Significance
Transportin 3 (Tnpo3) was shown to orchestrate nuclear import of splicing factors over a decade ago, but how it recognizes these cargoes remained unknown. Furthermore, the recently discovered role for Tnpo3 as a cofactor of HIV-1 replication requires mechanistic clarification. We show that Tnpo3 associates with a wide range of proteins involved in mRNA metabolism, the majority of which contain serine/arginine-rich domains. Using X-ray crystallography we determined the three-dimensional structures of Tnpo3 in its key functional states, explaining how this nuclear import factor binds and releases its cargoes. We also show that Tnpo3 mutants that are not able to interact with cleavage and polyadenylation specificity factor 6 do not facilitate HIV-1 infectivity, suggesting a potential route of pharmacological intervention in the treatment of AIDS.
Keywords: SR protein, Transportin-SR, importin, host factor
Abstract
Transportin 3 (Tnpo3, Transportin-SR2) is implicated in nuclear import of splicing factors and HIV-1 replication. Herein, we show that the majority of cellular Tnpo3 binding partners contain arginine-serine (RS) repeat domains and present crystal structures of human Tnpo3 in its free as well as GTPase Ran- and alternative splicing factor/splicing factor 2 (ASF/SF2)-bound forms. The flexible β-karyopherin fold of Tnpo3 embraces the RNA recognition motif and RS domains of the cargo. A constellation of charged residues on and around the arginine-rich helix of Tnpo3 HEAT repeat 15 engage the phosphorylated RS domain and are critical for the recognition and nuclear import of ASF/SF2. Mutations in the same region of Tnpo3 impair its interaction with the cleavage and polyadenylation specificity factor 6 (CPSF6) and its ability to support HIV-1 replication. Steric incompatibility of the RS domain and RanGTP engagement by Tnpo3 provides the mechanism for cargo release in the nucleus. Our results elucidate the structural bases for nuclear import of splicing factors and the Tnpo3–CPSF6 nexus in HIV-1 biology.
The transport of macromolecules between cytoplasm and nucleus is orchestrated by a family of nuclear import and export receptors (1). Referred to as importins and exportins, these proteins bind their specific cargoes and translocate them across the nuclear pore complex. The process is regulated by the small GTPase Ran that partitions between cytoplasm and nucleus in the predominantly GDP- and GTP-bound form, respectively. Importins associate with their cargoes in the cytoplasm, and the competitive binding of RanGTP induces them to release their cargoes in the nucleus (2). Most nuclear import/export receptors belong to the β-karyopherin family of proteins, with 22 members encoded in the human genome (3). The majority of β-karyopherins bind their cargoes directly, recognizing a linear nuclear localization or export signal and/or a specific tertiary/quaternary structural feature (4, 5).
A fundamentally important type of a nuclear localization signal (NLS), comprising sequences rich in Arg-Ser and/or Arg-Asp/Glu/Gly dipeptides (referred to as RS or RS-like domains), belongs to the family of Ser/Arg-rich (SR) proteins. These nuclear proteins also contain RNA recognition motif (RRM) domains and play essential roles in pre-mRNA splicing and 3′ processing and participate in transcription regulation, mRNA transport, translation, and nonsense-mediated mRNA decay (6). The splicing factors alternative splicing factor/splicing factor 2 (ASF/SF2) and SC35 along with the cleavage and polyadenylation specificity factor 6 (CPSF6, also known as CF-Im-68) are among the best-characterized metazoan SR proteins (7–10). The SR protein family can be further extended by inclusion of a structurally and functionally diverse group of nuclear proteins that possess RS repeats but lack RRM domains (11). The RS domains are processively phosphorylated on their Ser residues by a set of dedicated kinases (12–16). Phosphorylation of SR proteins is thought to trigger their import into the nucleus and is subsequently required for spliceosome assembly, whereas their dephosphorylation allows for splicing to proceed (17–20). The human β-karyopherin Transportin 3 (Tnpo3) (also known as Transportin-SR2) and its alternative splice form Transportin-SR, identified through their ability to bind the RS domain of ASF/SF2, were shown to execute nuclear import of ASF/SF2 and SC35 (21–23). Phosphorylation of the ASF/SF2 RS domain is required for nuclear import by Tnpo3 (23). Yeast, insect, and plant orthologs of Tnpo3 were similarly demonstrated to carry out nuclear import of SR and SR-like splicing factors (24–26).
In addition to its role in nuclear import of splicing factors, Tnpo3 was implicated in HIV-1 infection (27–29). Although early reports proposed a direct interaction between Tnpo3 and HIV-1 integrase (29), subsequent genetic evidence strongly suggested a functional interplay between Tnpo3 and viral capsid protein (30–33). A surprising discovery that a CPSF6 deletion mutant lacking the RS domain can potently inhibit HIV-1 replication through direct binding to capsid provides for a link between Tnpo3 and a viral component (34, 35).
Herein, we show that Tnpo3 interacts with a wide range of RS domain-containing proteins, the majority of which are involved in mRNA metabolism. To elucidate the mechanism of cargo engagement and release by Tnpo3 we determined the crystal structures of the β-karyopherin in its free, RanGTP-, and ASF/SF2-bound forms. We show that residues on and around the Arg-rich inner α-helix of HEAT repeat 15 are essential for the interaction with the phosphorylated RS domain of ASF/SF2. In addition, we show that mutations in the same region of Tnpo3 impair its interaction with CPSF6 and, concomitantly, its ability to facilitate HIV-1 infection.
Results and Discussion
Tnpo3 Binds a Wide Range of RRM and RS Domain-Containing Proteins.
To obtain an unbiased profile of potential Tnpo3 cargoes, we selected a human cell line stably expressing a Flag-tagged version of the β-karyopherin. Extracts of control and Flag–Tnpo3-expressing cells were immunoprecipitated with anti-Flag antibody in the absence and presence of a zwitterionic detergent, and copurifying proteins were identified by tandem mass spectrometry following trypsin digestion. In total, 113 human proteins were identified as specifically copurifying with Tnpo3 owing to their absence in the immunoprecipitations from control cells (SI Appendix, Table S1). We considered the 68 proteins identified in both the absence and presence of the detergent as strong candidates for being physiological Tnpo3 binding partners. Of these, over 70% contained a recognizable RS or RS-like domain, whereas 40% were canonical SR proteins, each containing additionally at least one RRM domain (SI Appendix, Table S1 A and B). In agreement with the role of Tnpo3 as a nuclear import factor, Ran and RanBP1 were also identified in the proteomic analyses (SI Appendix, Table S1E). The abundance of RS domain-containing proteins among Tnpo3 binding partners, including those that lack RRM domains, strongly supports the proposed role for RS domains in cargo recognition by the β-karyopherin (21–23). Notably, the bulk of proteins recovered in this experiment are known to be, or predicted to be, involved in splicing or in other aspects of mRNA metabolism. Plausibly, the necessity for transport of molecules with highly unbalanced charge distributions through the hydrophobic interior of the nuclear pore drove the evolution of a specialized nuclear import factor.
Crystal Structures of Tnpo3 in Its Free and RanGTP-Bound States.
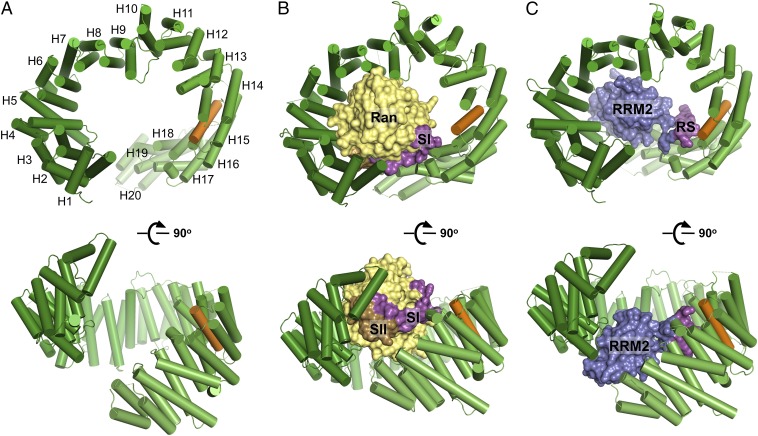
We obtained crystals and refined the structures of unliganded Tnpo3 to 3.0 Å resolution with an Rwork/Rfree of 23.6/26.6% and of the Tnpo3–RanGTP complex to 3.4 Å resolution with an Rwork/Rfree of 26.8/29.0% (SI Appendix, Table S2 and Figs. S1 and S2). The single-point mutation C511A was engineered in Tnpo3 to improve crystal packing of the unliganded form (see SI Appendix, Materials and Methods for more information), and the Q69L mutant of Ran was used to prevent GTP hydrolysis (36) during crystallization of the heterodimer. The closest paralog of Tnpo3 is Importin 13 (Imp13), with which it shares 21.6% amino acid sequence identity. The overall structures of isolated Tnpo3 and of its complex with RanGTP are predictably similar to the corresponding Imp13 structures reported previously (37–39). Akin to Imp13 and some other β-karyopherins, Tnpo3 is composed of 20 HEAT repeats and adopts an open circular/toroidal shape (Fig. 1A), with its N- and C-terminal arches facing each other. A HEAT repeat is an ∼40-residue structural element composed of two antiparallel α-helices connected by a loop of variable length (40, 41). Consecutive HEAT repeats pack against each other, usually with a slight clockwise twist, to form two layers of α-helices that line up the convex and concave surfaces of the molecule. Similar to Imp13 (37), Tnpo3 features three unusual inter-HEAT repeat interfaces: HEAT repeat 1 packs nearly perpendicularly to HEAT repeat 2; whereas HEAT repeats 4 and 10 stack against their respective preceding repeats with pronounced left-handed twists (Fig. 1A). As in Imp13 and some other β-karyopherins (37, 42), the C-terminal HEAT repeat of Tnpo3 is expanded by an additional α-helix. Unliganded Tnpo3 crystallized in the triclinic space group P1 with four molecules per unit cell, forming a pair of homodimers composed of interlocking protein chains (Movie S1 and SI Appendix, Fig. S1A). Tnpo3 is known to dimerize at high protein concentrations, and the overall shape of the observed dimers is compatible with the molecular envelope determined by small-angle X-ray scattering (43).
Fig. 1.
Overview of the crystal structures of unliganded Tnpo3 (A), Tnpo3–RanGTP (B), and Tnpo3–ASF/SF2 (C) complexes. Tnpo3 is shown as cartoons, and ASF/SF2 and Ran are in space-fill mode. Tnpo3 is colored green, except the R-helix, which is orange; Switch I (SI) and Switch II (SII) regions of Ran are magenta and brown, respectively, and the remainder of the Ran structure is yellow. RRM2 and RS domains of ASF/SF2 are blue and magenta, respectively.
Within its complex with Tnpo3, the Ras-like core domain of GTP-bound Ran is well defined in electron density maps (SI Appendix, Fig. S2 A and B), and its structure has been extensively characterized (44, 45). As expected, RanGTP binds to the concave side of Tnpo3 (Fig. 1B and SI Appendix, Fig. S3A), burying ∼3,700 Å2 of molecular surface. The structures of Tnpo3–RanGTP and Imp13–RanGTP complexes can be superposed with an rmsd between β-karyopherin Cα atom positions of 3.4 Å (SI Appendix, Fig. S3C). The most extensive contacts involve the Switch II region of Ran (residues 66–80) and HEAT repeats 1–3 of Tnpo3, whereas Switch I (Ran residues 32–45) interacts with HEAT repeats 17–18 (SI Appendix, Fig. S3 B and C). The switch regions of Ras-like GTPases change their conformations upon hydrolysis of GTP (46). Consistent with the structure, Tnpo3 bound RanGTP much more avidly than RanGDP (SI Appendix, Fig. S4).
Crystal Structure of Tnpo3 in Complex with ASF/SF2.
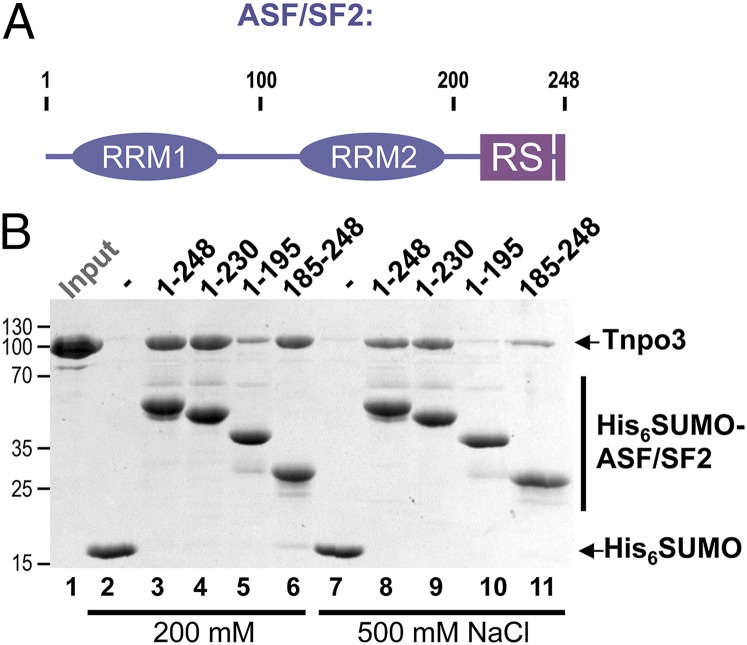
We used ASF/SF2, a canonical splicing factor containing a pair of RRM domains and a well-defined RS domain (Fig. 2A), to characterize Tnpo3–cargo interactions. RS domains are extensively phosphorylated in vivo, and the phosphorylation was reported to increase ASF/SF2 solubility (47). Concordantly, we were able to obtain soluble, monodispersed preparations of human ASF/SF2 by coexpression with CDC-like kinase 1 (CLK1) (13) or SR protein kinase 1 (SRPK1) (12) in bacteria. Bacteriophage λ protein phosphatase treatment of hexahistidine-SUMO (His6SUMO)-tagged ASF/SF2 produced in the presence of CLK1 shifted its migration in SDS/PAGE gels, confirming phosphorylation of the protein (SI Appendix, Fig. S5). Phosphorylated His6SUMO–ASF/SF2 readily interacted with Tnpo3 in His6 tag pull-down assays (Fig. 2B). Deletion mutants of ASF/SF2 containing the entirety or a part of the RS domain were likewise efficiently phosphorylated by coexpression with CLK1 (SI Appendix, Fig. S5). The mutant proteins lacking the RRM domains or the RS region recovered less Tnpo3 in pull-down experiments than did the full-length protein, a difference that became more evident under high-ionic-strength conditions (Fig. 2B, 500 mM NaCl). However, the removal of the RS region resulted in a considerably more profound reduction in Tnpo3 recovery, indicating that whereas both the N- and C-terminal portions of ASF/SF2 contribute to the interaction with Tnpo3, the C-terminal region, spanning the RS domain, is the dominant determinant.
Fig. 2.
Interaction of ASF/SF2 with Tnpo3 in vitro. (A) Schematic of ASF/SF2 domain organization. (B) Pulldown of Tnpo3 with His6SUMO (lanes 2 and 7), full length His6SUMO–ASF/SF2 (lanes 3 and 8), or its deletion constructs (lanes 4–6 and 9–11) on Ni-nitrilotriacetic acid agarose in buffer containing 200 mM (lanes 2–6) or 500 mM (lanes 7–11) NaCl. The input quantity of Tnpo3 is shown in lane 1.
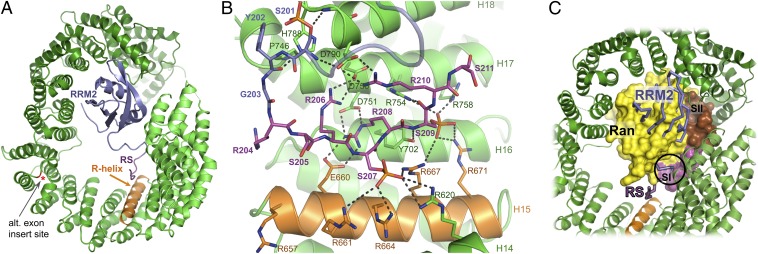
Following extensive trials with various recombinant SR proteins and synthetic RS peptides, we cocrystallized Tnpo3 with a recombinant construct spanning the second RRM domain (RRM2) and most of the RS domain of human ASF/SF2 (residues 106–230). This protein, produced in a phosphorylated form in bacteria (SI Appendix, Fig. S5), readily formed a stable complex with Tnpo3 (SI Appendix, Fig. S6). The structure refined to 2.6 Å resolution with an Rwork/Rfree of 22.1/26.9% (SI Appendix, Table S2) revealed two nearly identical heterodimers with an rmsd between Cα atom positions of 0.73 Å. Tnpo3 uses its inner concave surface to enclose the protein cargo (Figs. 1C and 3A), and ∼4,300 Å2 of molecular surface is buried upon formation of a tripartite binding interface. The RRM2 domain is wedged between Tnpo3 HEAT repeats 4–7 and 19–20 (Fig. 3A and SI Appendix, Fig. S7), whereas the RS region interacts with HEAT repeats 14–17 (Fig. 3B). The Tnpo3–ASF/SF2 interface is poor in hydrophobic contacts, with an estimated overall solvation energy gain of only −0.7 kcal/mol.
Fig. 3.
Details of the Tnpo3–ASF/SF2 structure. (A) Overview with both protein chains displayed as cartoons. The ASF/SF2 RRM2 and RS domains, the R-helix of Tnpo3, and the position of the insertion site of the alternative exon found in Transportin-SR (red asterisk) are indicated. (B) Details of the interface between Tnpo3 and the ASF/SF2 RS domain. (C) Superposition of the Tnpo3–RanGTP and Tnpo3–ASF/SF2 structures based on the HEAT repeats of the RS domain-binding region of Tnpo3. Ran, ASF/SF2, and Tnpo3 are shown in space-fill, Cα trace, and ribbon cartoon modes, respectively. The clash between Switch I of Ran and ASF/SF2 residues preceding the RS domain is indicated with a circle.
The linker between ASF/SF2 RRM2 and RS domains, and four Arg-Ser repeats of the RS domain, are well ordered in the structure (Fig. 3B and SI Appendix, Fig. S8A). Three ASF/SF2 Ser residues are phosphorylated in the crystallized complex, including Ser207 and Ser209 located within the ordered RS domain portion. We confirmed the presence and positions of the phosphate groups in the crystallized complex by anomalous X-ray scattering (SI Appendix, Fig. S8B). Residues 204–211 of the RS domain adopt an extended conformation, interacting with the inner helices of Tnpo3 HEAT repeats 12–16, engaging in a network of 11 salt bridges with complementary Tnpo3 residues (Fig. 3B). The inner helix of Tnpo3 HEAT repeat 15, which we refer to as R-helix (orange in the figures), projects an arginine side chain at each turn toward the concave surface of Tnpo3 and acts as a phospho-serine binding platform (Fig. 3B). The phosphate group attached to Ser207 of ASF/SF2 interacts with the side chains of Tnpo3 Arg620, Arg661, Arg664, and Arg667. The phosphate on Ser209 of ASF/SF2 bridges to side chains of Tnpo3 Arg667, Arg671, Arg754, and Arg758. The side chain of ASF/SF2 Arg208 rests on the hydrophobic side chain of Tnpo3 Tyr702 and bridges to Tnpo3 Glu660 and Asp751, whereas ASF/SF2 Arg206 and Arg210 interact with Tnpo3 Asp750 and Asp790, respectively. In addition, backbone carbonyl and amide groups of ASF/SF2 Ser209 are held in place by hydrogen bonds with the side chains of Tnpo3 Tyr702 and Arg754.
Overall, there is high-level sequence conservation among metazoan Tnpo3 orthologs, and some of the Tnpo3 residues involved in RS domain binding are conserved among all eukaryotes, including plants and yeasts (SI Appendix, Fig. S9). The RS domains are thought to have evolved from an Arg-rich sequence (48). ASF/SF2 Arg208 is situated at the center of the Tnpo3–RS interface; its side chain rests on a conserved aromatic residue of Tnpo3 (Tyr702 in humans) and is shared between invariant Tnpo3 Glu660 and Asp751 (Fig. 3B). It is tempting to speculate that Tyr702 along with Glu660 and Asp751 may compose the primordial kernel evolved for the recognition of an Arg-containing motif by the β-karyopherin.
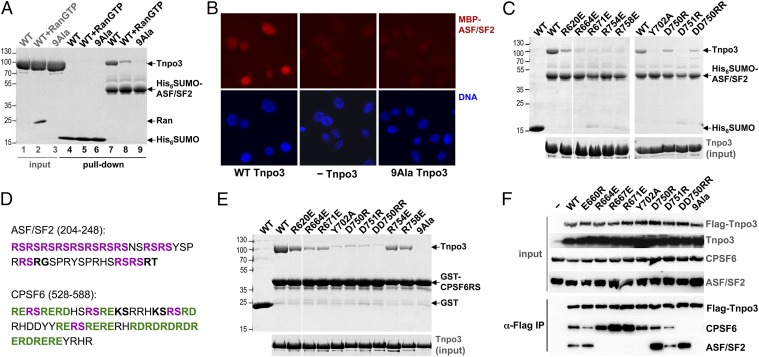
To verify the importance of the interactions between Tnpo3 and the RS domain observed in the structure we substituted nine Tnpo3 amino acids (Arg620, Glu660, Arg664, Arg667, Arg671, Tyr702, Asp750, Asp751, and Arg758) found in direct contacts with the RS domain for alanine residues. The resulting mutant (referred to as 9Ala) robustly and selectively bound RanGTP (SI Appendix, Fig. S4), indicating that the mutations did not disrupt the overall Tnpo3 structure. However, consistent with the crystal structure, the mutant was deficient for the interaction with His6SUMO–ASF/SF2 in pull-down assays (Fig. 4A). Furthermore, in contrast to WT protein, 9Ala Tnpo3 was unable to facilitate the import of maltose binding protein–ASF/SF2 fusion construct into nuclei of digitonin-permeabilized HeLa cells (Fig. 4B), confirming the functional importance of the Tnpo3–RS domain interface.
Fig. 4.
Mutagenesis of Tnpo3 residues involved in the interaction with the RS domain. (A) Pulldown of WT and 9Ala Tnpo3 with His6SUMO–ASF/SF2 in the presence and absence of RanGTP. (B) Nuclear import of fluorescently labeled MBP–ASF/SF2 in digitonin-permeabilized HeLa cells in the presence (Left) or absence (Center) of WT Tnpo3 or in the presence of the 9Ala Tnpo3 mutant (Right). The images show detection of MBP–ASF/SF2 (Upper) or DNA (Lower). (C) Pulldown of WT and mutant Tnpo3 proteins with His6SUMO–ASF/SF2. (D) Amino acid sequences of the C-terminal regions of human ASF/SF2 and CPSF6, spanning their RS domains. The canonical Arg-Ser dipeptides and the RS-like motifs Arg-Asp/Glu are in bold magenta and green, respectively, and RS-like Arg-Thr/Gly and Lys-Ser are in bold black. (E) Pulldown of WT and mutant Tnpo3 proteins with phosphorylated GST–CPSF6RS on glutathione beads. (F) Co-IP of endogenous ASF/SF2 and CPSF6 with WT and mutant Flag–Tnpo3 from cells depleted for expression of endogenous Tnpo3.
We next tested 10 single- and double-point mutations within the RS binding region of Tnpo3. Reverse charge mutations of Tnpo3 Arg664, Arg671, Arg754, Arg758, and Asp751 disrupted the interaction with ASF/SF2 (Fig. 4C). Similarly, the mutation of Tyr702, a residue at the center of the RS binding platform, to alanine also resulted in loss of the interaction. Because CPSF6 harbors an RRM domain alongside an RS region, we hypothesized that the mode of its interaction with Tnpo3 is similar to that of ASF/SF2. Whereas full-length CPSF6 was not stable when expressed in bacteria or insect cells, we were able to produce a GST fusion construct with residues 441–588 that span its RS domain (CPSF6RS). CPSF6RS is enriched in Arg-Glu/Asp dipeptides (Fig. 4D), which likely act as functional mimics of phosphorylated RS dipeptides. The construct could be expressed in bacteria in soluble form with and without phosphorylation by SR protein kinases as monodisperse preparations and high-molecular-mass aggregates, respectively (SI Appendix, Fig. S10 A and B). Both types of preparations robustly interacted with WT Tnpo3 in GST pull-down assays and responded to the mutations in the Tnpo3 RS binding region in similar ways (Fig. 4E and SI Appendix, Fig. S10C). From our panel of mutants, 9Ala and DD750RR Tnpo3 displayed the lowest affinity for GST–CPSF6RS. These results corroborate the importance of the charged residues on and around the R-helix for the interaction with ASF/SF2 and with divergent RS domains.
Although the contacts involving the RRM2 domain are responsible for ∼60% of the total buried surface between Tnpo3 and ASF/SF2, the RRM portion of the cargo is not the major contributor to the interaction (Fig. 2B). Indeed, mutations disrupting the interface with the RS domain are sufficient to abrogate the interaction with full-length ASF/SF2 (Fig. 4). Furthermore, about half of RS repeat containing Tnpo3 interactors identified in our proteomic experiment lacked recognizable RRM domains (SI Appendix, Table S1B), and the RS domain on its own can function as a transferrable Tnpo3-dependent NLS (21–23). Relatively sparse interactions across the RRM2–Tnpo3 interface (SI Appendix, Fig. S7) may help to explain its weaker contribution compared with that of the RS domain. We note, however, that experimental binding energy measurements would be required to ascertain contributions of the individual ASF/SF2 domains to the Tnpo3 binding affinity. An alternative splice form of Tnpo3, known as Transportin-SR (21), contains a 33-residue insertion in the loop connecting HEAT repeats 10 and 11. The amino acid sequence of the insert is not predictive of a stable secondary structure and the insertion site is remote from the interface with ASF/SF2 (Fig. 3A, red asterisk). Therefore, the modes of engagement of SR proteins by both splice forms of the β-karyopherin are likely to be similar.
Nuclear RanGTP competes with cargoes for binding to nuclear import receptors (2). Concordantly, the recovery of Tnpo3 in pull-down assays with His6SUMO–ASF/SF2 was greatly reduced in the presence of RanGTP (Fig. 4A, compare lanes 7 and 8). A comparison of the Tnpo3–ASF/SF2 and Tnpo3–RanGTP structures revealed that the binding platforms for RanGTP and ASF/SF2 partially overlap on the concave surface of Tnpo3, with the Switch I region of Ran obstructing the approach of the RS domain to the R-helix (Fig. 3C). Given the importance of the RS domain for the Tnpo3–ASF/SF2 interaction, this provides the structural basis for the dissociation of Tnpo3–cargo complexes via competitive binding of RanGTP. Although Tnpo3 and Imp13 are close paralogs, their modes of cargo engagement are very different. Imp13 has been cocrystallized with two of its nuclear import substrates: Ubc9 and the Mago–Y14 complex (37, 38). Unlike Tnpo3, which primarily relies on recognition of the flexible RS domains, Imp13 adapts to engage folded regions of its cargoes. While using its N-terminal arch to bind Ubc9, Imp13 commands 15 of its 20 HEAT repeats to bind Mago–Y14. However, it would be of interest to determine whether, like Imp13, Tnpo3 can function as a bidirectional transporter, and there is some evidence that Tnpo3 can also act as an export factor in human cells (49).
Tnpo3 Mutants Deficient for CPSF6 Binding Fail to Facilitate HIV-1 Infectivity.
To test effects of Tnpo3 mutations on interaction with cellular SR proteins and HIV-1 replication, we established a polyclonal human cell line wherein endogenous Tnpo3 levels were reduced ∼13-fold through the use of an shRNA (SI Appendix, Fig. S11A). In agreement with published observations (31), knockdown of Tnpo3 expression led to an increase in the steady-state level of cytoplasmic CPSF6 (SI Appendix, Fig. S11B). Stable ectopic expression of a Flag-tagged version of Tnpo3 using an shRNA-resistant construct in these cells reduced the cytoplasmic SR protein pool back to low/undetectable levels (SI Appendix, Fig. S11B). We selected a panel of complemented cell lines expressing a set of Flag–Tnpo3 mutants. The mutants were first tested for the interaction with endogenous ASF/SF2 and CPSF6 by coimmunoprecipitation (co-IP) using anti-Flag antibodies (Fig. 4F). Tnpo3 variants 9Ala, R664E, R671E, and Y702A failed to bind ASF/SF2, whereas D750R and DD750RR retained, if not enhanced, the interaction. In addition, R660E and R667E Tnpo3 scored as positive and as negative for binding ASF/SF2, respectively. In contrast, the interaction with CPSF6 was fully disrupted only by the DD750RR and 9Ala mutations. The results of the co-IP experiments are in good agreement with pull-down assays conducted with purified components (Fig. 4 A, C, and E) and underscore discordance of Tnpo3 mutant interaction profiles with ASF/SF2 and CPSF6. These observations suggest that although the ASF/SF2– and CPSF6–Tnpo3 interfaces differ in the specific hot-spot contacts, the region of and around the R-helix in Tnpo3 is critical for binding both SR proteins. We note that some of the mutations in Tnpo3 resulted in apparent interaction enhancements with the endogenous proteins. For example, co-IP of ASF/SF2 with D750R Tnpo3 was considerably more robust than with the WT protein, whereas the co-IP of CPSF6 was enhanced by the R671E mutation (Fig. 4F). These improved recoveries likely result from increased accessibility of the Tnpo3 mutants, with which the SR protein retained interaction (Fig. 4 C and E), in the face of competition with the excess of alternative cargoes in the cell.
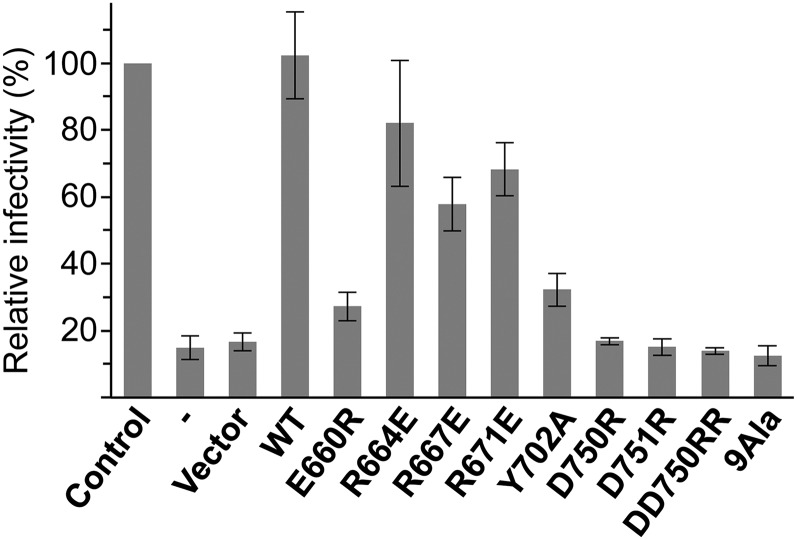
As expected, expression of 9Ala and DD750RR Flag–Tnpo3, which were deficient for the interaction with CPSF6, did not completely deplete the steady-state cytoplasmic pools of the SR protein in Tnpo3 knock-down cells (SI Appendix, Fig. S11B). To assess the effects of mutations within the RS-binding region of Tnpo3 on HIV-1 infectivity, we inoculated our panel of back-complemented cell lines with an HIV-1 vector pseudotyped with vesicular stomatitis virus glycoprotein G. In agreement with published observations (27–32, 34, 43, 50), Tnpo3-depeleted cells displayed a drastic defect in HIV-1 infectivity (Fig. 5). The infectivity was fully restored by back-complementation with WT Flag–Tnpo3 (Fig. 5). Strikingly, the ability of Flag–Tnpo3 mutants to support HIV-1 infection correlated with their propensity to interact with CPSF6, whereas no correlation was observed with ASF/SF2 binding. In particular, Flag–Tnpo3 mutants R664E, R667E, and R671E, which retained the interaction with CPSF6 but not with ASF/SF2, restored HIV-1 infectivity to 60–80% of the control condition. In contrast, DD750RR and 9Ala mutants did not rescue viral infectivity (Fig. 5), although they retained the ability to interact with HIV-1 integrase in vitro (SI Appendix, Fig. S12). These results further reinforce the functional relevance of the Tnpo3–RS domain interface and substantiate that the interplay between Tnpo3 and CPSF6 contributes to the defect in viral replication in the face of Tnpo3 depletion. Of note, notwithstanding the dramatically improved capacity of some Tnpo3 mutants to co-IP endogenous CPSF6 (Fig. 4F), none of them scored better than the WT protein to support HIV-1 infectivity (Fig. 5), arguing that HIV-1 replication does not rely on the steady-state level of Tnpo3–CPSF6 complexes. These results agree with the model whereby cytoplasmically delocalized CPSF6 inhibits HIV-1 infectivity via premature engagement of the viral capsid (31, 35). The antagonistic HIV-1 capsid–CPSF6 interaction is supported by a substantial weight of genetic evidence, and potential nuclear functions of CPSF6 as an HIV-1 host factor remain an intriguing possibility for further investigation (30–32, 34, 49, 50).
Fig. 5.
Effects of mutations within RS domain binding patch of Tnpo3 on HIV-1 infectivity. Relative HIV-1 infectivity measured in Tnpo3 knock-down cells before and after transduction with the empty vector, or vectors expressing WT or mutant Flag–Tnpo3 proteins, relative to that in control cell line (set as 100%). The error bars correspond to SDs from experiments done in triplicate.
In conclusion, our comprehensive crystallography studies establish the mode of binding and release of diverse RS domain-containing cargoes by Tnpo3. Although the RS domains of ASF/SF2 and CPSF6 engage the same region of the Tnpo3 surface, the marked discordance in their Tnpo3 mutant interaction profiles (Fig. 4) suggests that the β-karyopherin may be exploitable as a target for the development of small molecules to selectively inhibit its interactions with a subset of cargoes. Given the potent antiviral properties of cytoplasmic CPSF6 (31), redirecting even a small fraction of this protein into the cytoplasm could be a viable strategy to block HIV-1 replication.
Materials and Methods
Recombinant proteins were produced in bacteria, purified and crystallized as detailed in SI Appendix, Materials and Methods. X-ray diffraction data were acquired at the European Synchrotron Radiation Facility in Grenoble, France, beam lines ID14-4 and ID23-1, and Diamond Light Source beam line I03 in Oxfordshire, United Kingdom. The structures were solved by molecular replacement using Phaser (51) with fragments of Imp13 structures from PDB ID codes 2X19 (37), 2XWU (38), and ASF/SF2 3BEG (52) as search models. The final structures were refined in Phenix (53) and assessed using Molprobity (54). Examples of the electron density maps are given in SI Appendix, Figs. S1, S2, and S8. Additional experimental details are given in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank the staff of European Synchrotron Radiation Facility ID14-4 and ID23-1 and Diamond I03 beam lines for assistance with X-ray data collection, D. Frith for excellent technical assistance with mass spectrometry, L. Sansregret for help with fluorescent microscopy, and Jonathan Stoye for critical reading of the manuscript. This work was supported by US National Institute of General Medical Sciences P50 Grant GM082251-06 (to A.E. and P.C.), National Institute of Allergies and Infectious Disease R01 Grant AI052014-11 (to A.E.), and European Union FP7 HIVINNOV Consortium Grant 305137 (to A.F.). V.N.K. is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4C0O, 4C0P, and 4C0Q).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320755111/-/DCSupplemental.
References
- 1.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8(3):195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 2.Görlich D, Panté N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15(20):5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 3.Strom AC, Weis K. Importin-beta-like nuclear transport receptors. Genome Biol. 2001;2(6):reviews 3008.1–3008.9. doi: 10.1186/gb-2001-2-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. doi: 10.1146/annurev.biochem.76.052705.161529. [DOI] [PubMed] [Google Scholar]
- 5.Cook AG, Conti E. Nuclear export complexes in the frame. Curr Opin Struct Biol. 2010;20(2):247–252. doi: 10.1016/j.sbi.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Long JC, Caceres JF. The SR protein family of splicing factors: Master regulators of gene expression. Biochem J. 2009;417(1):15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 7.Ge H, Zuo P, Manley JL. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- 8.Krainer AR, Mayeda A, Kozak D, Binns G. Functional expression of cloned human splicing factor SF2: Homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- 9.Fu XD, Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- 10.Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: A conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 11.Boucher L, Ouzounis CA, Enright AJ, Blencowe BJ. A genome-wide survey of RS domain proteins. RNA. 2001;7(12):1693–1701. [PMC free article] [PubMed] [Google Scholar]
- 12.Gui JF, Lane WS, Fu XD. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369(6482):678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 13.Colwill K, et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 1996;15(2):265–275. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HY, et al. SRPK2: A differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol. 1998;140(4):737–750. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubol BE, et al. Processive phosphorylation of alternative splicing factor/splicing factor 2. Proc Natl Acad Sci USA. 2003;100(22):12601–12606. doi: 10.1073/pnas.1635129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velazquez-Dones A, et al. Mass spectrometric and kinetic analysis of ASF/SF2 phosphorylation by SRPK1 and Clk/Sty. J Biol Chem. 2005;280(50):41761–41768. doi: 10.1074/jbc.M504156200. [DOI] [PubMed] [Google Scholar]
- 17.Ding JH, et al. Regulated cellular partitioning of SR protein-specific kinases in mammalian cells. Mol Biol Cell. 2006;17(2):876–885. doi: 10.1091/mbc.E05-10-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mermoud JE, Cohen PT, Lamond AI. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 1994;13(23):5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao SH, Manley JL. Phosphorylation of the ASF/SF2 RS domain affects both protein-protein and protein-RNA interactions and is necessary for splicing. Genes Dev. 1997;11(3):334–344. doi: 10.1101/gad.11.3.334. [DOI] [PubMed] [Google Scholar]
- 20.Cao W, Jamison SF, Garcia-Blanco MA. Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro. RNA. 1997;3(12):1456–1467. [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka N, Bachorik JL, Dreyfuss G. Transportin-SR, a nuclear import receptor for SR proteins. J Cell Biol. 1999;145(6):1145–1152. doi: 10.1083/jcb.145.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai MC, Lin RI, Huang SY, Tsai CW, Tarn WY. A human importin-beta family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J Biol Chem. 2000;275(11):7950–7957. doi: 10.1074/jbc.275.11.7950. [DOI] [PubMed] [Google Scholar]
- 23.Lai MC, Lin RI, Tarn WY. Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc Natl Acad Sci USA. 2001;98(18):10154–10159. doi: 10.1073/pnas.181354098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senger B, et al. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 1998;17(8):2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allemand E, Dokudovskaya S, Bordonné R, Tazi J. A conserved Drosophila transportin-serine/arginine-rich (SR) protein permits nuclear import of Drosophila SR protein splicing factors and their antagonist repressor splicing factor 1. Mol Biol Cell. 2002;13(7):2436–2447. doi: 10.1091/mbc.E02-02-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, et al. Transportin-SR is required for proper splicing of resistance genes and plant immunity. PLoS Genet. 2011;7(6):e1002159. doi: 10.1371/journal.pgen.1002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brass AL, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 28.König R, et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135(1):49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ F, et al. Transportin-SR2 imports HIV into the nucleus. Curr Biol. 2008;18(16):1192–1202. doi: 10.1016/j.cub.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 30.De Iaco A, Luban J. Inhibition of HIV-1 infection by TNPO3 depletion is determined by capsid and detectable after viral cDNA enters the nucleus. Retrovirology. 2011;8:98. doi: 10.1186/1742-4690-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Iaco A, et al. TNPO3 protects HIV-1 replication from CPSF6-mediated capsid stabilization in the host cell cytoplasm. Retrovirology. 2013;10:20. doi: 10.1186/1742-4690-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan L, et al. The requirement for cellular transportin 3 (TNPO3 or TRN-SR2) during infection maps to human immunodeficiency virus type 1 capsid and not integrase. J Virol. 2010;84(1):397–406. doi: 10.1128/JVI.01899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valle-Casuso JC, et al. TNPO3 is required for HIV-1 replication after nuclear import but prior to integration and binds the HIV-1 core. J Virol. 2012;86(10):5931–5936. doi: 10.1128/JVI.00451-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K, et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7(3):221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price AJ, et al. CPSF6 defines a conserved capsid interface that modulates HIV-1 replication. PLoS Pathog. 2012;8(8):e1002896. doi: 10.1371/journal.ppat.1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci USA. 1994;91(7):2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bono F, Cook AG, Grünwald M, Ebert J, Conti E. Nuclear import mechanism of the EJC component Mago-Y14 revealed by structural studies of importin 13. Mol Cell. 2010;37(2):211–222. doi: 10.1016/j.molcel.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Grünwald M, Bono F. Structure of Importin13-Ubc9 complex: Nuclear import and release of a key regulator of sumoylation. EMBO J. 2011;30(2):427–438. doi: 10.1038/emboj.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grünwald M, Lazzaretti D, Bono F. Structural basis for the nuclear export activity of Importin13. EMBO J. 2013;32(6):899–913. doi: 10.1038/emboj.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrade MA, Bork P. HEAT repeats in the Huntington’s disease protein. Nat Genet. 1995;11(2):115–116. doi: 10.1038/ng1095-115. [DOI] [PubMed] [Google Scholar]
- 41.Cingolani G, Petosa C, Weis K, Müller CW. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature. 1999;399(6733):221–229. doi: 10.1038/20367. [DOI] [PubMed] [Google Scholar]
- 42.Petosa C, et al. Architecture of CRM1/Exportin1 suggests how cooperativity is achieved during formation of a nuclear export complex. Mol Cell. 2004;16(5):761–775. doi: 10.1016/j.molcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Larue R, et al. Interaction of the HIV-1 intasome with transportin 3 protein (TNPO3 or TRN-SR2) J Biol Chem. 2012;287(41):34044–34058. doi: 10.1074/jbc.M112.384669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chook YM, Blobel G. Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp. Nature. 1999;399(6733):230–237. doi: 10.1038/20375. [DOI] [PubMed] [Google Scholar]
- 45.Vetter IR, Arndt A, Kutay U, Görlich D, Wittinghofer A. Structural view of the Ran-Importin beta interaction at 2.3 A resolution. Cell. 1999;97(5):635–646. doi: 10.1016/s0092-8674(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 46.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294(5545):1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 47.Yue BG, Ajuh P, Akusjärvi G, Lamond AI, Kreivi JP. Functional coexpression of serine protein kinase SRPK1 and its substrate ASF/SF2 in Escherichia coli. Nucleic Acids Res. 2000;28(5):E14. doi: 10.1093/nar/28.5.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busch A, Hertel KJ. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip Rev RNA. 2012;3(1):1–12. doi: 10.1002/wrna.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou L, et al. Transportin 3 promotes a nuclear maturation step required for efficient HIV-1 integration. PLoS Pathog. 2011;7(8):e1002194. doi: 10.1371/journal.ppat.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fricke T, et al. The ability of TNPO3-depleted cells to inhibit HIV-1 infection requires CPSF6. Retrovirology. 2013;10:46. doi: 10.1186/1742-4690-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ngo JC, et al. A sliding docking interaction is essential for sequential and processive phosphorylation of an SR protein by SRPK1. Mol Cell. 2008;29(5):563–576. doi: 10.1016/j.molcel.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen VB, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.