Significance
The therapeutic potential of hematopoietic stem cell (HSC)-mediated gene therapy has been demonstrated in clinical trials, and yet the dilemma of the need for high transgene frequency to maximize expression while concurrently minimizing the associated increased risk of oncogenesis remains unresolved. Use of megakaryocytes (MKs)/platelets for transgene expression may take advantage of their rapid turnover and protective storage in platelets and reduce the risk of activating oncogenes in HSC. Here, we demonstrate that the MK/platelet lineage is capable of generating/storing a fully functional lysosomal enzyme and leads to highly efficient, continuous, and versatile transport/distribution of enzyme into the circulation and major diseased organs. This study provides proof of concept of MK/platelet as a depot for efficient production, delivery, and effective distribution of lysosomal enzymes.
Keywords: systemic and on-target delivery, platelet secretion, platelet clearance, lysosomal storage diseases, gene therapy
Abstract
Use of megakaryocytes/platelets for transgene expression may take advantage of their rapid turnover and protective storage in platelets and reduce the risk of activating oncogenes in hematopoietic stem and progenitor cells (HSCs). Here, we show that human megakaryocytic cells could overexpress the lysosomal enzyme, α-l-iduronidase (IDUA), which is deficient in patients with mucopolysaccharidosis type I (MPS I). Upon megakaryocytic differentiation, the amount of released enzyme increased rapidly and steadily by 30-fold. Using a murine MPS I model, we demonstrated that megakaryocyte/platelets were capable of producing, packaging, and storing large amounts of IDUA with proper catalytic activity, lysosomal trafficking, and receptor-mediated uptake. IDUA can be released directly into extracellular space or within microparticles during megakaryocyte maturation or platelet activation, while retaining the capacity for cross-correction in patient’s cells. Gene transfer into 1.7% of HSCs led to long-term normalization of plasma IDUA and preferential distribution of enzyme in liver and spleen with complete metabolic correction in MPS I mice. Detection of GFP (coexpressed with IDUA) in Kupffer cells and hepatocytes suggested liver delivery of platelet-derived IDUA possibly via the clearance pathway for senile platelets. These findings provide proof of concept that cells from megakaryocytic lineage and platelets are capable of generating and storing fully functional lysosomal enzymes and can also lead to efficient delivery of both the enzymes released into the circulation and those protected within platelets/microparticles. This study opens a door for use of the megakaryocytes/platelets as a depot for efficient production, delivery, and effective tissue distribution of lysosomal enzymes.
The potential of therapeutic benefits from genetically modified hematopoietic stem cells (HSCs) has been supported in recent gene therapy clinical trials (1, 2). High transgene dosage or selective growth of genetically corrected HSCs appears to be necessary for achieving clinical efficacy. However, genotoxic risk caused by proviral integration-associated oncogenesis is directly concomitant with high numbers of integration events or clonal expansion (3, 4). New approaches are needed to balance the need for high transgene frequency while limiting the associated increased risk of oncogenesis.
Platelets are anuclear, secretory particulate entities containing proteins stored in cytoplasmic granules that can be released upon activation (5). Healthy adults produce 2–5 × 1011 platelets daily with a baseline activation rate of 1–5% (6). Use of megakaryocytes (MKs)/platelets for transgene expression may (i) take advantage of this immense cell mass and its rapid turnover (5–9 d); (ii) provide protective storage of the transgene product, which is essential for proteins sensitive to plasma pH; and (iii) continuously dispense proteins via degranulation from platelet activation at baseline (without detectable injury) and/or at sites of vascular injury. Highly efficient protein production and delivery could further reduce the need for high transgene frequency and the risk of activating oncogenes in HSCs and all their progeny. Although using platelets as a delivery system has been demonstrated for the expression of coagulation factors to treat inherited bleeding disorders in mice (7, 8), there has been no report of the feasibility of using MKs/platelets for the generation of nonhematologic proteins.
Lysosomal storage diseases (LSDs) are a group of inherited disorders, often affecting multiple organs including the liver and spleen, with a cumulative incidence of 1 in 5,000–7,000 live births (9). Overexpressing lysosomal enzymes in platelets not only can provide the protection of pH-sensitive enzymes and continuous enzyme release via low physiological levels of platelet activation but may also offer the benefit of on-target delivery of platelet-derived enzymes to spleen and liver in the process of platelet clearance (10). However, maintaining proper posttranslational modifications for appropriate lysosomal trafficking and intercellular lysosomal enzyme transfer is essential for metabolic cross correction in treating these multiorgan diseases (11). It is not known whether lysosomal enzymes generated from the MK/platelet lineage would be fully functional and capable of correcting lysosomal deficits in diseased cells.
In this study, we used a mouse model of Hurler syndrome, which is the severe form of mucopolysaccharidosis type I (MPS I), one of most common LSDs. It is caused by the deficiency of α-l-iduronidase (IDUA) and consequent accumulation of glycosaminoglycans (GAGs) (12, 13). We show that MKs are capable of producing large amounts of IDUA with proper catalytic function, lysosomal trafficking and receptor-mediated uptake, which could be sorted to and stored within platelets. The IDUA can be released directly into the extracellular space or within microparticles (MPs) during MK maturation or platelet activation, while retaining its ability to cross-correct cells derived from patients with MPS I.
Results
Coexpression of GFP and the Release of IDUA Increase During ex Vivo Megakaryocytic Maturation in Human DAMI Cells.
To determine whether cells from megakaryocytic lineage could produce and release lysosomal IDUA, we transduced cells from the human megakaryocytic cell line (DAMI) with a lentiviral vector (LV) that coexpressed eGFP and IDUA bicistronically using a hybrid human ankyrin-1 promoter (IHK) (i.e., LV-KIiG) (Fig. 1). This promoter has been reported to induce transgene overexpression in erythroid cells but not in myeloid or lymphoid cells (14, 15). We found that the IHK promoter could also generate strong GFP expression, as well as robust intracellular and extracellular IDUA activity, in uninduced DAMI-KIiG cells (i.e., 517- and 127-fold above background levels in DAMI cells). During inductive culture with phorbol 12-myristate 13-acetate (PMA), ex vivo megakaryocytic maturation of DAMI cells was verified by increasing numbers of polyploid cells and enlargement in cell size of most cells (Fig. S1). Concomitant with maturation, GFP expression rose steadily as demonstrated by the increase of mean fluorescent intensity with a pattern similar to that of CD61, a maturation marker in MKs (Fig. 1A). Moreover, extracellular IDUA levels increased by 30-fold, while intracellular IDUA levels remained unchanged (Fig. 1B). These results demonstrate that human MKs are capable of overproducing and releasing lysosomal IDUA during maturation.
Fig. 1.
Human megakaryocytic DAMI cells express both GFP and lysosomal IDUA transgenes with increasing release of IDUA during ex vivo maturation. (A) Increasing expression of GFP in stably transduced DAMI-KIiG cells is shown by representative FACS plots stained with CD61-PE (Left) and by quantitative analysis of MFI levels (Right) before and after inductive culture with 50 nM PMA. Transduction efficiency is 94% in DAMI-KIiG cells. (B) Intracellular IDUA expression (solid lines) is elevated with increase in extracellular IDUA release (dotted lines) during in vitro megakaryocytic maturation. Data were derived from two experiments in duplicate wells and expressed as means ± SD.
Lysosomal IDUA Generated in Megakaryocytes Can Be Packaged for Storage in Platelets of Mice Without Perturbation of Platelet Function.
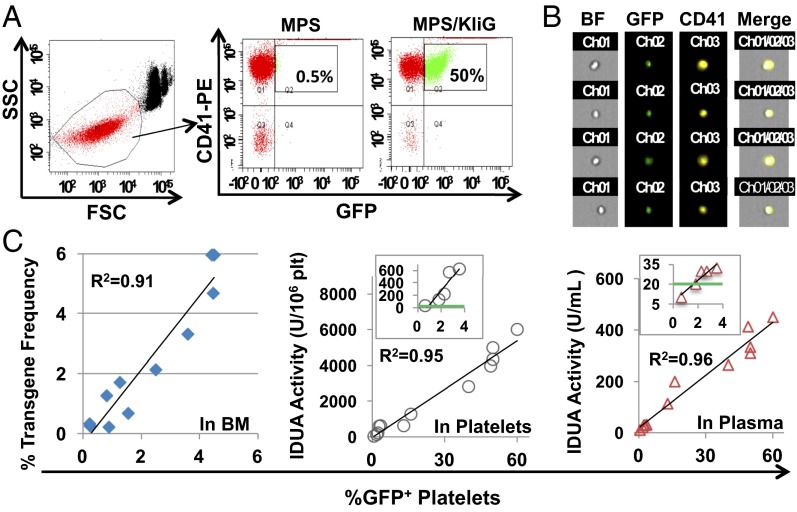
To determine whether MK-derived transgene products can be sorted and stored in platelets, we evaluated GFP and IDUA levels in platelets from MPS I mice 5–6 mo after transplantation of lineage-negative (Lin−) enzyme-deficient cells transduced with LV-KIiG, as well as from 2° bone marrow (BM) transplantation recipients with various gene dosages (Fig. 2). In vivo transgene expression in megakaryocytic lineage was demonstrated by significant percentages of GFP+ platelets using FACS assay and by views from ImageStream analysis (Fig. 2 A and B). Moreover, transgene dosages in BM of 2° MPS/KIiG mice, as determined by real-time quantitative (q) PCR, were linearly correlated with the percentages of GFP+ platelets (r2 = 0.91) at low transgene frequency (<6%) (Fig. 2C). The intraplatelet IDUA activities and plasma IDUA levels in MPS I mice were also directly associated with frequency of GFP+ platelets (r2 = 0.95 or 0.96). Notably, 1% gene transfer efficiency was sufficient to bring intraplatelet IDUA levels comparable to those found in wild-type (WT) platelets. Our results demonstrate that IDUA can be produced at extremely high levels in megakaryocytic progeny and stored in platelets with proper processing and catalytic function.
Fig. 2.
In vivo expression of both GFP and lysosomal IDUA in platelets of MPS I chimeras after transduction with erythroid/MK-specific LV. Lin− cells from MPS I mice were transduced with LV-KIiG and transplanted into lethally irradiated MPS I mice (MPS/KIiG). MPS/KIiG mice with various gene dosages were generated by 2° transplantation using a serial dilution of BM from 1° MPS/KIiG with MPS marrow 5 mo after BM transplantation. (A) Representative GFP expression in platelets is shown by FACS plots after staining with CD41-PE antibody. FSC, forward light scatter; SSC, side light scatter. (B) Representative GFP+ platelets are viewed by ImageStream analysis. (C) Correlation of GFP expression in platelets with gene transfer efficiency (GT) in BM cells as determined by real-time qPCR, IDUA enzyme activities in isolated platelets and in plasma from MPS/KIiG mice. The Insets show IDUA levels in mice at ≤4% GT, with green lines indicating WT levels. Each symbol represents data from an individual animal.
We also evaluated whether expression of IDUA transgene in MK/platelet lineage may have any effect on platelet formation or function in MPS/KIiG mice, in comparison with age-matched normal or untreated MPS I mice (Fig. S2). No significant difference was observed among these three groups for platelet counts of fresh whole blood, baseline status of platelet activation (percentage of Annexin V+ platelets), or stop time of tail bleeding. These data suggest that overexpression of lysosomal IDUA would not perturb platelet number, activation status, or function in vivo.
Platelet IDUA Can Be Released and Endocytosed for Correction of Lysosomal Deficit in Patients’ Cells.
Only a small portion of proteins identified in platelets by recent proteomic profiling are found in platelet releasate upon stimulation with different agonists (16–18). To determine whether transgene-derived platelet IDUA could be released, calcium-induced platelet activation was conducted, resulting in 94% activation efficiency measured by FACS analysis with Annexin V staining (Fig. 3A). The catalytically active IDUA levels in the releasate were linearly correlated with intraplatelet IDUA levels (r2 = 0.98), suggesting that functional IDUA could be released from platelets upon activation (Fig. 3B).
Fig. 3.
Platelet-derived IDUA can be released with intact functionality and capability to correct deficit in cells from MPS I patient. Freshly isolated platelets were resuspended in IDUA-negative platelet-free plasma isolated from MPS I mice, followed by incubation with 10 µg/mL calcium ionophore or buffer. (A) Platelet-activation status was determined by staining with CD41-PE and Annexin V–APC and is shown by representative FACS plots (Left). Data from all activation samples (n = 13) are summarized in the graph (Right). (B) Correlation of IDUA released by platelet activation with intraplatelet IDUA activities. The Inset highlights the correlation within low range of activities. The red circle indicates corresponding IDUA levels in platelets from WT mice. (C) M6P receptor-mediated uptake of platelet released IDUA in LCLmps upon culturing for 3 h in the presence or absence of M6P inhibitor. Data were derived from two independent experiments in duplicate wells. (D) Normalization of abnormal lysosomal morphology in LCLmps by platelet-released IDUA (40–50 U/mL). Representative morphological images from cytospin slides are shown following immunofluorescence staining with LysoTracker for lysosomes (red) and DAPI for nuclei (blue).
The mannose-6-phosphate (M6P) receptor system plays a key role in intracellular trafficking and intercellular exchange of lysosomal enzymes that require appropriate posttranslational modification (13). To determine whether platelet-released IDUA is properly processed for M6P receptor-mediated endocytosis, we performed a competitive-uptake assay using lymphoblastoid cells derived from an MPS I patient (LCLmps) (Fig. 3C). When exposed to increasing amounts of platelet-released IDUA, LCLmps exhibited a steady increase of intracellular IDUA toward a saturation plateau. This uptake process was inhibited by the presence of M6P inhibitor. These data demonstrate that platelet-released IDUA retains its intercellular trafficking capability and is suitable for uptake by cells via M6P receptor-mediated endocytosis.
To determine the functional integrity of platelet-released IDUA, we evaluated lysosomal morphology of lymphoblastoid cells by immunostaining with LysoTracker, a fluorescent dye that can be endocytosed into lysosomes (Fig. 3D). Unlike LCL from a healthy individual (LCLwt), untreated patient’s cells contained more lysosomes (i.e., stronger fluorescent intensity), and these compartments appeared smaller in size as suggested by more uniform staining. The majority of cells exposed to platelet-released IDUA exhibited a normalized lysosomal pattern that was comparable to normal LCLwt. This finding indicates that platelet-derived IDUA can correct defective lysosomal morphology in MPS I patient cells.
The IDUA Overexpressed in Megakaryocytes Can Be Delivered Within MK/Platelet-Derived MPs.
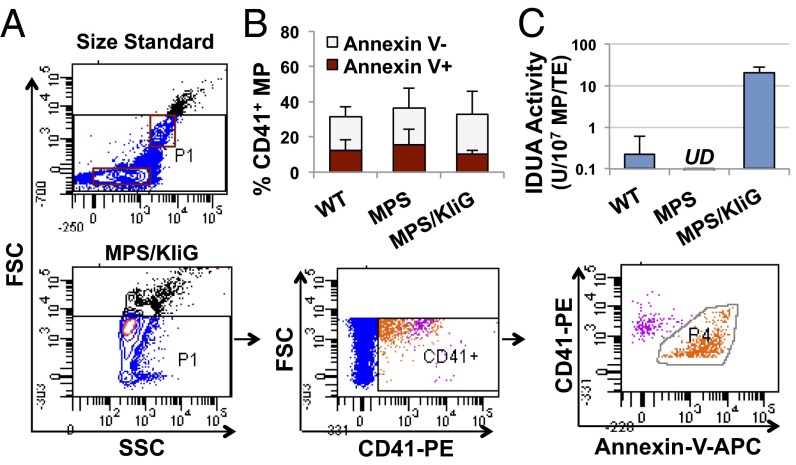
MKs have recently been shown to contribute to the generation of membrane-shed, submicron (<1 µm) microparticles in the blood stream (19, 20), in addition to the former view that most of the MPs in plasma are derived from activated platelets (21). To obtain insight into the pathway of MK-derived IDUA delivery, we isolated MPs from whole blood freshly collected by retroorbital venous plexus sampling (Fig. 4). Guided by the standard beads for appropriate FACS gating, submicron-sized MPs were analyzed by costaining with CD41 for MK/platelet origin and Annexin V as an activation marker (Fig. 4A). The portion of MK/platelet-derived MPs (mpMPs) was a mean of 33% in MPS/KIiG mice, which was comparable to that in age-matched WT (31%) or MPS I (36%) mice (Fig. 4B). Interestingly, more than half of mpMPs were negative for Annexin V in MPS/KIiG mice (68%), which was similar to that observed in WT (61%) or in untreated MPS I (58%) mice. Importantly, robust IDUA levels were detected in blood MPs from MPS/KIiG mice that were 91-fold higher than those detected in MPs from WT mice (Fig. 4C). These results suggest that mpMPs can be generated not only from platelet activation (as Annexin V+ mpMPs) but also from other processes (such as MK maturation or platelet formation) and that IDUA derived from MK overexpression can be stored and delivered within mpMPs.
Fig. 4.
Robust IDUA levels were found in mpMPs that heterogeneously expressed Annexin V. (A) Representative FACS plots using MPs isolated from fresh whole blood after staining with CD41-PE and Annexin V–APC. The FSC and SSC characteristics of standard beads (0.5 and 1 µm) (Upper) were used for MP gating (Left and Center of the lower row). (B) Percentage of CD41+ MPs in isolates with Annexin V status. Data were derived from three independent assays (error bars: SD). (C) IDUA activity in isolated MPs (n = 7−10). UD, undetectable.
Platelets Provide Preferential Distribution of IDUA to the Liver and Spleen and Complete Metabolic Normalization.
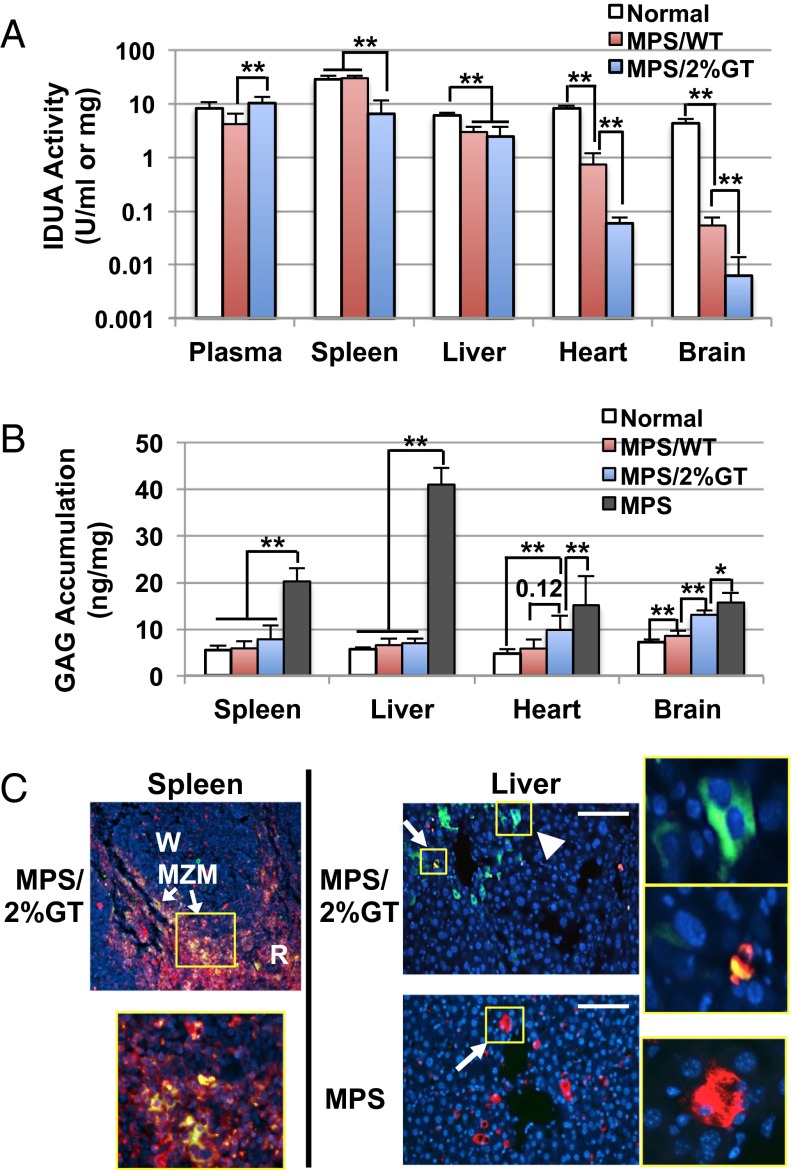
To assess the biodistribution of IDUA generated and delivered via MK/platelet lineage, we determined IDUA levels in plasma, spleen, and nonhematopoietic organs including heart and liver in MPS I mice with 1.7 ± 1.1% of HSC gene transfer (MPS/2%GT), a level attainable in most of current gene therapy clinical trials (Fig. S3). Compared with normal heterozygous mice, MPS/2%GT mice exhibited higher or similar IDUA levels in plasma that were 2.5-fold higher than MPS I mice with full engraftment of WT HSCs (MPS/WT) (Fig. 5A). In the spleen (a hematopoietic organ), MPS/2%GT mice showed 23% of normal IDUA levels, whereas normalized enzyme levels were found in MPS/WT. The livers of both MPS/2%GT and MPS/WT mice contained similar IDUA levels, which were ∼45% of those in normal mice. The heart of MPS/2%GT mice contained minimal IDUA levels (≤1% of normal), which were also significantly less than those in MPS/WT (9% of normal). Detectable levels of IDUA were observed in brain of MPS/2%GT mice, which were significantly less than those in MPS/WT group (1.3% of normal). These results demonstrated that the erythroid/MK-derived IDUA was distributed preferentially to the circulation, as well as to the spleen and liver, with significantly less to other nonhematopoietic organs, such as the heart.
Fig. 5.
Preferential distribution of platelet-derived IDUA in liver and spleen was associated with clearance of senile platelets. Five months after transplantation, organs were collected from MPS I mice that were transplanted with WT marrow (MPS/WT) or MPS/KIiG marrow with 1.7 ± 1.1% transgene frequency (MPS/2%GT). (A) Enzyme activities in plasma and organs of perfused animals (n = 5–7 per group). **P < 0.001. (B) GAG levels in spleen, liver, heart, and brain (n = 5–7 per group). **P < 0.01; *P < 0.05. (C) Representative images from immune-fluorescence staining of liver and spleen sections. Samples were stained with antibodies against GFP (green) and CD68 (red) and counterstained with DAPI for nuclei (blue). Areas within yellow Insets are amplified to show GFP-containing hepatocytes (arrowhead), Kupffer cells (arrow), or spleen macrophages. MZM, marginal zone macrophages; R, red pulp; W, white pulp. (Scale bars, 100 μm.)
We further evaluated the therapeutic potential of MK/platelet-derived IDUA by analyzing GAG levels in spleen, liver, heart, and brain of MPS I mice 5–6 mo after transplantation (Fig. 5B). The elevated GAG levels in spleen (3.7-fold of normal levels) or liver (7.4-fold of normal) of untreated MPS I mice were completely normalized to the normal heterozygous levels in the MPS/2%GT mice and MPS/WT mice. The GAG levels in heart of MPS/2%GT mice (8.2 ± 1.0 ng/mg protein) were significantly reduced (P = 0.0037) from untreated heart (16.6 ± 5.2) even through only less than 1% of IDUA activities were found in these mice. These GAG levels were not significantly different from those detected in MPS/WT (P = 0.124) but were also not normalized to nondiseased carrier mice (4.6 ± 1.0). Interestingly, GAG accumulation in brain of MPS/2%GT mice (13.0 ± 1.0 ng/mg protein) were significantly reduced from untreated brain (15.7 ± 1.9), albeit only residual IDUA activities were detected. This observation is consistent with the previous report that supraphysiological levels of IDUA in blood stream could lead to normalization of brain GAG and significant improvement of brain pathology and behavioral deficits in MPS I mice (15), although the precise mechanism of CNS entry of plasma IDUA is to be determined.
To determine whether the clearance of IDUA-containing platelets contributes to the preferential distribution, immunofluorescent analysis was conducted using frozen sections from spleen and liver of MPS/2%GT mice costained with anti-CD68 for tissue macrophages and anti-GFP for producer tracking (as GFP cannot be released) (Fig. 5C). Strong colocalization of GFP+ and CD68+ signals was observed around the marginal zone and red pulp of the spleen. This observation is consistent with the concept that the clearance of senile platelets is accomplished via antibody-mediated phagocytosis by spleen macrophages (22). In MPS/2%GT mice, GFP+/CD68+ Kupffer cells, as well as cubically shaped, GFP+ hepatocytes were detected, suggesting the likely involvement of both Kupffer cells and hepatocytes in the clearance of IDUA-containing platelets. Interestingly, the numerous abnormally enlarged CD68+ Kupffer cells typically found in untreated MPS I disappeared in the liver of MPS/2%GT mice, a sign of correction of disease-associated pathology.
Discussion
We have demonstrated a unique and somewhat surprising delivery pathway for lysosomal enzymes that leads to highly efficient, continuous, and versatile (discretely or membrane-protected) transport/distribution of enzyme into the circulation and major diseased organs. We show that human megakaryocytic DAMI cells could overexpress a lysosomal enzyme and, upon in vitro megakaryocytic differentiation, release increasing amounts of enzyme rapidly and steadily (up to 30-fold) above those from unstimulated cells. Using a murine MPS I model, we verified in vivo that lysosomal IDUA could be generated at high levels (WT intraplatelet IDUA was achieved in mice with 1% gene transfer efficiency) in megakaryocytic progeny and stored with proper processing and catalytic function in platelets and MPs. The intraplatelet IDUA could be released upon platelet activation with full functional proficiency to cross-correct deficits in patient’s cells. Moreover, normalized plasma IDUA and robust therapeutic benefits were achieved in liver and spleen of MPS I mice with only 1.7% HSC gene transfer, comparable to those found in MPS I mice fully engrafted with WT HSCs. These findings provide proof of concept that the MK and platelet lineage is capable of generating and storing fully functional lysosomal enzyme and also can lead to efficient use of all enzymes generated, including the soluble enzymes released into the circulation and also enzymes protected/immobilized within mpMPs or platelets.
It is only in recent years that lysosomes have been considered one of the three granule types normally present in platelets. However, their numbers are few, and their function is not well-studied (23). Additionally, among more than 1,500 platelet proteins identified by proteomic profiling (18), less than 20% are found in platelet releasate upon stimulation with different agonists (16, 17). Moreover, preservation of appropriate pH in lysosomes and other cellular vesicles is important for cargo release and lysosomal hydrolase maturation (9, 24). Among a short list of lysosomal enzymes found in platelets, β-hexosaminidase is one of the few that have been reported to be released in vitro or “in vivo” at localized sites of vessel wall damage (25, 26). In the present study, we have demonstrated that not only endogenous IDUA enzyme is detectable in normal mouse platelets but also transgene-derived, overexpressed IDUA can be stored in platelets and retain normal function. The intraplatelet IDUA can be released upon activation and mediate correction of lysosomal defects in cells from patients or MPS I mice. These results support the suitability of platelets for storage, delivery, and discharge of lysosomal enzymes while conserving their corrective functions.
The IDUA derived from MK/platelet lineage is not only fully functional catalytically but also highly effective (corrective) with on-target delivery to affected cells in spleen and liver. The IDUA in nonhematopoietic organs of MPS/WT were derived from two sources, resident macrophages/monocytes and infiltrated lymphocytes of donor origin and uptake of IDUA from the circulation (27). Comparing to MPS/WT, the first source of IDUA is absent in both heart and liver of MPS/2%GT mice because of lineage restriction. However, much better enzyme distribution was found in liver than in heart (compared with MPS/WT), although they are both nonhematopoietic organs. That this phenomenon may, in part, be attributable to the involvement of liver in the clearance of senile, transduced platelets is suggested by the detection of GFP+ Kupffer cells and GFP+ hepatocytes throughout the liver in MPS/2%GT mice. Although antibody-mediated clearance by spleen macrophages is a well-known clearance mechanism for senile platelets (22), glycan-lectin–mediated clearance by Kupffer cells and hepatocytes in liver has recently been recognized to participate in the clearance of transfused platelets (28, 29). Our data indicate that the clearance of transgene-overexpressing platelets (GFP+ platelets) from the circulation is mediated not only by macrophages in spleen and liver but also by hepatocytes. These observations highlight the advantage of using MK lineage for highly efficient systemic protein production and preferred protein distribution in spleen and liver, with subsequent therapeutic benefits.
The MK/platelet lineage can provide multiple forms of lysosomal enzymes, including soluble/discrete enzyme, MP-bound enzyme, and intraplatelet enzyme. Importantly, these enzymes are distributed systemically via the circulation and also locally (into liver and spleen) in multiple steps during MK/platelet “life cycle,” as summarized in Fig. 6.
Fig. 6.
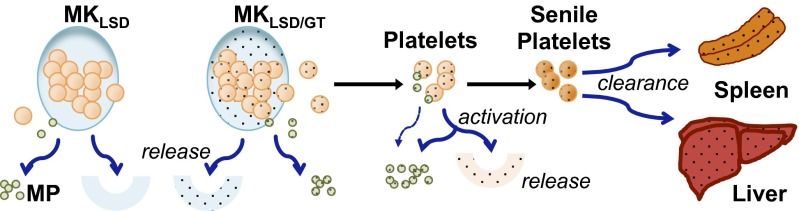
Scheme showing the delivery pathway for use and distribution of MK-derived lysosomal protein. MKLSD, enzyme-deficient MKs of LSD patient; MKLSD/GT, transduced MKLSD cells that overexpressing therapeutic lysosomal protein; MP, microparticles.
All three forms of enzyme can contribute to the correction of disease as follows. First, during MK maturation, enzyme overproduced in genetically corrected autologous MKs (MKLSD/GT) can be released directly into extracellular space (and then blood stream). This is consistent with the DAMI-KIiG data demonstrating that human MKs were capable of overproducing and releasing IDUA during in vitro maturation. Secondly, the MK-derived enzyme will also be packaged and stored in MPs and/or platelets and circulate in the blood as MP- or platelet-associated enzyme sources. This is supported by the observations that less than half of mpMPs (CD41+) was derived from platelet activation (Annexin V+) and that resting platelets from MPS/GT mice contained robust levels of IDUA activities. Thirdly, circulating platelets will release MPs and dispense functional enzymes via degranulation from baseline and/or injury site activation at a rate of 1–5% in human (6). Finally, the clearance of senile platelets is mediated by the mononuclear phagocyte system that primarily resides in spleen and lymph nodes, as well as Kupffer cells of the liver (30). For many LSDs, the delivery of MK-derived enzyme to this system presents the therapeutic enzyme directly to the important target tissues of involvement (e.g., the spleen, liver, and BM). The enzyme released into the blood stream will be taken by other cells via M6P receptor-mediated endocytosis. We have shown cross-correction of cells derived from a patient with MPS I by platelet-released IDUA. This corrective potency is consistent with previous reports that human platelets were the richest source of corrective “high uptake” (M6P-enriched) β-glucuronidase (deficient in MPS type VII) among all human tissues tested (31–33). Taken together, these results show that MK-derived lysosomal enzyme can be delivered/distributed continuously by multiple routes and effectively benefit multiple organs, especially the BM, spleen, and liver.
It has been reported that LV-mediated HSC gene therapy using an ubiquitous cellular promoter with gene transfer efficiency of ∼15 copies per HSC completely corrected CNS disease manifestations and peripheral organ abnormalities in MPS I mice, and the IDUA from overexpressing brain macrophages/microglial cells was essential for the observed CNS effects (34). Moreover, a recent HSC gene therapy clinical study for metachromatic leukodystrophy disease (a neurological LSD) has shown that a clear arrest of CNS disease progression could be achieved in patients with vector copy number (VCN) in various cell populations ranging from 2.5 to 4.4 VCN per genome (35). These studies demonstrate that enzyme dosage dictated by high gene transfer efficiency is essential for therapeutic efficacy; however, multiple independent integrations of the vector in each HSC genome will also increase the theoretical risk of insertional mutagenesis (36). Using transgene expression in MK/platelet lineage, which can be combined with other lineages such as maturing red blood cells (15) and/or monocytes/macrophages (37), may help to tackle genotoxicity associated with proviral insertion during HSC-mediated gene therapy in several ways. First, lineage-restricted transgene expression may lessen its susceptibility to gene silencing, which is often found in HSCs and progenitors, leading to sustained protein production. Secondly, the high-level, efficient protein generation can be achieved from a relatively low vector copy number by exploiting the enormous cell mass and quick turnover. Thirdly, therapeutic benefits can be maximized by versatile enzyme transport and on-target protein delivery. Together, the risk of insertional oncogenesis can be reduced by achieving sufficient protein production/delivery/distribution with lower copy number insertions, thereby having less chance of activating oncogenes in HSCs and their progeny in all lineages.
In summary, we have provided a proof of principle study in developing a unique depot (the MK/platelet lineage) for efficient production, protective delivery, and effective distribution of lysosomal enzymes. This production/delivery pathway opens the door for new therapeutic strategies, using MK/platelet lineage alone or combined with other blood cell lineages, for optimization of transgene expression/disease treatment and for reduction of the risk of oncogenesis. These findings warrant further evaluation of this approach in treating other lysosomal storage diseases where protection of pH-sensitive enzyme in platelets is desirable that may lead to continuous delivery of enzyme with on-target protein distribution and favorable therapeutic benefits.
Materials and Methods
SI Materials and Methods include additional information on experimental methods.
LV.
The LV-KIiG coexpressing human IDUA cDNA and eGFP bicistronically under a hybrid human ankyrin-1 promoter was constructed, packaged, and concentrated using the previously reported 4-plasmids system (15, 38).
HSC Isolation, Transduction, and Transplantation.
To isolate HSCs, low-density BM cells were stained with the Biotin Mouse Lineage Panel Kit (BD Pharmingen), followed by lineage depletion using anti-biotin microbead-mediated LS column (Miltenyi Biotech). Lin− cells were cultured for a 12-h prestimulation period in serum-free StemSpan medium (StemCell Technologies) supplemented with 100 ng/mL hFlt3, 100 ng/mL mouse stem cell factors, 100 ng/mL hIL-11, and 20 ng/mL mIL-3. Cells were then transduced twice within 24 h with LV-KIiG, followed by transplantation into lethally irradiated mice at 1–2 × 105 cells per mouse. All animal procedures were approved by Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital Medical Center.
IDUA Activity Assay.
The catalytic activity of IDUA was measured as previously reported (15, 39). One unit of enzyme activity is defined as the release of 1 nmol of 4-methylumbelliferyl in a 1-h reaction at 37 °C, and IDUA-specific activity was calculated as unit per milligram of protein or per milliliter of liquid.
Statistical Analysis.
Quantitative assays were performed in duplicate or triplicate from at least two individual experiments. Data are presented as means ± SD, unless otherwise specified. Comparisons between two groups were performed using two-tailed Student t tests, unless otherwise specified. P values of less than 0.05 were considered as statistical significance.
Supplementary Material
Acknowledgments
We thank Phuong Cao, Meghan Bromwell, Mara Kohls, and the Comprehensive Mouse Core for technical assistance. This work was supported by National Institutes of Health Grants NS064330, NS086134, and U54 HL06-008.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323155111/-/DCSupplemental.
References
- 1.Verma IM. Medicine. Gene therapy that works. Science. 2013;341(6148):853–855. doi: 10.1126/science.1242551. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee S, Thrasher AJ. Gene therapy for PIDs: Progress, pitfalls and prospects. Gene. 2013;525(2):174–181. doi: 10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum C, Modlich U, Göhring G, Schlegelberger B. Concise review: Managing genotoxicity in the therapeutic modification of stem cells. Stem Cells. 2011;29(10):1479–1484. doi: 10.1002/stem.716. [DOI] [PubMed] [Google Scholar]
- 4.Rivière I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119(5):1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, et al. Factor IX ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia B mice. Blood. 2010;116(8):1235–1243. doi: 10.1182/blood-2009-11-255612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, et al. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112(7):2713–2721. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz ML, Tecedor L, Chang M, Davidson BL. Clarifying lysosomal storage diseases. Trends Neurosci. 2011;34(8):401–410. doi: 10.1016/j.tins.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(Suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox TM, Cachón-González MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226(2):241–254. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld ES, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, et al., editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; 2007. Available at http://ommbid.mhmedical.com/book.aspx?bookid=474. Accessed January 21, 2014. [Google Scholar]
- 13.Pan D. Cell- and gene-based therapeutic approaches for neurological deficits in mucopolysaccharidoses. Curr Pharm Biotechnol. 2011;12(6):884–896. doi: 10.2174/138920111795542679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau-Gaudry F, et al. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood. 2001;98(9):2664–2672. doi: 10.1182/blood.v98.9.2664. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, et al. Reprogramming erythroid cells for lysosomal enzyme production leads to visceral and CNS cross-correction in mice with Hurler syndrome. Proc Natl Acad Sci USA. 2009;106(47):19958–19963. doi: 10.1073/pnas.0908528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppinger JA, Maguire PB. Insights into the platelet releasate. Curr Pharm Des. 2007;13(26):2640–2646. doi: 10.2174/138161207781662885. [DOI] [PubMed] [Google Scholar]
- 17.Senis Y, García A. Platelet proteomics: State of the art and future perspective. Methods Mol Biol. 2012;788:367–399. doi: 10.1007/978-1-61779-307-3_24. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AH, et al. Proteomic and phospho-proteomic profile of human platelets in basal, resting state: Insights into integrin signaling. PLoS ONE. 2009;4(10):e7627. doi: 10.1371/journal.pone.0007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaumenhaft R, et al. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113(5):1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rank A, et al. Cellular origin of platelet-derived microparticles in vivo. Thromb Res. 2010;126(4):e255–e259. doi: 10.1016/j.thromres.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Siljander PR. Platelet-derived microparticles - an updated perspective. Thromb Res. 2011;127(Suppl 2):S30–S33. doi: 10.1016/S0049-3848(10)70152-3. [DOI] [PubMed] [Google Scholar]
- 22.Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia: Increased platelet destruction and/or decreased platelet production. Br J Haematol. 2009;146(6):585–596. doi: 10.1111/j.1365-2141.2009.07717.x. [DOI] [PubMed] [Google Scholar]
- 23.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol. 2013;20(5):464–471. doi: 10.1097/MOH.0b013e3283632e6b. [DOI] [PubMed] [Google Scholar]
- 24.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: Targeting, regulation and function. Curr Opin Cell Biol. 2008;20(4):415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciferri S, et al. Platelets release their lysosomal content in vivo in humans upon activation. Thromb Haemost. 2000;83(1):157–164. [PubMed] [Google Scholar]
- 26.Chen D, Lemons PP, Schraw T, Whiteheart SW. Molecular mechanisms of platelet exocytosis: Role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96(5):1782–1788. [PubMed] [Google Scholar]
- 27.Hawkins-Salsbury JA, Reddy AS, Sands MS. Combination therapies for lysosomal storage disease: Is the whole greater than the sum of its parts? Hum Mol Genet. 2011;20(R1):R54–R60. doi: 10.1093/hmg/ddr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen AL, et al. Role of sialic acid for platelet life span: Exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114(8):1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17(6):585–589. doi: 10.1097/MOH.0b013e32833e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmaier AH, Lazarus HM. Concise Guide to Hematology. Chichester, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 31.Brot FE, Glaser JH, Roozen KJ, Sly WS, Stahl PD. In vitro correction of deficient human fibroblasts by beta-glucuronidase from different human sources. Biochem Biophys Res Commun. 1974;57(1):1–8. doi: 10.1016/s0006-291x(74)80349-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan A, Achord DT, Sly WS. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci USA. 1977;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natowicz MR, Chi MM, Lowry OH, Sly WS. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci USA. 1979;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visigalli I, et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;116(24):5130–5139. doi: 10.1182/blood-2010-04-278234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biffi A, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 36.Williams DA. Broadening the indications for hematopoietic stem cell genetic therapies. Cell Stem Cell. 2013;13(3):263–264. doi: 10.1016/j.stem.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Montiel-Equihua CA, et al. The β-globin locus control region in combination with the EF1α short promoter allows enhanced lentiviral vector-mediated erythroid gene expression with conserved multilineage activity. Mol Ther. 2012;20(7):1400–1409. doi: 10.1038/mt.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther. 2006;14(4):514–524. doi: 10.1016/j.ymthe.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, et al. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc Natl Acad Sci USA. 2013;110(8):2999–3004. doi: 10.1073/pnas.1222742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.