Abstract
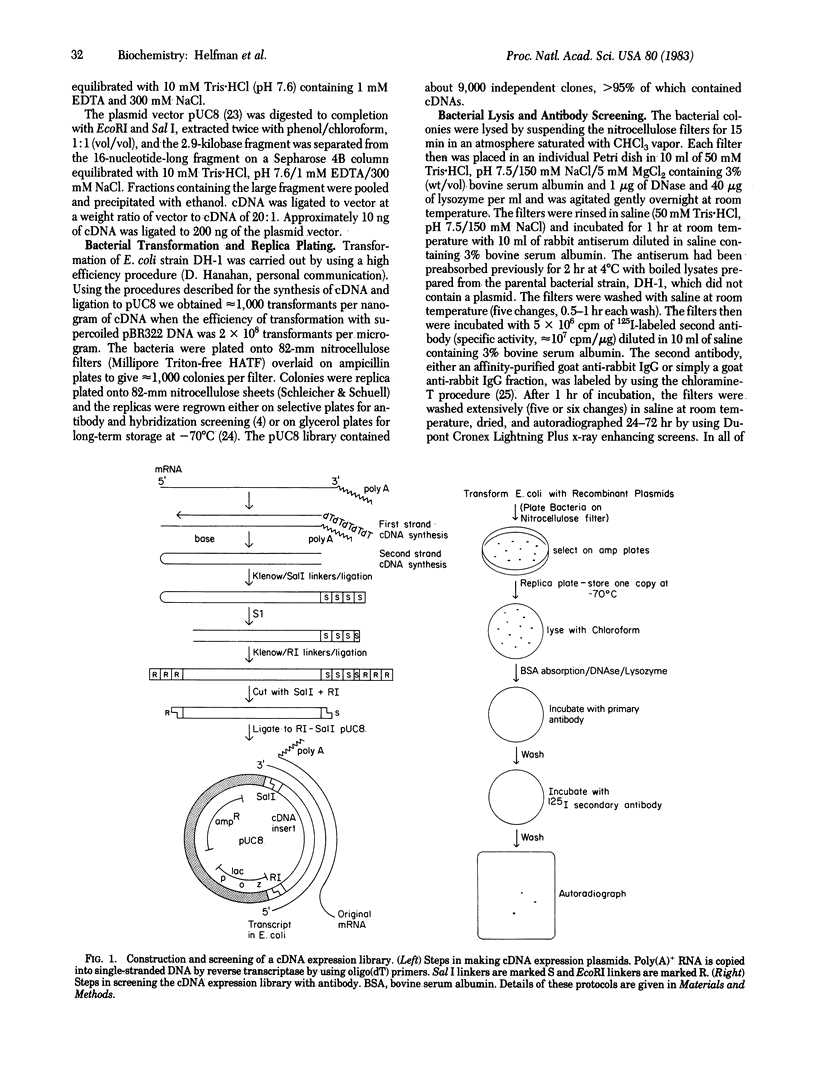
A cDNA library of approximately equal to 9,000 members has been prepared from chicken smooth muscle mRNA by using the plasmid expression vector pUC8. Addition of Sal I and EcoRI linkers at different stages during the preparation of the cDNA resulted in a population of molecules, most of which had EcoRI linkers at the end of the cDNA that corresponded to the 5' end of the template mRNA and Sal I linkers at the end that corresponded to the 3' end of the mRNA. The cDNA molecules then were inserted into a EcoRI-Sal I-cut plasmid vector, pUC8, that contains the transcriptional and translational start sequences from the lacZ gene upstream of the EcoRI site. The sequential addition of the linkers to the cDNA ensured that most of the cDNAs were inserted into pUC8 in the proper orientation for expression. The colonies were replica plated onto nitrocellulose filters and lysed in situ with chloroform vapor. The library was screened for colonies producing products immunologically related to chicken tropomyosin by incubating the filters first with a rabbit antitropomyosin antibody and second with a 125I-labeled goat anti-rabbit IgG. Two colonies were detected that reacted specifically with the antisera. Plasmids from both clones have been partially subjected to sequence analysis; both plasmids contain cDNAs that encode tropomyosin. These protocols are potentially useful for the identification of cDNA clones for genes expressed at low levels from large cDNA expression libraries.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Shapiro L., Skalka A. M. In situ immunoassays for translation products. Methods Enzymol. 1979;68:428–436. doi: 10.1016/0076-6879(79)68032-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clarke L., Hitzeman R., Carbon J. Selection of specific clones from colony banks by screening with radioactive antibody. Methods Enzymol. 1979;68:436–442. doi: 10.1016/0076-6879(79)68033-3. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Cohen S. N., McDevitt H. O. Immunological detection and characterization of products translated from cloned DNA fragments. Methods Enzymol. 1979;68:443–453. doi: 10.1016/0076-6879(79)68034-5. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. Isolation, cloning and sequence analysis of the cDNA for the alpha-subunit of human chorionic gonadotropin. Nature. 1979 Oct 4;281(5730):351–356. doi: 10.1038/281351a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiland I., Gething M. J. Cloned copy of the haemagglutinin gene codes for human influenza antigenic determinants in E. coli. Nature. 1981 Aug 27;292(5826):851–852. doi: 10.1038/292851a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Nicodemus C. F. Cloning of alpha 2u globulin cDNA using a high efficiency technique for the cloning of trace messenger RNAs. Gene. 1981 Mar;13(2):145–152. doi: 10.1016/0378-1119(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Mevarech M., Stein R., Agarwal K. L. Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1770–1774. doi: 10.1073/pnas.76.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M. The isolation of eukaryotic messenger RNA. Annu Rev Biochem. 1979;48:681–717. doi: 10.1146/annurev.bi.48.070179.003341. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]





