Abstract
Renal cell therapy has shown clinical efficacy in the treatment of acute renal failure (ARF) and promise for treatment of end-stage renal disease (ESRD) by supplementing conventional small solute clearance (hemodialysis or hemofiltration) with endocrine and metabolic function provided by cells maintained in an extracorporeal circuit. A major obstacle in the widespread adoption of this therapeutic approach is the lack of a cryopreservable system to enable distribution, storage, and therapeutic use at point of care facilities. This report details the design, fabrication, and assessment of a Bioartificial Renal Epithelial Cell System (BRECS), the first all-in-one culture vessel, cryostorage device, and cell therapy delivery system. The BRECS was loaded with up to 20 cell-seeded porous disks, which were maintained by perfusion culture. Once cells reached over 5×106 cells/disk for a total therapeutic dose of approximately 108 cells, the BRECS was cryopreserved for storage at −80°C or −140°C. The BRECS was rapidly thawed, and perfusion culture was resumed. Near precryopreservation values of cell viability, metabolic activity, and differentiated phenotype of functional renal cells were confirmed postreconstitution. This technology could be extended to administer other cell-based therapies where metabolic, regulatory, or secretion functions can be leveraged in an immunoisolated extracorporeal circuit.
Keywords: Extracorporeal cell therapy, Progenitor, Cryopreservation, Bioreactor, Acute renal failure, End-stage renal disease
INTRODUCTION
One of the most promising current approaches within regenerative medicine is cell-based therapy. While many modalities of functional organ replacement continue to face hurdles and therapeutic efficacy has yet to be realized, renal epithelial cell (REC) therapy, utilizing RECs derived from adult renal epithelial progenitor cells, along with dialysis has already shown clinical promise as a more complete ex vivo substitute for renal function (11,26). Lifesaving renal replacement therapy, utilizing extracorporeal dialysis systems, pioneered the field of biomedical engineering. Current dialysis outcomes, however, are disappointing with an annual patient mortality exceeding 20% in patients with chronic end-stage renal disease (ESRD) and greater than 50% mortality in patients in the intensive care unit with acute renal failure (ARF) (15,28). The disease state arising from renal failure is the result of more than the loss of blood volume regulation and the clearance of small solutes and toxins, which are replaced by conventional dialysis therapy. Although dialysis improves small solute clearance, it fails to address the metabolic, endocrine, and immunomodulatory functions of the kidney that are diminished in the disease state. Mortality in ARF is more directly related to the failure of extrarenal organs, rather than an insufficient dialysis dose (4). This suggests that a more complete treatment method, which provides metabolic, endocrine, and immunologic signaling function of the kidney would be clinically beneficial. REC therapy, when used in conjunction with dialysis, is a novel multifactorial approach that addresses the shortcomings of conventional therapy alone.
Current cell-based therapies differ in approach, cell sourcing, and mode of administration. Direct administration of stem cells to injured organs relies on the cells' inherent capabilities for differentiation, organization, and integration into existing tissues to restore function. This method has been shown to be efficacious for some disease states, but safety issues have not been fully addressed (3,12). Tissue engineering approaches are based on in vitro differentiation of stem/progenitor cells and organization within matrices that mimic natural cellular environments (1,14) or through the implantation of biomaterials to organize native cells into new tissue growth (23). These tissue constructs augment or replace function following implantation at the location of the defect. Alternatively, some cell therapies may be applied from outside the body as part of an extracorporeal circuit (10). The choice of the mode of administration as well as the choice of the cell source (xenogenic, allogenic, or autogenic) for cell-based therapies is important in determining efficacy (2).
An extracorporeal bioartificial kidney consisting of a renal tubule assist device (RAD) and a conventional hemofilter has been tested in US Food and Drug Administration (FDA)-approved human clinical trials and demonstrated early clinical efficacy in ARF, secondary to acute kidney injury (AKI), with a 50% reduction in the mortality rates (11,26). The conventional hemofilter provided an immunoisolated extracorporeal circuit for cell therapy in which the metabolic and endocrinologic activity of the renal progenitor-derived REC was leveraged to modulate the return of therapeutic factors to the patients. The immunoisolation provided by the filter in the extracorporeal circuit circumvented many of the inflammatory and immune rejection issues faced by other cell therapies that utilize injected cells or implanted cell constructs. An allogenic cell source allowed for the preparation of therapeutic cell devices ahead of time for on-site, immediate treatment of patients experiencing ARF. Despite the success of this translational strategy of REC therapy; a major obstacle in the widespread clinical use of cell-based devices was the inability to cryopreserve the device for storage, distribution, and therapeutic use at point of care facilities (6). To address this hurdle, the Bioartificial Renal Epithelial Cell System (BRECS) has been developed.
The BRECS is the first all-in-one culture vessel, cryopreservation storage device, and cell therapy system. This unique design allows for long-term storage and on-demand use for acute clinical applications. The BRECS was designed to maintain a dense population of adult human RECs grown on porous, niobium-coated carbon disks (CytoMatrix, Woburn, MA) (27) within a bioreactor housing maintained by perfusion culture through the disks. After the cells reach an optimum density, the BRECS can be cryopreserved, transported, and stored at a clinical site for on-demand use in an immunoisolated extracorporeal hemodialysis or peritoneal dialysis circuit, alleviating the many practical limitations previously encountered by cell-based therapies.
MATERIALS AND METHODS
BRECS Design, Fabrication, and Flow Visualization
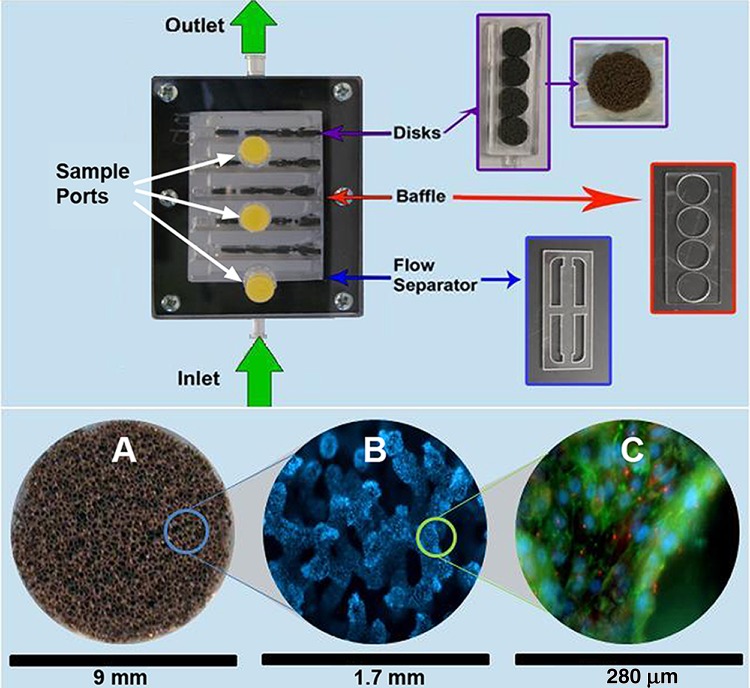
The BRECS, a perfusion, attached cell bioreactor, was designed to be fully cryopreservable. Commercially available porous niobium (Nb)-coated carbon disks were chosen to support cell adhesion and growth within the bioreactor due to their high porosity, ability to adhere matrix proteins to promote various cell type attachment (16,19,27), and ability to be cryopreserved. Briefly, the BRECS housing consisted of a top lid (polycarbonate), a bottom chamber casing (polycarbonate) with an inlet and outlet (fabricated for Innovative BioTherapies by ARL Service, LLC, Clarkston, MI), a gasket (ethylene propylene diene monomer, EPDM), and hardware (stainless steel) to hold the assembly together (Fig. 1). The BRECS as tested was approximately 9 cm×7.5 cm×3 cm, with an approximate internal fill volume of 30 ml. Cell culture media containing oxygen and nutrients were perfused through the porous disks as directed by flow baffles housed within the BRECS. An inlet flow separator, in conjunction with flow baffles, were used to create homogenous, laminar flow within the bioreactor, as visualized using 1% Trypan Blue (Sigma, St. Louis, MO) and assessed by time-lapse photography using a Canon camera.
Figure 1.
Bioartificial Renal Epithelial Cell System (BRECS) design, including depiction of internal components (top). Cells were grown on niobium-coated carbon disks (A), which were held in place within the BRECS by tuning forks. Baffles were used to critically space tuning forks and direct flow through disks. A flow separator was positioned at the inlet to maintain steady and homogeneous flow throughout the BRECS. Cells shown in (B) were stained with DAPI (blue), and cells shown in (C) were stained for zona occludens-1 (ZO-1; green) and acetylated tubulin (AT-1; red).
REC Isolation and Culture
Primary porcine (P) and human (H)RECs were isolated and expanded. PRECs were isolated from freshly excised pig kidneys, and HRECs were isolated from human kidneys procured from the National Disease Research Interchange (NDRI, Philadelphia, PA) found unsuitable for transplantation due to anatomic or fibrotic defects, utilizing established methods (21). Progenitor/adult stem cells were isolated either from porcine or human renal cortex tissue to seed BRECS units to evaluate a chronic ovine model of uremia (17,22), an acute porcine model of sepsis and multiorgan failure (9), and for eventual clinical application similar to the RAD (11,26). A recently developed technique of enhanced propagation (EP) allowed for the amplification of kidney progenitor cells from primary isolates. Application of the defined EP method resulted in an increase of up to 8 orders of magnitude in cell yield over historic, standard propagation techniques (30). Historic averages using standard propagation yielded 1.26×107 to 1.00×108 cells/g cortex, in comparison to EP yields of 1.12×109 to 9.20×1016 cells/g cortex.
Briefly, cells were cultured in Ultra MDCK media (Lonza, Walkersville, MD) supplemented with 0.5× the manufacturer's recommended dilution for insulin, transferrin, ethanolamine, and selenium supplement (ITES, Lonza), 60 ng/ml epidermal growth factor (EGF,#E1257 Sigma-Aldrich, St. Louis, MO), 10−9 M triiodothyronine (T3, #T6397, Sigma-Aldrich, St. Louis, MO), and antibiotic-antimycotic (15240-062, Invitrogen, Carlsbad, CA). PRECs were maintained under standard REC culture protocol, with continuous exposure to retinoic acid (RA)-supplemented media. HRECs were maintained under EP REC culture protocol, in which non-RA media are used to allow for maximal HREC expansion. Cells were passaged upon 70–80% confluence for a maximum of three passes, followed by seeding on carbon disks or cryopreservation for future disk seeding.
Extracellular Matrix Coating of Porous Disks
Porous, niobium (Nb)-coated carbon disks were coated with collagen IV (#354233, BD Biosciences, San Jose, CA) utilizing a two-side dynamic coating process with centrifugation: one side of a disk was loaded drop-wise with 75 µl of collagen IV, placed into a sterile 24-well plate, and centrifuged at 14.1×g for 1 min followed by a 1-h incubation. The method was then repeated on the other side of the disk.
REC Seeding of Extracellular Matrix-Coated Carbon Disks
RECs were seeded onto the collagen IV-coated disks utilizing a two-side static seeding process: a drop-wise loading of a 75-μl cell suspension in Ultra MDCK media containing 106 cells was applied onto one side of a disk followed by 1.5-h incubation at 37°C. A second identical cell loading was then applied to the other side of the disk. Following seeding, disks were loaded into tuning forks, four disks per fork, and placed in a BRECS or, for comparison, a commercially available Starwheel spinner flask (Cytomatrix, Woburn, MA). The REC-seeded disks were expanded up to an average of 5×106 cells/disk.
Maintenance of PRECs on Carbon Disks in Starwheel Spinner Flask
PREC-seeded carbon disks were cultured in a Starwheel spinner flask system proven to be efficacious for in vitro culture of cell covered Nb-coated carbon disks (27). Starwheel systems were filled with 175 ml of supplemented Ultra MDCK media at 2–4 rpm, per the manufacturer's recommendation. Media changes were performed three times a week. Weekly samples were collected to evaluate lactate production. PRECs were maintained under standard REC culture protocol, with continuous exposure to retinoic acid (RA)-supplemented media.
Maintenance of RECs on Carbon Disks in BRECS
Cell-seeded, disk-loaded tuning forks were transferred into sterilized BRECS housing units under sterile conditions. The BRECS was then placed in a 500 ml, 25 or 50 ml/min perfused, recirculation system. Medium was exchanged on the BRECS twice per week. PREC-seeded BRECS (PBRECS) was maintained under standard REC culture protocol, with continuous exposure to RA-supplemented media. HREC-seeded BRECS (HBRECS) was maintained under EP REC culture protocol, in which non-RA media were initially used to allow for optimal HREC expansion on the carbon disks, followed by continuous exposure to RA-supplemented media at 2 weeks in BRECs culture to promote renal tubule cell differentiation.
Metabolic Activity Assessment
Metabolic activity was evaluated using lactate production and glutathione degradation, with qualitative oxygen consumption rates measured via a portable clinical iSTAT analyzer (Abbott Laboratories, Abbott Park, IL). Concentrations of produced lactate were determined using a colorimetric assay (13,29) and used to estimate cell number through a correlation of lactate production and DNA quantification. Gamma-glutamyltranspeptidase (γGT) activity was determined by measuring the rate of degradation of exogenously added glutathione (GSH) in the BRECS unit. Completed Ultra MDCK media (70 ml) containing an initial concentration of 20 μM GSH (#G6257 Sigma-Aldrich) was recirculated through the BRECS unit for 1 h at 37°C, with samples collected at t=0 and 60 min for determination of GSH degradation rates. Samples were analyzed for GSH by the method of Tietze (25).
Cell Number by DNA Quantification
Quantification of total cellular DNA in BRECS was performed following the protocol as described by Sales et al. (20), with slight modifications. Each individual disk from BRECS was placed in a 15-ml centrifuge tube and extracted in 5 ml of a solution of 0.125 mg/ml papain (P4762, Sigma-Aldrich) and 10 mM/l l-cysteine dihydrochloride (Sigma-Aldrich) in phosphate-buffered EDTA (Sigma) in a 60°C water bath for 16 h. After incubation, each tube was vortexed for 3 min, aliquoted, and stored at −20°C until ready for assays. Total DNA per disk was determined using the PicoGreen dsDNA quantification kit as per the manufacturer's instructions (Molecular Probes, Eugene, OR). Standard curve was constructed by using the DNA extracted from parallel dish cultures with known cell numbers. Lactate production and DNA content were then correlated.
Cell Distribution and Cell Viability on Disks
Cell distributions on disks after culture were visualized by fluorescent 2-(4-amidinophenyl)-6- indolecarbamidine dihydrochloride (DAPI, #D9542, Sigma-Aldrich) staining of the nuclei. Cell viability was assessed by adding 1 µg/ml fluorescein diacetate and propidium iodide to individual disks in 24-well plates. Living cells and dead cells were visualized immediately with a Zeiss Axiovert 200 inverted fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with corresponding filter sets and micrographs obtained using Zeiss AxioCam MRm and ICc1 cameras (Carl Zeiss, Inc.). The disks were rinsed with sterile phosphate-buffered saline (PBS) and fixed with cold, freshly made 4% paraformaldehyde (PFA, #15700 Electron Microscopy Sciences, Hatfield, PA), placed on ice for 20 min and stained with 1 µg/ml DAPI in 0.1% Triton X-100 for 1 h dark incubation.
Immunohistochemical Analysis of HREC-Seeded Disks
Immunohistochemical analysis was performed to assess cell coverage and renal cell differentiated phenotype of the cell-seeded disks. At designated time points during BRECS culture, both before and after cryopreservation, HREC-seeded disks were removed from BRECS units, rinsed with sterile PBS, and fixed in cold, freshly made 4% paraformaldehyde for 20 min on ice, permeated with 0.1%Triton X-100 for 10 min on ice, and subsequently blocked for 2 h using 5% goat serum (#G9023, Sigma-Aldrich) and 0.1% NaN3 in PBS. Following the blocking incubation, the disks were incubated overnight at 4°C with primary antibodies (7): rabbit anti-human zona occludens-1 (ZO-1; #61-7300, Zymed, San Francisco, CA); mouse anti-human acetylated tubulin (AT-01; #32-2700 Zymed); mouse anti-aminopeptidase-N (CD13; #347830 BD Pharmingen, San Jose, CA); and mouse anti-human γGT (#MS-1272 BioVision, Mountain View, CA). After overnight incubation, the primary antibodies were removed, followed by a 2-h dark exposure to DAPI and fluorescent secondary antibodies: anti-rabbit IgG AlexaFluor 488 (Invitrogen, Carlsbad, CA) to label ZO-1 and anti-mouse IgG AlexaFluor 594 (Invitrogen) to label AT-1, CD13, and γGT. Extensive PBS washes were performed prior to visualization.
BRECS Cryopreservation and Reconstitution
Individual Disk Cryopreservation
To initially assess the cryopreservation of RECs on porous disks, studies with various cryopreservation buffers and purge solutions were conducted on single disks seeded with PRECs or HRECs. Disks were cryopreserved in 1.2 ml cryovials (Corning, Corning, NY) in an Ultralow Freezer at −80°C (Thermo Fisher Scientific, Waltham, MA), followed by transfer to the vapor phase of liquid nitrogen.
BRECS Cryopreservation
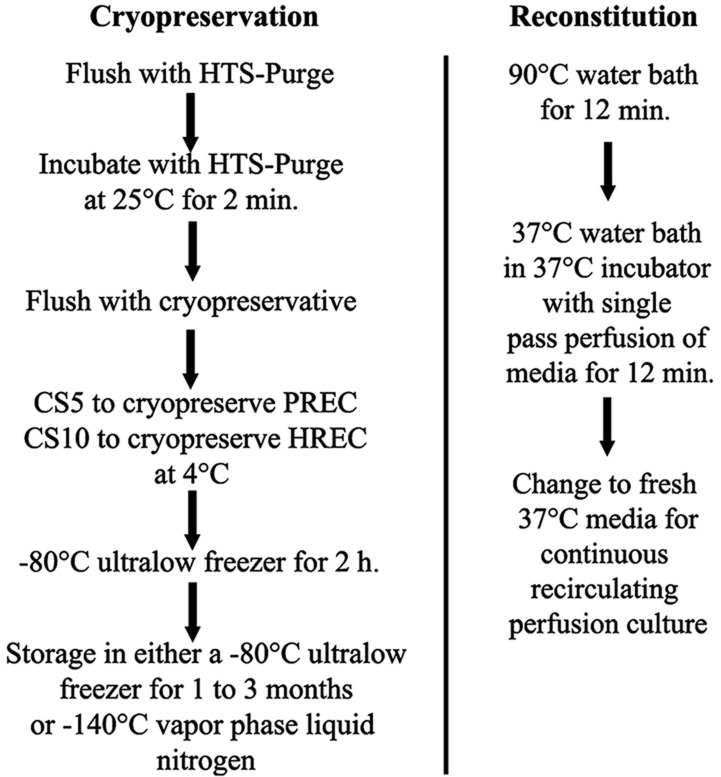
BRECSs were flushed and incubated with room temperature hypothermosol (HTS)-Purge Solution (# 637112 BioLife Solutions, Inc., Bothell, WA) for 2 min. HTS-Purge was then replaced with cryopreservation buffer, CryoStor 10 (CS10, #640222 BioLife Solutions) at 4°C to prepare HBRECS for cryostorage. PBRECS was cryopreserved with CryoStor 5 (CS5, #610202, Biolife Solutions). The temperature drop in the unit, as summarized by Figure 2, was conducted by placing the BRECS directly into an Ultralow Freezer at −80°C for 2 h, followed by transfer to the vapor phase of liquid nitrogen (−140°C) for long-term cryostorage.
Figure 2.
Flow chart of BRECS cryopreservation and reconstitution. HTS, Hypothermosol; CS5, CryoStor 5; P (or H)REC, porcine (or human) renal epithelial cell.
BRECS Reconstitution
Cryopreserved BRECS units were placed in a 90°C water bath for 12 min and then transferred to a 34–37°C beaker of water inside of a 37°C/5% CO2 incubator. Immediate single pass perfusion was initiated with 37°C media at a flow rate of 50 ml/min for up to 12 min for removal of the cryopreservation buffer. The BRECS units were then switched to a recirculation system with supplemented Ultra MDCK media for the duration of the study.
RESULTS
BRECS Flow Visualization Studies
Flow visualization studies indicated a uniform flow distribution throughout the BRECS. At a flow rate of 30 ml/ min, residence time was approximately 1 min. At flow rates tested, no significant pressure drop was measured across the BRECS, demonstrating that incorporation of the BRECS in an extracorporeal circuit would not restrict flow. All external and internal BRECS components have remained intact, with no indication of degradation during continuous culture up to 6 months tested to date. BRECS remained patent and maintained a hermetic seal during cryopreservation, reconstitution, and subsequent perfusion culture.
PREC Cultivation in BRECS and Starwheel Culture Systems
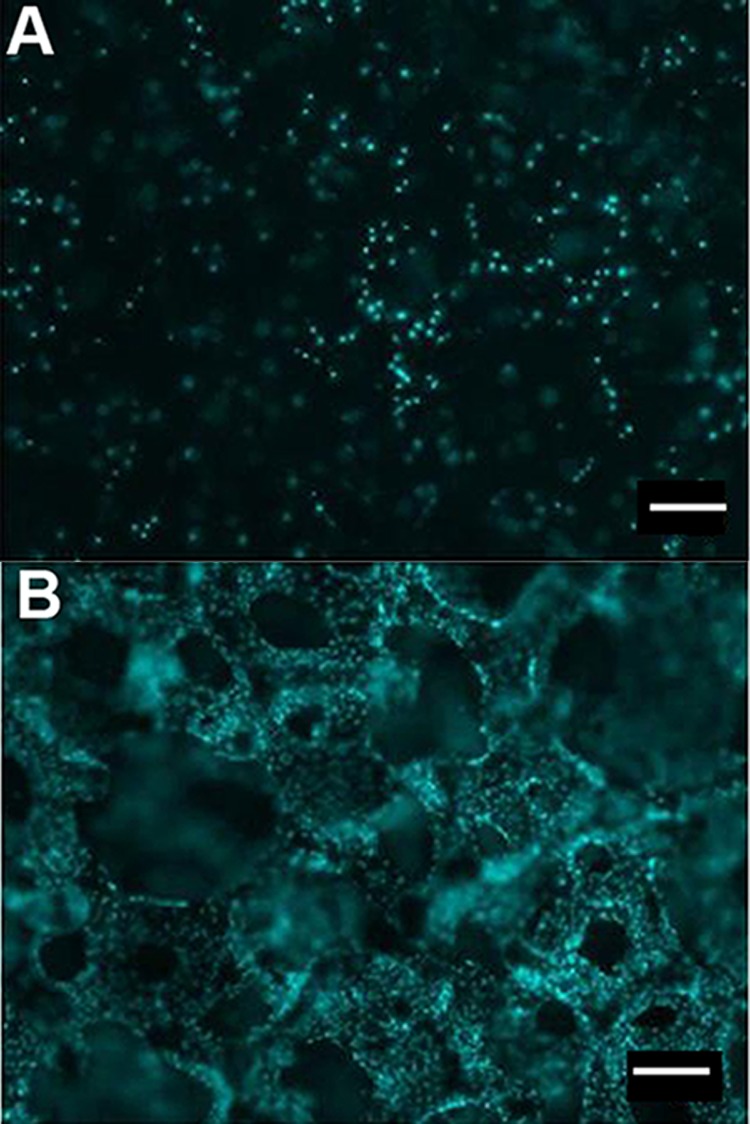
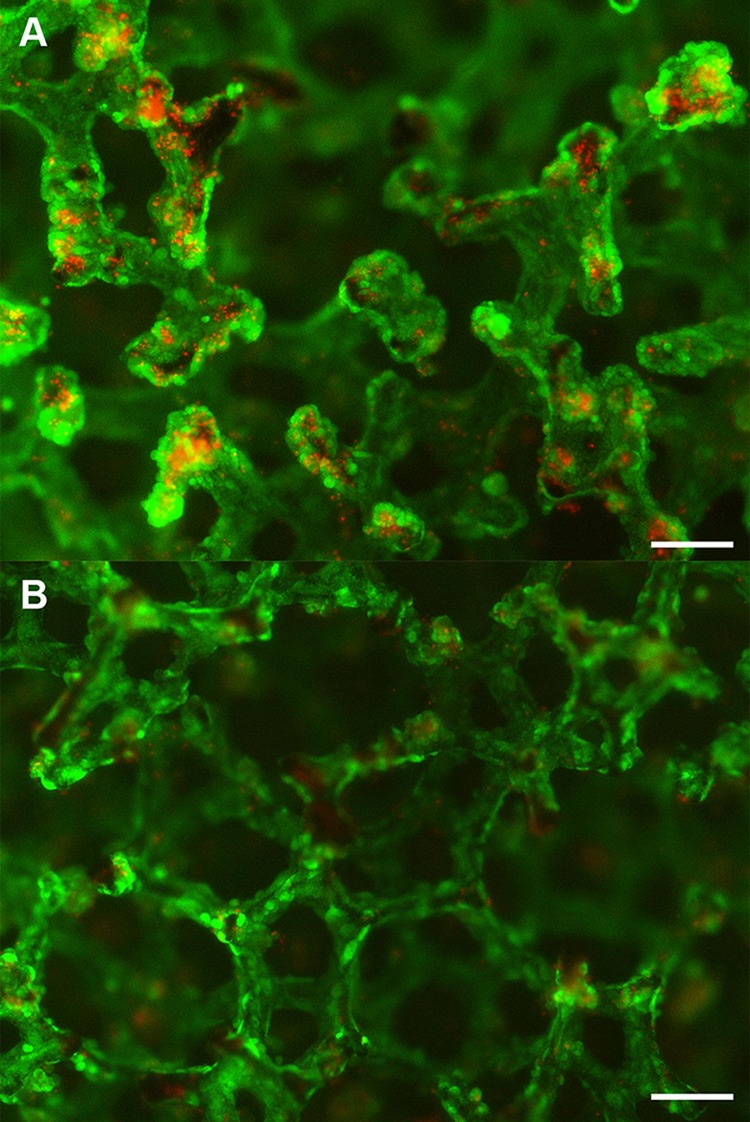
Starwheel culture systems displayed significantly lower lactate production (701.5 ± 117.9 µmol/day) throughout the period tested, accompanied by greater variability when compared to BRECS culture (926.1 ± 38.0 µmol/day). Fluorescent nuclear staining using DAPI (blue stain) demonstrated increased cell coverage on disks grown in BRECS (Fig. 3A) over those cultivated in Starwheel spinner flasks (Fig. 3B).
Figure 3.
Cell retention on Starwheel cultured disks (A) and BRECS cultured disks (B) are, respectively, shown by nuclear stain (DAPI). Of note, the significantly lower renal epithelial cell (REC) density on the Starwheel cultured disk (A) does not allow for visualization of the trabeculated disk structure, as opposed to the well-defined trabeculation observed on the BRECS cultured disk, due to high REC density (B). Scale bars: 100 µm.
REC Metabolic Activity in BRECS Perfusion Culture
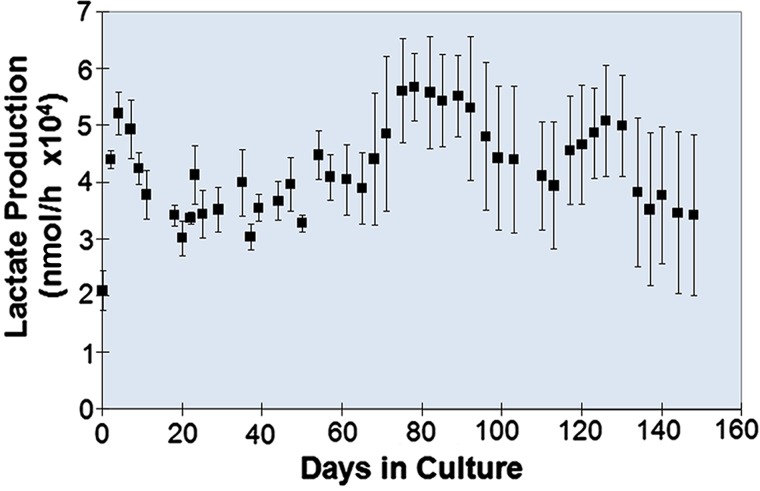
Lactate and oxygen measurements over 5 months in BRECS perfusion culture suggest that perfusion rates between 25 and 50 ml/min are adequate to maintain PREC metabolic activity. Average lactate production rates in BRECS over this 5-month time period (1004±31.5 μmol/day) were consistent with a cell number of approximately 108 cells per BRECS unit (Fig. 4), as verified by correlation of lactate to cell number by DNA quantification (10.6±2.2 μmol/106 cells/day). Qualitative assessments of oxygen consumption rates within sealed BRECS units over a 30-min, nonperfused time period demonstrated consistent oxygen concentration decreases, indicative of oxygen consumption. HBRECS had similar metabolic activity as PBRECS during culture durations studied.
Figure 4.
Lactate production and estimated cell number over the time course of PREC-seeded BRECS perfusion culture (n = 4) as averages ± standard error.
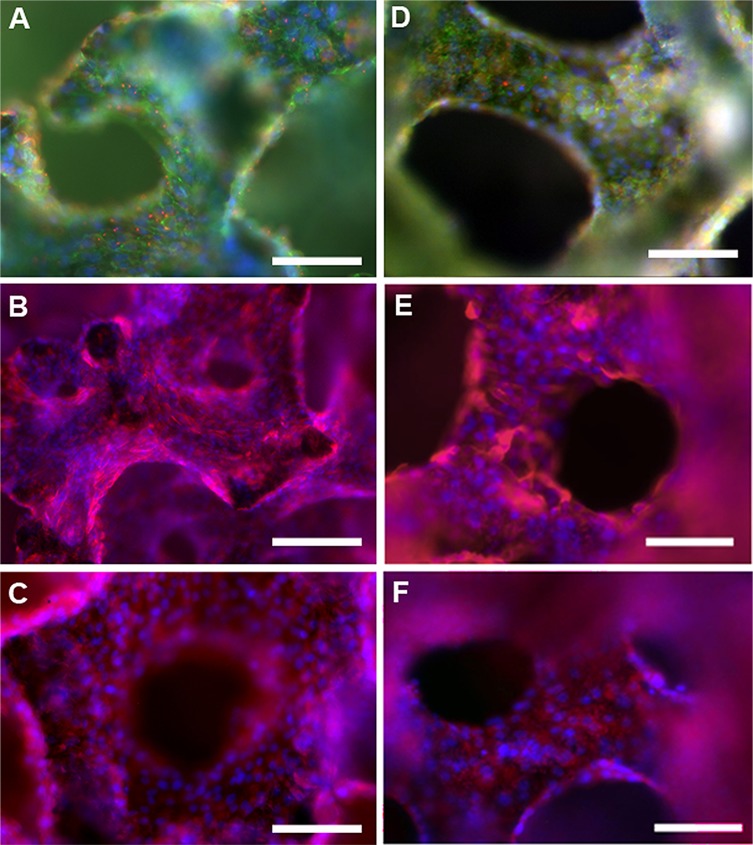
HREC Phenotype and Functionality in BRECS Perfusion Culture
HREC-seeded disks from BRECS units in culture for 3–6 weeks were processed for immunohistochemical (IHC) analysis of selected renal cell differentiated markers both before cryopreservation (Fig. 5A–C) and after reconstitution (Fig. 5D–F). In Figure 5 A and D, AT-1 (shown in red), a marker for apical central cilia of proximal tubule cells, exhibited regular staining in central regions of cells grown on disks, and ZO-1 (shown in green), a marker for epithelial tight junctions, displayed strong expression along the surface of the cells with DAPI-stained nuclei shown in blue, before cryopreservation and after reconstitution (Fig. 5A and D). ZO-1-positive tight junctions and punctate AT-1-positive central cilia are indicative of polarized epithelium and were evident in all disks tested to date. Aminopeptidase-N (CD13 shown in red, with DAPI nuclei counterstain in blue for Fig. 5B and E), a brush border enzyme that facilitates amino acid transport, and γGT (shown in red), a brush border enzyme that facilitates glutathione metabolism, were prominent along the entire disk surface (Fig. 5C and F). For all renal cell differentiation markers, similar staining patterns were observed before cryopreservation and after reconstitution.
Figure 5.
Immunohistochemical study of HRECs grown on collagen IV-coated carbon disks from BRECS units, post 4 weeks culture, before cryopreservation (A–C) and 2 weeks postreconstitution (D–F). In (A) and (D), tight junctions (ZO-1, green fluorescence), centralized cilia (AT-1, red punctate fluorescence), and nuclei (DAPI, blue) can be seen in cells across the surface area of the porous disk. In (B) and (E), prominent CD13 (red) distribution indicates presence of brush border enzyme for Na+-dependent amino acid transport, a marker for renal tubule cell phenotype, with nuclei stained (DAPI, blue). In (C) and (F), presence of Gamma-glutamyltranspeptidase (γGT; red) suggests glutathione metabolism capability of the cells, with nuclei stained by DAPI, also shown in blue). Scale bars: 100 µm.
A nondestructive glutathione (GSH) degradation assay, utilizing media supplemented with exogenous glutathione, demonstrated a consistent average GSH degradation rate of 1025.2±63.1 nmol GSH/h/BRECS over the duration of culture, indicative of sustained differentiated renal cell function.
BRECS Cryopreservation, Storage, and Cell Viability Upon Reconstitution
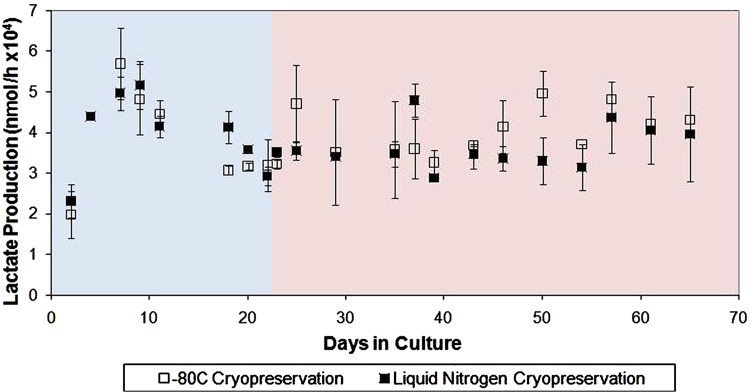
Single-disk cryopreservation studies with PRECs and HRECs achieved maximal post-thaw cell retention and subsequent cell metabolic activity of PRECs with CryoStor 5 and for HRECs, CryoStor 10. Cryopreservation studies with BRECS containing REC-seeded disks demonstrated an average cell viability greater than 80% achieved for PREC-seeded disks (Fig. 6A), after storage for 1 month at −80°C. Immediate postcryopreservation, HREC retention was approximately 90% after storage for 1 month at −80°C (Fig. 6B). PBRECS units after thaw from 1 month cryostorage at −80°C and 3 months at −140°C had similar metabolic activity pre- and postcryopreservation, as assessed by lactate production (Fig. 7). Qualitative measurements of oxygen demonstrated similar consumption rates precryopreservation and postreconstitution (n=4). BRECS have been routinely cryopreserved and cryostored for less than 1 month (n=6), 1–3 months (n=5), 3–6 months (n=2), and 6–12 months (n=2), with successful reconstitution as suggested by lactate production rates pre- and postcryopreservation.
Figure 6.
PREC (A) and HREC (B) viability after cryopreservation demonstrate a high density of living cells (fluorescein diacetate, green) and relatively low density of dead cells (propidium iodide, red). Scale bar: 100 µm.
Figure 7.
Evaluation of PREC metabolism in seeded BRECS prior to and after liquid nitrogen gas phase cryopreservation (−140°C ) for 3 months and cryopreservation in an ultralow −80°C freezer for 1 month. Blue background section designates the precryopreservation culture period, and the pink background section marks the postconstitution culture period for both cryostorage methods. Note that the representation of cryostorage has been truncated to allow for direct comparison of metabolic parameters while in culture.
DISCUSSION
BRECS therapy is built on a proven approach using an immunoisolated, extracorporeal blood circuit to deliver renal epithelial cell products to patients with acute kidney injury (AKI). The pathophysiology of AKI is due to toxic and/or ischemic damage to the lining epithelium of the renal tubules, which promote acute organ failure. Previous clinical studies with a renal assist device (RAD) that utilizes similar cells as used in the BRECS suggest that if the patient can be maintained over the subsequent 7–10 days after AKI, survival outcomes are improved, likely due to the innate ability of renal epithelium to regenerate and repair the organ (11,24,26).
The key differences between the BRECS and the RAD are as follows: (1) the BRECS supports cells on porous disks and the RAD supported cells on hollow fibers; (2) the BRECS can be cryopreserved, whereas the RAD did not have this capability due to polysulfone hollow fiber fracture during the freeze-thaw process; and (3) the BRECS utilizes both an immunoisolation prefilter to generate ultrafiltration (UF) and a separate postfilter after the BRECS to retain cell debris, whereas the RAD utilized an immunoisolation prefilter to generate UF but did not require a separate postfilter, as the hollow fiber-based RAD acted as its own postfilter to retain cell debris. Other salient features of RAD therapy remain intact for BRECS treatment. The relevant features, with respect to the therapeutic efficacy, shared by the BRECS and RAD include (1) both cell-based devices that provide supplementary metabolic and secretory renal functions, (2) both utilize an extracorporeal circuit as a platform for therapy, (3) small solute clearance is afforded by hemofiltration, and (4) reabsorption/reclamation is based on hydraulic forces generated by pumps and not active transport (11).
The cell seeding scaffold for the BRECS, niobium-coated carbon disks was selected based on their ability to adsorb matrix materials to promote cell growth. In addition, they are nonbiodegradable, so they remain intact during long-term perfusion culture and have favorable thermo-mechanical properties that enable cryopreservation. Growth of adequate cell numbers to achieve a therapeutic impact was allowed due to disks' high surface area. High porosity (80–90%) was allowed for sufficient, homogeneous delivery of oxygen and nutrients to the cells on the disks through a balance of convective and diffusive flow across and through the scaffold. The other BRECS components (the polycarbonate housing, the gasket, nuts, bolts, and access ports) were carefully selected and thoroughly tested to withstand cryogenic temperatures while still maintaining an uncompromised, sterile internal BRECS environment. For example, the specific durometer of the chosen elastomeric gaskets allowed for compression of greater than the elongation of the stainless steel screws during thaw, allowing for internal seal and device integrity throughout the cryopreservation and reconstitution process.
The BRECS design reported in this manuscript was able to be cryopreserved for long-term storage at −140°C, transitioned to −80°C for short-term storage, thawed and maintained at 37°C for potential clinical application, accompanied by an average loss in HREC viability of no greater than 10%. This data demonstrate that the BRECS is the first single device that can serve as a culture vessel to maintain cells, reach cryopreservation temperatures as a full unit, and, lastly, be reconstituted to provide cell therapy. This all-in-one design is a viable solution for current cell-based device manufacturing, storage, distribution, and end therapeutic use challenges. BRECS units can be mass-produced and seeded with a high density of HRECs from a plentiful source of human renal progenitor cells (30). The ability to cryopreserve and maintain the BRECS at liquid nitrogen temperatures will allow a clinically relevant supply of devices to be available. Short-term storage in ultralow −80°C freezers available at most clinics enables frozen distribution and on-site storage at the clinical site for on-demand therapy. Having this storage capacity makes both emergent and acute use feasible.
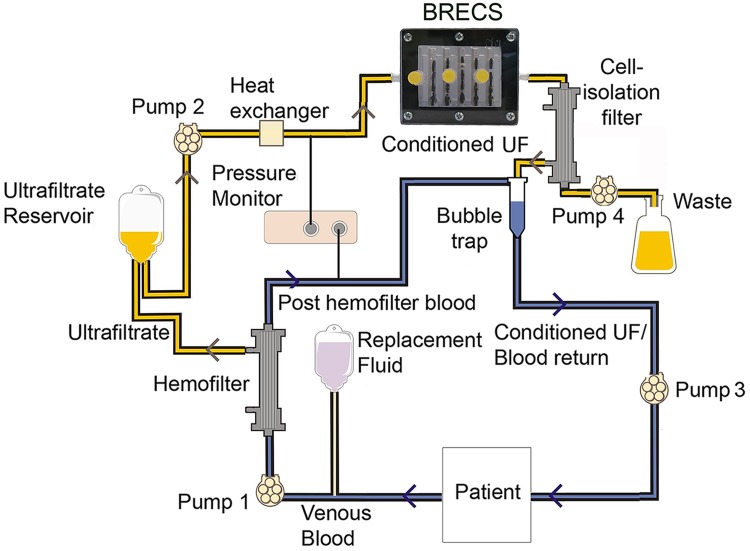
The BRECS was designed for clinical perfusion with either ultrafiltered blood or body fluids, such as peritoneal fluid, to minimize the numerous problems associated with maintaining a continuous acute or chronic extracorporeal blood circuit. The setup of an extracorporeal circuit for clinical treatment is shown in Figure 8. Immunoisolation is afforded by a 65-kDa molecular weight cut off (MWCO) pre- and postcell therapy filters, which allow circulating factors such as small proteins and hormones to be presented to the cells of the therapy device, while protecting the device from immune system components. BRECS cell products, such as secreted small proteins and hormones, are allowed to pass through the postfilter and return to the patient. Furthermore, in the BRECS circuit, the uremic state can be controlled by modulating the small solute clearance through hemofiltration dose via a change in prefilter surface area, filtration rate, and flow rate to waste. In preclinical testing of the BRECS for the treatment of ARF caused by septic shock in a porcine model (17), PBRECS was demonstrated to be therapeutically efficacious in this extracorporeal circuit, along with improved cardiovascular performance and prolonged survival time (31).
Figure 8.
Proposed extracorporeal circuit for future clinical treatment. Pre- and post-BRECS filters allow immunoisolation of the therapeutic cells from the patient's body. UF = ultrafiltration.
The fluid dynamics of the BRECS was optimized so that perfusion rates from 25 to 50 ml/min, both within an extracorporeal circuit or in cell culture perfusion, provide adequate nutrient and oxygen delivery without the use of an oxygenator. In comparison to a commercially available culture system available through the carbon disk manufacturer to cultivate cells grown on porous disks, the Starwheel system, the BRECS was superior with respect to cell attachment and viability over culture duration, suggestive that perfusion culture may be more amenable for RECs over centrifugal, spinning culture. In the longest in vitro studies to date, cell viability was maintained within BRECS units for over 5 months. The general metabolic activity of cells within the BRECS, assessed with lactate production and qualitative oxygen consumption, demonstrated a consistent metabolic profile throughout the in vitro culture duration. The presence of ZO-1, a renal epithelial tight junction marker, and AT-1, as a component of the highly differentiated central cilium, confirmed epithelial characteristics (7). The presence of aminopeptidase (CD13) and γ-GT, differentiated apical membrane enzymes, confirmed a highly differentiated functional phenotype both before cryopreservation and after reconstitution. These cells also maintained renal cell-specific glutathione metabolism, a critical function to maintain glutathione homeostasis in vivo. In the preclinical testing of the BRECS for the treatment of ESRD, BRECS maintained viability and exhibited continued glutathione metabolism in a peritoneal dialysis extracorporeal circuit in a chronic ovine model of uremia for 7 days (22).
The fill volume (30 ml) and footprint (9 cm×7.5 cm × 3 cm) make the pocket-sized BRECS easy to handle, ship, and store. Furthermore, a modified BRECS design to be mass manufactured will have an even smaller footprint and fill volume. This portability also allows for the future incorporation into a wearable dialysis system. Although no current wearable dialysis system exists, recent advancements in pump miniaturization and technology to reduce the volume of regenerated dialysate during continuous peritoneal dialysis are important steps to an engineered solution (5,8,18). In this regard, the BRECS current cell durability suggests that it could be utilized for at least 6 months in a chronic use application without need for replacement, ideal for a renal assist device in a continuous peritoneal dialysis circuit.
The current BRECS design utilizes renal epithelial cells; however, this technology could be extended to administer other cell-based therapies. Prerequisites for use of other cells within this system are that the cell type (1) is adherent; (2) can be cultured in isolation or simple coculture; (3) is amenable to being maintained by low-shear perfusion culture; and (4) has anabolic, catabolic, regulatory, or secretion functions that can be leveraged in an extracorporeal circuit for therapeutic impact.
ACKNOWLEDGMENTS
We thank Brian Matty, Tim Angeli, Mark Neitzke, Min Wang, Allen Zeitlin, Miller Tsai, Tyson Delandsheer, and Simin Abrishami for providing invaluable technical support toward the findings in these studies.
This work was supported by the US Army Medical Research and Materiel Command, Contract W81XWH-05-2-0010 and W81XWH-10-2-0137, and the Small Business Innovation Research program of the National Institutes of Health, Grants NIDDK R44 DK074289 and NIDDK R43 DK082050.
We acknowledge the provision of human kidneys, used to isolate renal epithelial cells, by the National Disease Research Interchange (NDRI) with support by grant number 5 U42 RR006042 from NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
The Michigan Memorial Phoenix Project (MMPP) provided services for the completion of this study.
HDH is a shareholder of Innovative BioTherapies, Inc., and CytoPherx, Inc. DAB, CJP, LC, AJW, and GH are employees or former employees of Innovative BioTherapies, Inc.
REFERENCES
- 1. Alsberg E.; Anderson K. W.; Albeiruti A.; Rowley J. A.; Mooney D. J. Engineering growing tissues. Proc. Natl. Acad. Sci. USA 99(19):12025–12030; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atala A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 7(1):15–31; 2004. [DOI] [PubMed] [Google Scholar]
- 3. Brodie J. C.; Humes H. D. Stem cell approaches for the treatment of renal failure. Pharmacol. Rev. 57(3):299–313; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Casino F. G. [Dialysis dose quantification in critically ill patients]. G. Ital. Nefrol. 27(4):383–390; 2010. [PubMed] [Google Scholar]
- 5. Davenport A.; Gura V.; Ronco C.; Beizai M.; Ezon C.; Rambod E. A wearable haemodialysis device for patients with end-stage renal failure: A pilot study. Lancet 370(9604):2005–2010; 2007. [DOI] [PubMed] [Google Scholar]
- 6. Fahy G. M.; Wowk B.; Wu J. Cryopreservation of complex systems: The missing link in the regenerative medicine supply chain. Rejuvenation Res. 9(2):279–291; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Fissell W. H.; Manley S.; Westover A.; Humes H. D.; Fleischman A. J.; Roy S. Differentiated growth of human renal tubule cells on thin-film and nanostructured materials. ASAIO J. 52(3):221–227; 2006. [DOI] [PubMed] [Google Scholar]
- 8. Gura V.; Ronco C.; Nalesso F.; Brendolan A.; Beizai M.; Ezon C.; Davenport A.; Rambod E. A wearable hemofilter for continuous ambulatory ultrafiltration. Kidney Int. 73(4):497–502; 2008. [DOI] [PubMed] [Google Scholar]
- 9. Humes H. D.; Buffington D. A.; Lou L.; Abrishami S.; Wang M.; Xia J.; Fissell W. H. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Crit. Care Med. 31(10):2421–2428; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Humes H. D.; Buffington D. A.; MacKay S. M.; Funke A. J.; Weitzel W. F. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat. Biotechnol. 17(5):451–455; 1999. [DOI] [PubMed] [Google Scholar]
- 11. Humes H. D.; Weitzel W. F.; Bartlett R. H.; Swaniker F. C.; Paganini E. P.; Luderer J. R.; Sobota J. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 66(4):1578–1588; 2004. [DOI] [PubMed] [Google Scholar]
- 12. Imai E.; Iwatani H. The continuing story of renal repair with stem cells. J. Am. Soc. Nephrol. 18(9):2423–2424; 2007. [DOI] [PubMed] [Google Scholar]
- 13. Krieg A. F.; Rosenblum L. J.; Henry J. B. Lactate dehydrogenase isoenzymes a comparison of pyruvate-to-lactate and lactate-to-pyruvate assays. Clin. Chem. 13(3):196–203; 1967. [PubMed] [Google Scholar]
- 14. Levenberg S.; Huang N. F.; Lavik E.; Rogers A. B.; Itskovitz-Eldor J.; Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA 100(22):12741–12746; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta R. L.; Pascual M. T.; Soroko S.; Savage B. R.; Himmelfarb J.; Ikizler T. A.; Paganini E. P.; Chertow G. M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 66(4):1613–1621; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Poznansky M. C.; Evans R. H.; Foxall R. B.; Olszak I. T.; Piascik A. H.; Hartman K. E.; Brander C.; Meyer T. H.; Pykett M. J.; Chabner K. T.; Kalams S. A.; Rosenzweig M.; Scadden D. T. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol. 18(7):729–734; 2000. [DOI] [PubMed] [Google Scholar]
- 17. Rojas A.; Hageman G.; Lou L.; Ding F.; Song J.; Jung J.; Cook K.; Buffington D.; Humes H. Animal model of a wearable bioartificial kidney using peritoneal dialysis ASAIO 2009 Annual Meeting; 2009. [Google Scholar]
- 18. Ronco C.; Davenport A.; Gura V. Toward the wearable artificial kidney. Hemodial. Int. 12 Suppl. 1:S40–S47; 2008. [DOI] [PubMed] [Google Scholar]
- 19. Rosenzweig M.; Pykett M.; Marks D. F.; Johnson R. P. Enhanced maintenance and retroviral transduction of primitive hematopoietic progenitor cells using a novel three-dimensional culture system. Gene Ther. 4(9):928–936; 1997. [DOI] [PubMed] [Google Scholar]
- 20. Sales V. L.; Engelmayr Jr. G. C.; Mettler B. A.; Johnson Jr. J. A.; Sacks M. S.; Mayer J. E. Jr. Transforming growth factor-beta1 modulates extracellular matrix production, proliferation, and apoptosis of endothelial progenitor cells in tissue-engineering scaffolds. Circulation 114(1Suppl.):I193–I199; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Smith P.; Buffington D.; Humes H. Kidney epithelial cells. In: Klimanskaya I.; Lanza R., eds. Methods In Enzymology. San Diego: Elsevier Academic Press; 2006:194–207. [DOI] [PubMed] [Google Scholar]
- 22. Song J.; Jung J.; Ding F.; Westover A.; Hageman G.; Lou L.; Rojas A.; Charles L.; Smith P.; Buffington D.; Humes H. Uremic animal model of bioartifical renal cell system (BRECS) using continuous flow peritoneal dialysis-based extracorporeal circuit. American Society of Nephrology; 2009. [Google Scholar]
- 23. Stevens M. M.; Marini R. P.; Schaefer D.; Aronson J.; Langer R.; Shastri V. P. In vivo engineering of organs: The bone bioreactor. Proc. Natl. Acad. Sci. USA 102(32):11450–11455; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swann R. C.; Merrill J. P. The clinical course of acute renal failure. Medicine 32(2):215–292; 1953. [DOI] [PubMed] [Google Scholar]
- 25. Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27(3):502–522; 1969. [DOI] [PubMed] [Google Scholar]
- 26. Tumlin J.; Wali R.; Williams W.; Murray P.; Tolwani A. J.; Vinnikova A. K.; Szerlip H. M.; Ye J.; Paganini E. P.; Dworkin L.; Finkel K. W.; Kraus M. A.; Humes H. D. Efficacy and safety of renal tubule cell therapy for acute renal failure. J. Am. Soc. Nephrol. 19(5):1034–1040; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Upton T.; von Schild E.; Arnold L.; Finchinger J. Biomimetic 3-D matrix for high-density cell culture. [White paper]. Cytomatrix, Woburn, MA; 1997. [Google Scholar]
- 28. US Renal Data System. USRDS 2006 annual data report: Atlas of end-stage renal disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 29. Van Den Hamer C. J.; Elias R. W. A method for the determination of D(-)-lactic acid. Biochim. Biophys. Acta 29(3):556–562; 1958. [DOI] [PubMed] [Google Scholar]
- 30. Westover A. J.; Buffington D. A.; Humes H. D. Enhanced propagation of adult human renal epithelial cells to improve cell sourcing for tissue-engineered therapeutic devices for renal diseases. J. Tissue Eng. Regen. Med. 6(8):589–597; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westover A.; Lou L.; Smith P.; Jung J.; Buffington D.; Johnston K.; Humes H. Evaluation of bioartificial renal epithelial cell system therapy in a porcine septic shock model. American Society of Nephrology: Renal Week 2010 Denver, CO; 2010. [Google Scholar]