi: 10.1097/MED.0b013e32833aba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity. 2010;32(4):446–456. doi: 10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Yi W, et al. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120(4):1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Stadinski BD, Michels A, Kappler JW, Eisenbarth GS. Immunization with an insulin peptide-MHC complex to prevent type 1 diabetes of NOD mice. Diabetes Metab Res Rev. 2011;27(8):784–789. doi: 10.1002/dmrr.1252. [DOI] [PubMed] [Google Scholar]
- 23.Erlich H, et al. Type 1 Diabetes Genetics Consortium HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: Analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aly TA, et al. Genetic prediction of autoimmunity: Initial oligogenic prediction of anti-islet autoimmunity amongst DR3/DR4-DQ8 relatives of patients with type 1A diabetes. J Autoimmun. 2005;25(Suppl):40–45. doi: 10.1016/j.jaut.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Wicker LS, et al. Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun. 2005;25(Suppl):29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Suri A, Unanue ER. The murine diabetogenic class II histocompatibility molecule I-Ag7: Structural and functional properties and specificity of peptide selection. Adv Immunol. 2005;88:235–265. doi: 10.1016/S0065-2776(05)88007-1. [DOI] [PubMed] [Google Scholar]
- 27.Kanagawa O, Shimizu J, Unanue ER. The role of I-Ag7 beta chain in peptide binding and antigen recognition by T cells. Int Immunol. 1997;9(10):1523–1526. doi: 10.1093/intimm/9.10.1523. [DOI] [PubMed] [Google Scholar]
- 28.Stadinski BD, et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107(24):10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178(10):6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 30.Crawford F, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108(40):16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stadinski BD, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11(3):225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delong T, et al. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes. 2012;61(12):3239–3246. doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan JF, Unanue ER. A novel pathway of presentation by class II-MHC molecules involving peptides or denatured proteins important in autoimmunity. Mol Immunol. 2013;55(2):166–168. doi: 10.1016/j.molimm.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan JF, et al. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11(4):350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama M, et al. Germline TRAV5D-4 T-cell receptor sequence targets a primary insulin peptide of NOD mice. Diabetes. 2012;61(4):857–865. doi: 10.2337/db11-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18(2):313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 37.Alleva DG, et al. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol. 2006;63(1):59–69. doi: 10.1111/j.1365-3083.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 38.Thrower SL, et al. Proinsulin peptide immunotherapy in type 1 diabetes: Report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009;155(2):156–165. doi: 10.1111/j.1365-2249.2008.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy DB, et al. Monoclonal antibody detection of a major self peptide. MHC class II complex. J Immunol. 1992;148(11):3483–3491. [PubMed] [Google Scholar]
- 40.Eastman S, et al. A study of complexes of class II invariant chain peptide: Major histocompatibility complex class II molecules using a new complex-specific monoclonal antibody. Eur J Immunol. 1996;26(2):385–393. doi: 10.1002/eji.1830260218. [DOI] [PubMed] [Google Scholar]
- 41.Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med. 2012;2(9):a007765. doi: 10.1101/cshperspect.a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higashide T, et al. T cell epitope mapping study with insulin overlapping peptides using ELISPOT assay in Japanese children and adolescents with type 1 diabetes. Pediatr Res. 2006;59(3):445–450. doi: 10.1203/01.pdr.0000200803.72985.3c. [DOI] [PubMed] [Google Scholar]
- 43.Alleva DG, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107(2):173–180. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassainya Y, et al. Identification of naturally processed HLA-A2—restricted proinsulin epitopes by reverse immunology. Diabetes. 2005;54(7):2053–2059. doi: 10.2337/diabetes.54.7.2053. [DOI] [PubMed] [Google Scholar]
- 45.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 46.Todd JA, Bell JI, McDevitt HO. A molecular basis for genetic susceptibility to insulin-dependent diabetes mellitus. Trends Genet. 1988;4(5):129–134. doi: 10.1016/0168-9525(88)90135-7. [DOI] [PubMed] [Google Scholar]
- 47.Latek RR, et al. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12(6):699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 48.Corper AL, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288(5465):505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 49.Arif S, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113(3):451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109(7):895–903. doi: 10.1172/JCI14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA. 1989;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michels AW, et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol. 2011;187(11):5921–5930. doi: 10.4049/jimmunol.1100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, et al. Analysis of T cell receptor beta chains that combine with dominant conserved TRAV5D-4*04 anti-insulin B:9-23 alpha chains. J Autoimmun. 2009;33(1):42–49. doi: 10.1016/j.jaut.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulman M, Wilde CD, Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 55.Yu L, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: Evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA. 2000;97(4):1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208(12):2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.