Abstract
Cotinine, a major metabolite of nicotine, has produced improved learning and memory in rodents and non-human primates and corrects apomorphine-induced loss of pre-pulse startle inhibition in rats. The present study assessed cotinine, both acute and chronic (7-day), in the sensory inhibition paradigm in DBA/2 mice. These mice spontaneously show a deficit in hippocampal sensory inhibition, as assessed by the P20-N40 EEG paradigm, which models the deficit observed in schizophrenia patients. Anesthetized DBA/2 mice were recorded in the CA3 region of hippocampus for inhibition of paired, identical auditory stimuli, then administered cotinine (0.33, 0.1, 0.33, 1.0 or 3.3 mg/kg SQ) and recorded for 90 minutes. At doses of 0.1, 0.33 and 1.0 mg/kg, there were significant increases in conditioning amplitude, with no changes in test amplitude or TC ratio. Blockade of α4β2 nicotinic receptors with central administration of DHβE blocked the increase in conditioning amplitude induced by the 1.0 mg/kg dose of cotinine, as did blockade of α7 nicotinic receptors with α-bungarotoxin. Daily injections of 0.33, 1.0 or 3.3 mg/kg for 7 days produced similar increases in conditioning amplitude on the 7th day, but only at the 0.33 and 3.3 mg/kg doses. Determination of the “carry over” effect of the previous 6 daily doses of cotinine, prior to the 7th dose, showed that there was a significant increase in conditioning amplitude as compared to the baseline data for mice receiving the equivalent acute dose. There were no significant effects on test amplitude or TC ratio for any of the chronic doses. These data suggest that cotinine modulates the conditioning amplitude in the sensory inhibition paradigm through the α4β2 nicotinic receptor and possibly also through the α7 nicotinic receptor, as well. However the data do not suggest that cotinine is a potential therapeutic for the treatment of sensory inhibition deficits in schizophrenia.
Keywords: nicotinic receptors, schizophrenia, sensory processing, auditory gating
1. Introduction
(S)-(−)Cotinine is the primary oxidative metabolite of nicotine. More than 80% of nicotine is metabolized to cotinine by cytochrome P450 2A6 and cytochrome P450 2A5 (Lewis et al., 1999; Donato et al 2000; Visoni et al, 2008). Although nicotine and cotinine share structural similarities, their pharmacological properties, both in vitro and in vivo, are different. It has been proposed that cotinine, like nicotine, binds to, and activates nicotinic acetylcholine receptors (Dwoskin et al, 1999, Vainio and Tuominen, 2001), whereas other reports indicate no pharmacological action of cotinine (Linville et al, 1993, Radek et al, 1993). Cotinine has a much longer half-life (15 – 19 hours) compared to the half-life of nicotine (2 – 3 hours) (Davis et al, 1988; Crooks and Dwoskin, 1997) and has been proposed to be safe at doses up to 10 times greater than that attained during cigarette smoking (Hatsukami et al., 1997).
Recent data indicate that acute administration of cotinine improves cognitive deficits induced by the NMDA receptor antagonist MK-801 in rodents (Terry et al 2012). It has also been found to increase working memory and attention in non-human primates (Terry et al., 2005) and to attenuate apomorphine-induced deficits in pre-pulse startle inhibition in rats (Buccafusco and Terry, 2003, Terry et al, 2005). Due to cotinine’s prolonged effects in improving working memory and attention in non-human primates, cotinine may be a potential therapeutic for neuropsychiatric disorders involving impairments in attention and memory (Terry et al, 2012).
Deficits in sensory processing (sensory inhibition or auditory gating) are common in certain mental disorders such as schizophrenia and bi-polar disorder (Adler et al 1998; Franks et al 1983; Freedman et al 1983; 1987; Sanchez-Morla et al 2008). Deficient sensory inhibition can be measured using a paired-click paradigm, known as the P50 paradigm, which measures the level of circuit inhibition in response to repetitive stimuli. The paradigm measures and compares the auditory evoked responses to the 2 closely-paired, identical stimuli. In normal individuals, the response to the second stimulus is reduced compared to the response to the first stimulus. Schizophrenia patients show responses of similar magnitude to both stimuli, in other words, they fail to inhibit the second response (Franks et al 1983). Failure to suppress extraneous stimuli can lead to sensory overload or “flooding” (Venables, 1992), which may result in poor attention and deficits in learning and memory (Cullum et al 1993). The deficit in P50 sensory inhibition has been linked to reduced numbers of α7 nicotinic receptors in the hippocampus (Adler et al 1998; Freedman et al 2001) and to polymorphisms in the promoter for the α7 nicotinic receptor gene (Leonard et al 2002).
The DBA/2 mouse strain has been used to model the sensory inhibition deficit using a paradigm similar to P50 in humans, the P20-N40 paradigm (Stevens et al 1996). DBA/2 mice show abnormal, schizophrenia-like sensory inhibition (Stevens et al 1996), and have reduced levels of hippocampal α7 nicotinic acetylcholine receptors (Stevens et al, 1996) as well as a polymorphism in the promoter for the α7 nicotinic receptor gene (Stitzel et al 2003). Nicotinic agonists, whether selective for the α7 and/or the α4β2* subtype, of nicotinic acetylcholine receptors, produce improvements in sensory inhibition in these mice (Stevens and Wear, 1997, Stevens et al., 1998, Wildeboer and Stevens, 2008). Given that cotinine may have activity at nicotinic receptors (Dwoskin et al, 1999, Vainio and Tuominen, 2001) and has produced improvements in attention and working memory (Terry et al 2005; 2012), which may be related to nicotinic receptor activation (Levin and Simon 1998), we chose to test cotinine in the P20-N40, sensory inhibition paradigm. Cotinine was administered either as an acute dose, in the presence or absence of nicotinic receptor antagonists, or as a daily injection for seven days.
2. Methods
2.1 Animals
Male DBA/2 mice (20–25 g) were obtained from Harlan SD (Indianapolis, IN) and housed five to a shoebox cage with aspen wood chip bedding, in the colony at UC Denver, Anchutz Medical Campus. The mice were provided ad libitum water and food (Harlan Teklad, Indianapolis, IN). Lighting was cycled at 12 hour intervals (lights on at 0600 hours). All studies were performed in accordance with the Principles of Laboratory Animal Care (Institute of Laboratory Animal Research 1996) with approval from the Institutional Animal Care and Use Committee of UC Denver, Anchutz Medical Campus.
2.2 Surgery
As previously described, (Stevens et al, 1996) mice were anesthetized with chloral hydrate (400 mg/kg, IP) and pyrazole (400 mg/kg, IP) to retard the metabolism of the chloral hydrate. During recording, the anesthetic and pyrazole were supplemented as necessary (5 mg/kg, IP) to maintain a plane of anesthesia as evidenced by lack of reflexive limb withdrawal in response to toe pinch. Anesthetized mice were placed in a Kopf stereotaxic instrument (Kopf Instruments, Tujunga, CA) with hollow earbars, attached to miniature earphones connected to a sound amplifier, which were placed adjacent to the externalization of the aural canal. A stable core temperature was maintained at 35° C by a heating pad.
The scalp was incised and a burr hole opened over the dorsal CA3 region of the hippocampus [−1.8 mm posterior from bregma, ±2.7 mm lateral from midline (Paxinos and Franklin, 2001)]. A Teflon-coated stainless-steel cut wire recording electrode (0.127 mm diameter) was inserted, 1.5 to 1.7 mm ventral from the dorsal brain surface, into the CA3 pyramidal cell layer of the hippocampus. Final placement was determined by the presence of complex action potentials typical of hippocampal pyramidal neurons (Miller et al, 1992). A second burr hole was drilled anterior to bregma and contralateral to the recording electrode for placement of the reference electrode on dura. Electrical responses were amplified 1000× with analog to digital conversion (SciWorks, DataWave, Loveland, CO) for averaging and analysis by computer.
2.3 Experimental Protocols
Auditory stimuli in the form of tones (3000 Hz, 10 milliseconds, 70 dB) generated as a sine wave, were presented in pairs with a 500 millisecond interval between the paired tones and 10 s between pairs of stimuli. Responses to 16 pairs of tones were averaged at 5-minute intervals and digitally bandpass filtered with between 10 and 5000 Hz. The maximum negativity between 20 and 60 milliseconds after the stimulus was selected as the N40 wave and measured relative to the proceeding positivity, the P20 wave. This complex has been shown to be less variable than either component alone (Hashimoto et al, 2005). The ratio of amplitudes of the response to the second tone (test amplitude) to the response of the first tone (conditioning amplitude), yielded the TC ratio, the measure of the level of circuit inhibition. A TC ratio less than 0.5 indicated normal inhibition (Stevens et al, 1996). Four to five baseline records were obtained prior cotinine administration. Acute cotinine, dissolved in 0.9 % NaCl, and administered at five doses (0.033 mg/kg, n = 4; 0.1 mg/kg, n =8; 0.33 mg/kg, n = 8; 1 mg/kg; n = 8; 3.3 mg/kg, n = 8, all SQ). After injection, recordings continued at 5-minute intervals, for 95 minutes. For chronic administration studies, once daily injection of 0.33 mg/kg, SQ (n = 8), 1.0 mg/kg, SQ (n = 8) or 3.3 mg/kg, SQ (n = 8) mg/kg was administered for 6 consecutive days. On the 7th day mice were tested for effects on auditory gating. Following five baseline recordings each animal received a final (7th) injection of cotinine and records were obtained at 5-minute intervals for 90 minutes.
For antagonist experiments involving dihydro-β-erythroidine (DHβE, selective α4β2 antagonist) or α-bungarotoxin (αBTX, a selective α7 receptor antagonist), a third burr hole was drilled over the anterior lateral ventricle [+0.8 mm anterior from bregma, −0.5 mm lateral from midline (Paxinos and Franklin, 2001)] ipsilateral to the recording electrode. A 26-gauge needle attached to a 10 μl Hamilton syringe (Hamilton, Reno, NV) was inserted into the ventricle 2.0 mm below dura for intracerebroventricular (ICV) administration of antagonist. A 1 μl volume of either 1.25 nM alpha-BTX (n = 9) or 30 nM DhβE (n = 11) was administered following baseline recordings (Stevens and Wear 1997). After injection of antagonist, a single record was obtained at 5 minutes post ICV injection, after which cotinine, 1 mg/kg, SQ, was administered and an additional 90 minutes of records obtained.
2.4 Compounds
(−)-Cotinine was obtained from Sigma-Aldrich (St. Louis, MO). α-BTX and DHβE hydrobromide were obtained from Tocris (Minneapolis, MN). DHβE dosing was based on salt weight. (−)-Cotinine and alpha-BTX dosing was according to the free base weights. All compounds were dissolved in 9% NaCl.
2.5 Statistical Analysis
The time course data for cotinine, alone or in conjunction with an antagonist, were analyzed, for each dose, using repeated measures MANOVA. Where appropriate, Fisher’s protected least-significant difference (PLSD) a posteriori analysis was used to compare individual post injection time points to the average baseline values.
3. Results
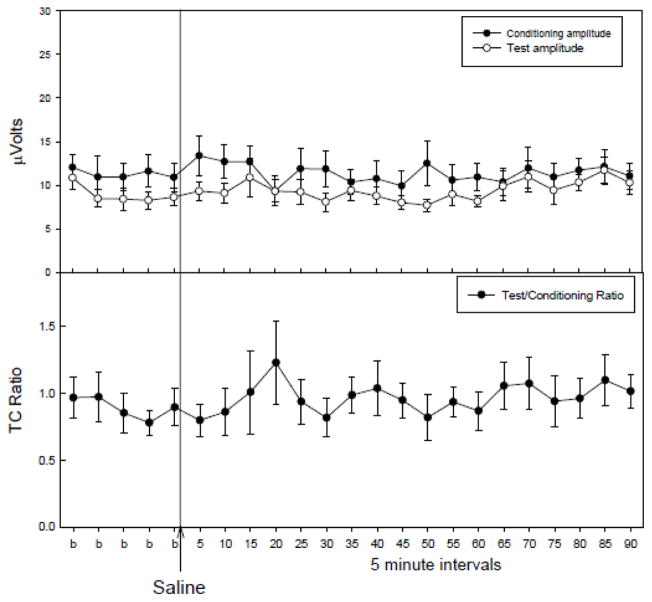
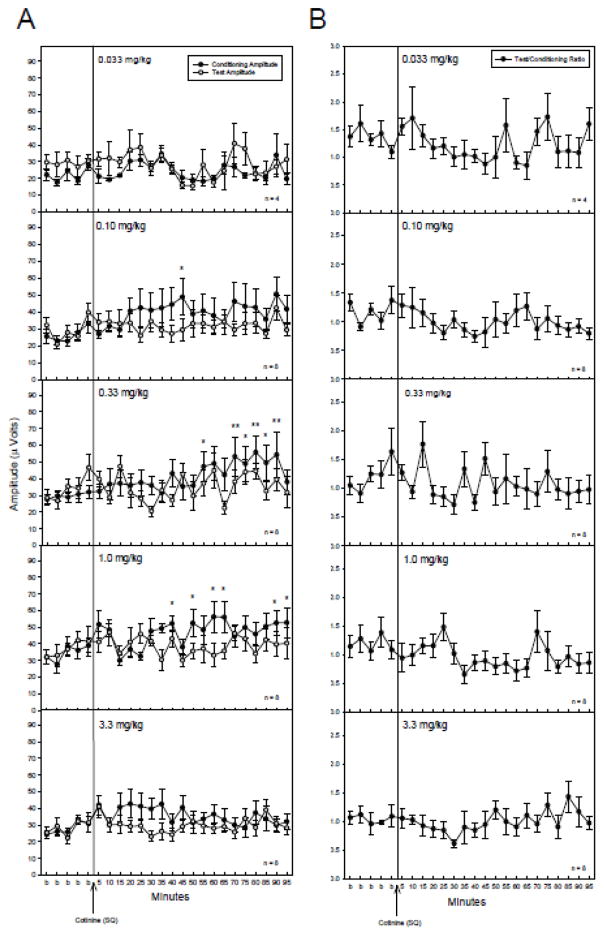
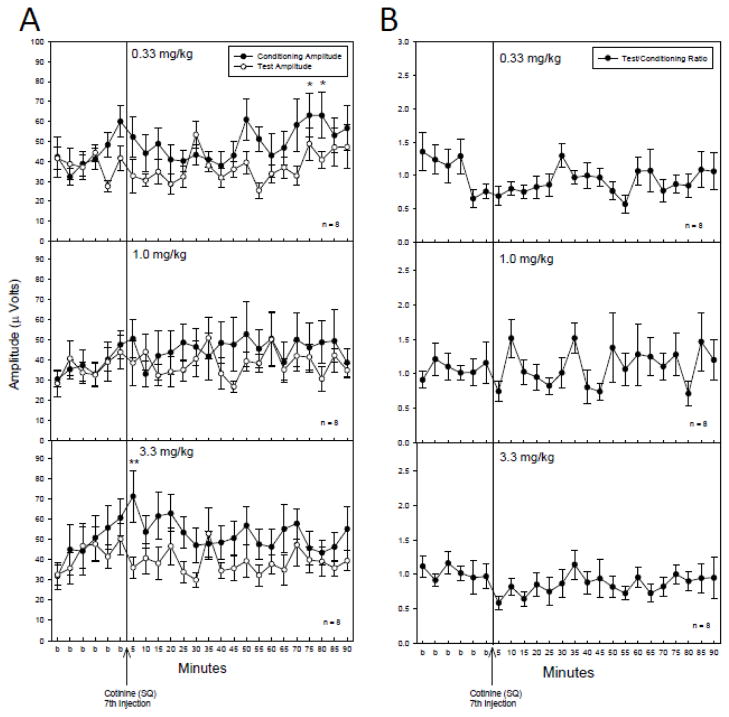
Saline administration failed to alter any parameter tested (Figure 1), therefore any effects of cotinine administration could be attributed to the drug and not due to the injection procedure. Acute administration of cotinine produced increases in the conditioning amplitude at three of the five doses tested (Figure 1A). At 0.1, 0.33 and 1 mg/kg there were significant changes in conditioning amplitude following cotinine administration with no effects on test amplitude (Figure 2A) or TC ratio (Figure 2B) as determined by a repeated measures MANOVA [0.1 mg/kg: F(23,161) = 1.96, p = 0.009; 0.33 mg/kg: F(23,161) = 2.15, p = 0.003; 1 mg/kg: F(23,161) = 2.76, p < 0.001]. Fisher’s PLSD a posteriori analysis revealed several time points with significant increases in conditioning amplitude post cotinine administration relative to the average of baseline measurements (Figure 2A). The earliest time point for significant increases in conditioning amplitude occurred 40 minutes post-cotinine injection for the 0.1 and 1 mg/kg doses and at 55 minutes for the 0.33 mg/kg doses. The effects on conditioning amplitude were evident through the 90 to 95 minute time points following injection (Figure 2A). The highest and lowest doses of cotinine, 0.033 and 3.3 mg/kg produced no significant changes in conditioning amplitude, or test amplitudes and consequently no changes in T/C ratio.
Figure 1.
The effects of acute saline injections (4 ml/kg, ip). Saline administration failed to alter conditioning, or test amplitudes, or the TC ratio. Data are mean ± SEM, n=8.
Figure 2.
A) Conditioning (●) and test (○) amplitudes before and after cotinine (SQ) administration. The 0.10, 0.33 and 1 mg/kg doses produced significant increases in the conditioning amplitude. Asterisks indicate time points following cotinine administration where a significant effect, as determined by Fisher’s PLSD, on the conditioning amplitude were found as compared to an averaged baseline (b) for the corresponding dose. B) TC ratios before and after cotinine administration. There were no significant effects of cotinine on TC ratio at any dose tested. Data are mean ± SEM. For 0.33 mg/kg, n = 4; for all other doses, n = 8. *p < 0.05, **p < 0.01.
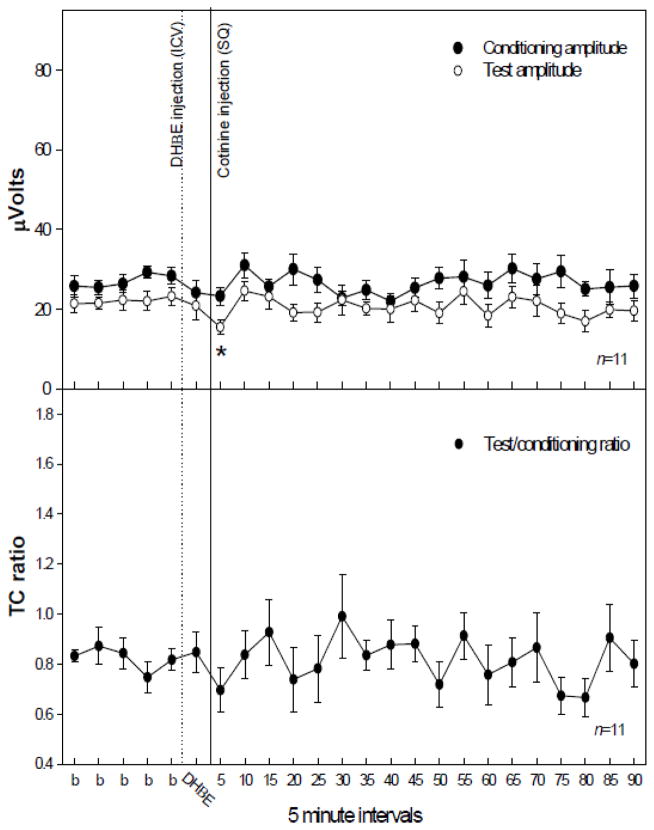
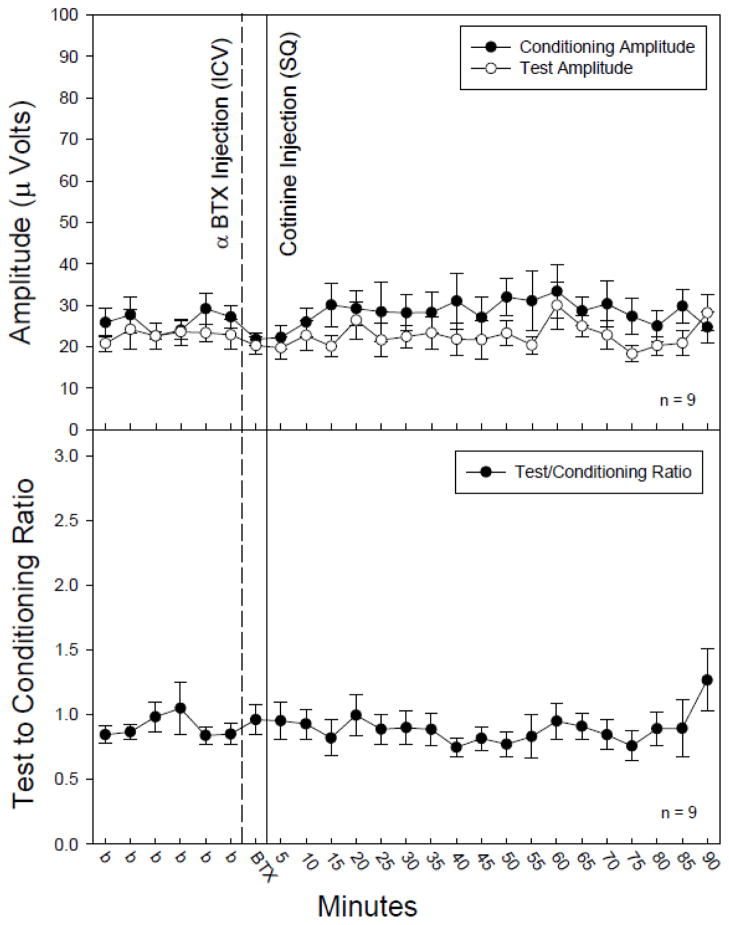
In order to determine involvement of specific nicotinic receptors following cotinine administration, selective antagonists for either the α7 or α4β2* subtypes of nicotinic receptor were administered, ICV, five minutes prior to the injection of cotinine following five baseline recordings. Blockade of the α4β2* subtype by DHβE (30 nM, ICV) prevented the significant increase in conditioning amplitude following cotinine administration (1.0 mg/kg, SQ) (Figure 3) which had been observed with cotinine (Figure 2A). However, there were significant changes in test amplitude [F(23,161) = 1.88, p = 0.013] following administration of DHβE with cotinine (Figure 3). Post-hoc analysis revealed a single time point, 5 minutes post injection, at which the test amplitude was significantly decreased as compared to the baseline averages (Figure 3). Blockade of the a7* nicotinic receptor subtype with αBTX (1.25 nM, ICV) prevented significant changes in the conditioning amplitude following cotinine (1.0 mg/kg, SQ) administration (Figure 4).
Figure 3.
The effect of dihydro-β-erythroidine (DHβE) injection (1 μl of 30 nM, ICV), an α4β2* nicotinic receptor antagonist, on conditioning (●) and test (○) and amplitudes (top figure) and TC ratio (bottom figure) prior to and following cotinine (1.0 mg/kg, SQ) administration. DHβE prevented cotinine induced increases in conditioning amplitude. The test amplitude was significantly different following cotinine administration with asterisks indicating time points significantly lower as compared to an averaged baseline (b) as determined by Fisher’s PLSD. Data are mean ± SEM. n = 11. *p < 0.05.
Figure 4.
The effect of α-bungarotoxin (αBTX) injection (1 μl of 1.25 nM, ICV), an α7 nicotinic receptor antagonist, on conditioning (●) and test (○) amplitudes (top figure) and TC ratio (bottom figure) prior to and following cotinine (1.0 mg/kg, SQ) administration. αBTX prevented cotinine induced increases in conditioning amplitude. There was no significant impact of αBTX or cotinine on TC ratio as compared to an averaged baseline (b) as determined by Fisher’s PLSD. Data are mean ± SEM. n = 9.
Chronic daily administration of cotinine produced significant effects on the conditioning amplitude after the 7th injection for only the 0.33 and 3.3 mg/kg doses. Cotinine was administered once daily for six consecutive days. On the seventh day auditory gating measurements were performed. Following six baseline recordings a seventh and final injection of cotinine was administered. A repeated measures MANOVA of the conditioning amplitude revealed significant changes for the conditioning amplitude at the 0.33 mg/kg dose [F(23,161) = 1.63, p = 0.044] (Figure 5A). Fisher’s PLSD a posteriori analysis revealed the 75 and 80 minute time points of the conditioning amplitude were significantly increased as compared to the baseline recording. The 3.3 mg/kg dose also produced significant changes for the conditioning amplitude as determined by a repeated measures MANOVA [F(23,161) = 1.72, p = 0.028] (Figure 5A). Fisher’s PLSD a posteriori analysis revealed the five minute time point of the conditioning amplitude following the 7th day injection of cotinine (3.3 mg/kg, SQ) was significantly increased as compared to the baseline recording. There were no significant effects of chronic administration of cotinine on the test amplitude (Figure 5A) or TC ratios (Figure 5B) at any dose tested. Vehicle chronic control injections of saline had no impact on conditioning or test amplitudes or T/C ratios in the DBA/2 mice (data not shown). MANOVA to compare across doses of cotinine did not reveal any significant differences between dose effects for any of the 3 parameters tested.
Figure 5.
A) The effect of chronic cotinine administration (0.33, 1.0 and 3.3 mg/kg/day) 6 days prior to the P20-N40 inhibition measurement on the 7th day of cotinine administration. There was a significant difference in the conditioning amplitude(●) at the 0.33 and 3.3 mg/kg doses with asterisks indicating specific time points at which the conditioning amplitude was significantly higher as compared to an averaged baseline (b) as determined by Fisher’s PLSD. B) There was no effect of chronic cotinine on TC ratio. Data are mean ± SEM. n = 8. *p < 0.05, **p < 0.01.
4. Discussion
The results of the present study indicate that acute cotinine produces increases the conditioning amplitude at the 0.1, 0.33 and 1.0 mg/kg doses in the P20-N40 inhibition paradigm. This increase in conditioning amplitude is evident even with 7 daily injections of cotinine, at the 0.33 and 3.33 mg/kg doses, suggesting that cotinine does not produce long-term (24 hr) receptor desensitization; the current paradigm could not determine if receptor desensitization was evident at shorter injection intervals. In models of deficient sensory inhibition, such as the DBA/2 mouse stain, a significant improvement in sensory inhibition occurs if the TC ratio decreases following administration of a drug/ligand as compared to the averaged baseline values. A TC ratio of 0.5 or less is categorized as normal sensory inhibition. A TC ratio greater than 0.5 defines abnormal or deficient sensory inhibition (Leonard et al, 1996, Freedman et al, 1997). In the present study, there were no significant changes of TC ratio following any dose of cotinine tested for either the chronic or acute administration suggesting that there were no improvements in sensory inhibition produced by cotinine administration even though there were significant changes in the conditioning amplitude.
As noted, although cotinine did not improve overall sensory inhibition in the DBA/2 mice, it did produce a significant effect on the conditioning amplitude with both chronic and acute administration. Both human and rodent studies indicate a role for the α7 nicotinic receptor in modulating sensory inhibition of the P20-N40 inhibition paradigm. Agonists selective for the α7 nicotinic receptor transiently improve sensory inhibition via suppression of the test amplitude (Stevens et al, 1998, Simosky et al. 2001, Olincy et al. 2006) purportedly through activation of this receptor on hippocampal interneurons (Alkondon et al, 1999, Alkondon and Albuquerque, 2001). Cotinine administration had only minor effects on test amplitude suggesting only minimal activity at the α7 nicotinic receptor.
Recent studies in rodents have also demonstrated a role for α4β2* nicotinic receptors in sensory inhibition. Agonists for this receptor produced increases in conditioning amplitude in the DBA/2 mouse (Radek et al, 2006; Stevens and Wear, 1997; Wildeboer and Stevens, 2008). α4β2* receptors have been observed in the molecular layer of the dentate gyrus (Clarke et al, 1985, Pauly et al, 1989). Mossy fiber axons of cells in the dentate gyrus synapse onto both pyramidal neurons and interneurons in the CA3 region of the hippocampus (Henze et al, 2000). α4β2* receptors are also found on cells within the ventral tegmental area (Clarke et al, 1985;, Swanson et al, 1987; Pauly et al, 1989), which projects diffusely to, and may be activating cells, in the dentate gyrus resulting in an increase in conditioning amplitude. It is possible that activation of α4β2* receptors resulting in an increase of the conditioning amplitude, may reflect an increase in a pre-attentional state prior to the first auditory stimulus. P50 sensory inhibition (the human equivalent to rodent P20-N40) has been shown to be related to pre-attentional state (Wan et al, 2008) so that increasing or heightening awareness could manifest as an increase in the response to the first stimulus and possibly improve learning. Cotinine accumulation in the brain following nicotine administration occurs much more slowly than nicotine and has a longer residence time in the central nervous system than nicotine (Crooks and Dwoskin, 1997). This may account for the delayed effect following acute administration of cotinine.
To specifically examine the impact of cotinine on both the α7 and α4β2* nicotinic receptor subtypes, antagonists selective for each subtype were centrally administered 5 minutes prior to the administration of 1 mg/kg cotinine. The administration of DHβE, selective for the α4β2* receptor subtype, blocked cotinine’s effect on conditioning amplitude, further suggesting involvement of this receptor subtype receptors in the effect of cotinine. Antagonism of the α4β2* receptor, in the absence of exogenous agonist administration, in this model did not alter the conditioning amplitude (Simosky et al, 2003), suggesting that the α4β2* receptors do not provide tonic control of the conditioning amplitude. The effects of agonists for the α4β2* receptor demonstrate that activation of this receptor can modulate the conditioning response (Radek et al 2006; Wildeboer and Stevens, 2008). At two time-points following cotinine and DHβE administration, the test amplitude was significantly impacted. Although there was a decrease in test amplitude, suggesting involvement of the α7 receptor, the decrease in test amplitude was not reflected in TC ratio.
Unexpectedly, increases in conditioning amplitude were blocked by α-BTX [which centrally-administered alone, did not effect sensory inhibition parameters (Simosky et al 2003)], as well as by DHβE. Dwoskin et al. found that both mecamylamine [which blocks α7 as well as α4β2* nicotinic receptors (Moon et al, 2008)], and DHβE prevented cotinine evoked dopamine release from rat striatal slices, although the effective concentrations of cotinine were higher than that normally found in the plasma of cigarette smokers (Dwoskin et al., 1999). Thus, it is possible that α7 receptors are involved in the cotinine induced effect on the conditioning amplitude. Because α4β2* receptors do not appear to underlie the tonic control of the conditioning amplitude, α7 activation may directly or indirectly affect the conditioning amplitude. It does not appear that simply injecting into the lateral ventricle, in-of-itself, blocks agonist effects on sensory inhibition because the study by Simosky et al (2003) failed to show blockade of clozapine’s effects with ICV administration of DHβE. Thus, the current data suggest that blockade of either α4β2 or α7 nicotinic receptors can alter the effects of cotinine on sensory inhibition parameters.
Although there is no data to support desensitization of the α7 receptor by cotinine, it has been postulated that desensitization of these receptors on GABAergic neurons in the hippocampus allows for activation of glutamatergic receptors which translate to improvements in cognitive abilities (Buccafusco et al, 2007; 2009).
Chronic administration of cotinine at the 0.33 and 3.33 mg/kg doses tested had a significant effect on conditioning amplitude after the 7th injection. There was also a carryover effect from the six daily injections on the baseline conditioning amplitude (prior to the 7th injection), as compared to baseline values for animals receiving an acute corresponding dose of cotinine. This is in concert with studies demonstrating a long half-life for cotinine (Davis et al, 1988; Crooks and Dwoskin, 1997). There were no effects at any chronic dose of cotinine on the test amplitude or TC ratio. A recent study in a mouse model of Alzheimer’s disease, showed that chronic cotinine stimulates hippocampal pathway which is down stream of α7 nicotinic receptor activation, the Akt/GSK3β pathway (Echeverria et al, 2011). A more recent study from this group suggests the possibility that cotinine may be functioning as a positive allosteric modulator for the α7 nicotinic receptor (Echeverria and Zeitlin, 2012). They postulate that positive modulation of the α7 nicotinic receptor, through its associated pathways, may explain improvements in learning and memory as well as the reversal of apomorphine induced deficits in prepulse inhibition (Buccafusco and Terry, 2003).
Although, it is postulated that cotinine may have positive effects on cognition in humans, based upon learning and memory improvements in non-human primate studies (Terry et al 2005), there have been only two studies utilizing direct cotinine administration to humans. One was a safety study in which high doses of cotinine were administered with no detrimental physical effects (Hatsumaki et al 1997). The other study, also with high doses, suggested mild levels of impairment in long-list recall, coupled with a mild depressant effect (Herzig et al 1998). Two studies of the effects of second-hand smoke in non-smokers over 50 years of age, assessed serum cotinine levels as well as cognition. Both studies correlated higher levels of serum cotinine with poorer cognitive performance, even when scores were adjusted for comorbid complications such as diabetes or hypertension (Llewellyn et al 2009; Akhtar et al 2013). The present studies, which suggest potential improvement in a pre-attentional state, also suggest that there would not be overall improvement in inhibition, even if improvements in cognition were to be observed. Thus, the Terry paper not withstanding, cotinine administration to schizophrenia patients may not produce beneficial outcomes.
In summary, acute administration of cotinine significantly impacted the conditioning amplitude of the P20-N40 paradigm at three of the doses tested. However, it produced no effects on either the test amplitude or TC ratio demonstrating no efficacy in improving sensory inhibition in the DBA/2 mouse model. Chronic administration of cotinine also significantly impacted the conditioning amplitude of the P20-N40 paradigm after the 7th and final injection at two of the doses tested and there is also a carryover effect from the six prior daily injections at these two doses. Antagonism of either the α4β2* or α7 nicotinic receptors prevented the significant effect of cotinine on the conditioning amplitude, indicating an involvement, either directly or indirectly, of these receptors. These results, coupled with studies of cotinine in humans suggest that this drug may not be of particular benefit to schizophrenia patients.
Highlights.
cotinine, the primary metabolite of nicotine alters sensory inhibition parameters in DBA/2 mice
the primary effect was an increase in conditioning amplitude
antagonism of α4β2 or α7 nicotinic receptors blocked the improvement
systemic cotinine was effective with either acute or chronic administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;2:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Akhtar WZ, Andresen EM, Cannell MB, Xu X. Association of blood cotinine level with cognitive and physical performance in non-smoking older adults. Environ Res. 2013;121:64–70. doi: 10.1016/j.envres.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Shuster LC, Terry AV., Jr Disconnection between activation and desensitization of autonomic nicotinic receptors by nicotine and cotinine. Neurosci Lett. 2007;413:68–71. doi: 10.1016/j.neulet.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci. 2003;72:2931–2942. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;4:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10:131–41. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- Donato MT, Viitala P, Rodriguez-Antona C, Lindfors A, Castell JV, Raunio H, Gomez-Lechon MJ, Pelkonen O. CYP2A5/CYP2A6 expression in mouse and human hepatocytes treated with various in vivo inducers. Drug Metab Dispos. 2000;28:1321–1326. [PubMed] [Google Scholar]
- Davis RM, Novotny TE, Lynn WR, editors. The health consequences of smoking: Nicotine addiction. Washington, D.C: A Report of the Surgeon General, U.S. Government Printing Office; 1999. DHHS Publication No. (CDC) 88–8406. [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Crooks PA. (S)-(−)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther. 1999;288:905–911. [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R. Cotinine: A Potential New Therapeutic Agent against Alzheimer’s disease. CNS Neurosci Ther. 2012;18:517–523. doi: 10.1111/j.1755-5949.2012.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirschner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-beta aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis. 2011;24:817–835. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R. Neurophysiological studies of sensory gating in mania: comparison with schizophrenia. Biol Psychiatry. 1983;18:989–1005. [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–78. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, Farone SV, Malaspina D, Svrakic DM, Sanders A, Gejman P. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105:20–2. [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav. 1997;57:643–650. doi: 10.1016/s0091-3057(97)80001-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Herzig KE, Callaway E, Halliday R, Naylor H, Benowitz NL. Effects of cotinine on information processing in nonsmokers. Psychopharmacology (Berl) 1998;135:127–32. doi: 10.1007/s002130050493. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R. Nicotinic receptor function in schizophrenia. Schizophr Bull. 1996;22:431–445. doi: 10.1093/schbul/22.3.431. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–96. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Dickins M, Lake BG, Eddershaw PJ, Tarbit MH, Goldfarb PS. Molecular modelling of the human cytochrome P450 isoform CYP2A6 and investigations of CYP2A substrate selectivity. Toxicology. 1999;133:1–33. doi: 10.1016/s0300-483x(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Linville DG, Williams S, Raszkiewicz JL, Arneric SP. Nicotinic agonists modulate basal forebrain control of cortical cerebral blood flow in anesthetized rats. J Pharmacol Exp Ther. 1993;267:440–448. [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Naughton F, Matthews FE. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. Brit Med J. 2009;338:b462. doi: 10.1136/bmj.b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Kim SY, Lee HG, Kim SU, Lee YB. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1–42)-stimulated microglia. Exp Mol Med. 2008;40:11–8. doi: 10.3858/emm.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–459. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Radek RJ. Effects of nicotine on cortical high voltage spindles in rats. Brain Res. 1993;625:23–28. doi: 10.1016/0006-8993(93)90133-8. [DOI] [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacology (Berl) 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Sánchez-Morla EM, García-Jiménez MA, Barabash A, Martínez-Vizcaíno V, Mena J, Cabranes-Díaz JA, Baca-Baldomero E, Santos JL. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2008;117:313–8. doi: 10.1111/j.1600-0447.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiat. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Farnham DA, Collins AC. Linkage of strain-specific nicotinic receptor alpha 7 subunit restriction fragment length polymorphisms with levels of alpha-bungarotoxin binding in brain. Brain Res Mol Brain Res. 1996;43:30–40. doi: 10.1016/s0169-328x(96)00149-0. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012 Apr 1;83(7):941–51. doi: 10.1016/j.bcp.2011.12.043. Epub 2012 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005;11:229–252. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Schade R, Callahan PM, Chapman JM, Bartlett MG. Society for Neuroscience. Washington, DC: Neuroscience Meeting Planner; 2011. Chronic treatment with nicotine metabolit, cotinine, improves sustained attention and recognition memory in rats and attenuates glutamate (NMDA) antagonist-related impairments. (568.06/Z9, P. N., ed) [Google Scholar]
- Vainio PJ, Tuominen RK. Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res. 2011;3:177–182. doi: 10.1080/14622200110043095. [DOI] [PubMed] [Google Scholar]
- Venables PH. Hippocampal function and schizophrenia. Experimental psychological evidence. Ann N Y Acad Sci. 1992;658:111–127. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- Visoni S, Meireles N, Monteiro L, Rossini A, Pinto LF. Different modes of inhibition of mouse Cyp2a5 and rat CYP2A3 by the food-derived 8-methoxypsoralen. Food Chem Toxicol. 2008;46:1190–1195. doi: 10.1016/j.fct.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wan L, Friedman BH, Boutros NN, Crawford HJ. P50 sensory gating and attentional performance. Intl J Psychophysiol. 2008;67:91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeboer KM, Stevens KE. Stimulation of the alpha4beta2 nicotinic receptor by 5-I A-85380 improves auditory gating in DBA/2 mice. Brain Res. 2008;1224:29–36. doi: 10.1016/j.brainres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]