Abstract
BACKGROUND
Cardiovascular disease is the major cause of morbidity, mortality, and disability in Iranian people. Inflammation and oxidative processes are key components of cardiovascular disease. The aim of this study was to evaluate the effect of conjugated linoleic acids (CLA) and omega-3 fatty acid (ω-3 fatty acids) supplementation on inflammation markers and oxidative stress in atherosclerotic patients.
METHODS
This study was a two-month clinical, randomized trial. 90 volunteers who referred to Emam Reza Heart Clinic of Shiraz University of Medical Sciences (Shiraz, Iran) from February to March 2011 and had the inclusion criteria of this study were selected. Participants were classified into 3 groups receiving 3 g/d CLA, 1920 mg/d ω-3, or placebo for 2 months. C-reactive protein (CRP), interleukin-6 (IL-6), malondialdehyde (MDA), and glutathione peroxidase (GPx) were measured before and after supplementation.
RESULTS
The hs-CRP level decreased significantly in both the omega-3 and CLA group (P < 0.05). IL-6 reduced significantly in the ω-3 group, but the reduction of IL-6 levels in the CLA group was not significant. GPx increased in the CLA and omega-3 groups (P < 0.05). MDA level decreased significantly in both omega-3 and CLA groups (P < 0.05). Comparison between the groups indicates a significant change in CRP levels in the ω-3 group relative to the control group. However, other indices did not cause any significant change in the ω-3 and CLA groups in comparison to the control group.
CONCLUSION
Diet supplementation with CLA and ω-3 can have a beneficial effect on some indices of inflammatory and oxidative stress.
Keywords: Atherosclerosis, Inflammation, Oxidative Stress, Conjugated Linoleic Acids
Introduction
Cardiovascular disease is the serious cause of mortality in developed and developing countries.1 This disease is also the major cause of morbidity, mortality, and disability in Iranian people and accounts for nearly 50% of mortality each year.2 Recent research has found atherosclerosis to be a chronic inflammation that leads to an acute clinical event by plaque rupture.3 Inflammation appears by different stimuli, such as oxidative stress. Oxidative metabolites can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and increase induction of proinflammatory cytokines.4-6 NF-κB is a group of transcription factors that regulate the inflammatory and immune response.7 inflammation and oxidative stress are crucial in the atherosclerosis process.8
The anti-inflammatory and antioxidant effect of nutrients may improve cardiovascular disease.7,8 Today, there is a widespread interest in the health beneficial properties of conjugated linoleic acids (CLA) and ω-3 fatty acids.9 CLA was found naturally in food from ruminant animals such as dairy and meat products.10 For nearly a decade, the health benefits of CLA have been investigated in animal models. It has been observed that animals fed an atherogenic diet and supplemented with CLA had significantly less aortic lesions.11,12 Nagao and Yanagita have also found 30% regression of atherosclerotic lesions in CLA supplemented rabbits.11 ω-3 fatty acids are essential fatty acids that the human body needs for metabolic function.13 There are considerable evidence from randomized controlled trials (RCTs) indicating that ω-3 fatty acids from fish and fish oil are protective against atherosclerosis.14 Some studies attributed these prospective properties of omega-3 fatty acids on atherosclerosis to its anti-inflammatory effect.15,16 To the best of our knowledge, this paper was the first human study which assessed the effect of CLA supplementation on atherosclerosis patients. Due to high prevalence of atherosclerosis in the Iranian population, this study was carried out to evaluate the effect of omega-3 fatty acids and CLA on inflammatory and oxidative stress markers in atherosclerotic patients.
Materials and Methods
This was a 2-month clinical randomized trial. To determine the sample based on power = 80% and α = 0.05, the results of the study of Omrani et al. was used.17 The sample size in each group was calculated as 30. Therefore, 90 atherosclerotic patients (40 males and 50 females) aged 30 to 60 years with angiographically diagnosed coronary atherosclerosis who were referred to Emam Reza Heart Clinic from February to March 2011 were recruited for this study. Volunteers had the following criteria: history of angina, myocardial infarction or bypass surgery, body mass index (BMI) of 18.5-24.9 kg/m2, no pregnancy, and no dietary supplements. Volunteers with acute heart failure, acute arrhythmia, or chronic inflammatory disease were excluded from the study. Most of the patients consumed lipid lowering drugs; thus, the dosage and type of these drugs were kept consistent. Participants followed their regular diet and physical activity during the study. To determine the food intake and macro- and micronutrient consumptions of participants, the food frequency questionnaire (FFQ) was completed for each patient at the beginning of the study.
The study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran. All participants gave a written informed consent. The volunteers were randomly divided into 3 groups using balanced block randomization (BBR) protocol. They were allocated to receive 3 g/d CLA (3 × 1 g soft gel, a 50:50 isomer blend of cis-9 trans-11 and trans-10 cis-12), 1920 mg/d omega-3 fatty acids (3 × 640 mg soft gel blend of 210 mg DHA and 310 mg EPA), and the placebo.
CLA soft gel was obtained from Puritan's Pride (USA) and omega-3 fatty acids soft gel was produced by Seven Seas Ltd (UK). Placebo (olive oil) was produced by Zahravi Pharmaceutical Company (Tehran, Iran). Each group was invited separately to take their supplements every two weeks and the researcher supervised ingestion of supplements every week.
Procedure
Blood sampling: Fasting blood samples (5 cc) were collected at the beginning and the end of the study and immediately centrifuged (3000 × g, 10 min, 4ºC); then, the plasma was placed into a tube and stored at -70oC until analysis for high sensitivity C-reactive protein (hs-CRP), IL-6, malondialdehyde (MDA), and glutathione peroxidase (GPx).
Anthropometric assessment: Body weight was measured by Seca 713 scale while the subjects were minimally clothed and their height was determined using measuring tape without shoes. Then, BMI [weight (kg) / hight2 (m)] was calculated.
Biochemical analysis: Hs-CRP measurement was done by a highly sensitive enzyme-linked immunosorbent assay kit (IBL, Minnesota, USA), and IL-6 assay was performed by radioimmunoassay kit (IRMA source, Belgium, Louvain-la-Neuve). GPx enzyme activity was measured by the coupled enzyme assay commercial kit (Cayman, Michigan, USA). GPx catalyzes the oxidation of glutathione (GSH) by cumene hydroperoxide, in the presence of glutathione reductase (GR) and Nicotinamide adenine dinucleotide phosphate (NADPH); oxidized glutathione (GSSG) is immediately converted into the reduced form with concomitant oxidation of NADPH to NADP+. Decrease in absorbance at 340 nm is measured.18 MDA was determined using the thiobarbituric acid (TBA) method.
Statistical analysis
Data were analyzed using SPSS for Windows (version 19; SPSS Inc., Chicago, IL, USA). Normality of the data was evaluated by the Kolmogorov-Smirnov test. Normality distributed data were expressed as mean ± standard deviation. Paired t-test was used for within-group effects from baseline. Differences between groups from baseline to 8 weeks were assessed using ANOVA followed by a post-hoc Dunnett analysis. FFQ was analyzed using Food Processor Nut4 software. P values < 0.05 were considered statistically significant.
Results
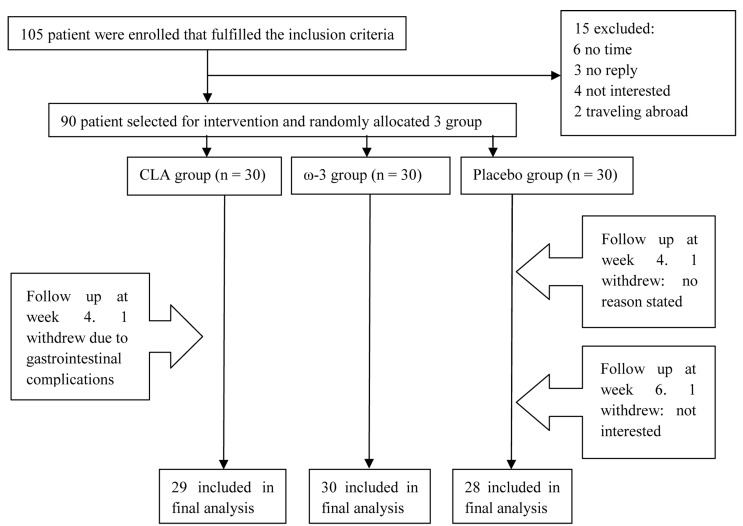
As shown in figure 1 three patients were excluded during the study and finally data from 87 patients (39 men and 48 women) were collected and analyzed and with on average over 95% of supplements being apparently consumed by trial participants. Moreover, there were no significant differences in terms of dosage and type of lipid lowering drugs between the groups. As shown in tables 1 and 2, age, weight, height, body mass index, disease duration, and biochemical markers did not differ significantly between the groups. Concerning differences in food intake between patients, analysis of food frequency questionnaire showed no differences in food intake between the patients (results will be presented in a separate article). At the end of the study, CRP differed significantly in CLA group as compared to baseline. However, this was not the case with IL-6, although a decreasing trend was seen in IL-6 status (16.1 ± 10.2 vs 12.9 ± 8.1). ω-3 supplementation reduced both CRP and IL-6 significantly during the study compared to baseline (Table 3).
Figure 1.
Flow chart of a randomized control trial CLA: Conjugated linoleic acids
Table 1.
Baseline characteristics of the study population
| Control group (n = 28)* | CLA group (n = 29) | Omega-3 group (n = 30) | P*** | |
|---|---|---|---|---|
| Age (year) | 55.85 ± 14.13** | 52.79 ± 14.11 | 54.53 ± 15.21 | 0.29 |
| Weight (kg) | 68.21 ± 7.82 | 67.06 ± 8.01 | 67.66 ± 7.96 | 0.89 |
| Height (cm) | 166.21 ± 5.75 | 167.51 ± 9.57 | 166.80 ± 6.33 | 0.48 |
| BMI (kg/m2) | 24.66 ± 2.34 | 24.02 ± 2.76 | 24.30 ± 2.34 | 0.68 |
| Cardiovascular disease duration (year) | 3.89 ± 2.00 | 3.50 ± 2.05 | 4.10 ± 1.96 | 0.56 |
CLA: Conjugated linoleic acids; BMI: Body mass index;
N refers to the number of participants in each group
All values are mean ± SD
Significance was determined using one-way ANOVA
Table 2.
Baseline biochemical markers of the study population
| Control group (n = 28)* | CLA group (n = 29) | Omega-3 group (n = 30) | P*** | |
|---|---|---|---|---|
| hs-CRP (mg/l) | 5.08 ± 5.02** | 7.48 ± 5.64 | 4.43 ± 4.13 | 0.05 |
| IL-6 (pg/ml) | 12.88 ± 9.13 | 16.13 ± 10.21 | 18.59 ± 11.12 | 0.11 |
| MDA (mol/l) | 4.46 ± 2.52 | 3.7 ± 1.77 | 3.98 ± 1.50 | 0.37 |
| GPx (nmol/ml/min) | 172.06 ± 55.84 | 125 ± 46.06 | 144.57 ± 56.89 | 0.07 |
CLA: Conjugated linoleic acids; Hs-CRP: High-sensitivity C-reactive protein; IL-6: Interleukin-6; MDA: Malondialdehyde; GPx: Glutathione peroxidase
N refers to the number of participants in each group;
All values are mean ± SD
Significance was determined using one-way ANOVA
Table 3.
Effect of supplementation on biochemical indices at the end of the study
| Indices | Control group (n = 28)* | CLA group (n = 29) | Omega-3 group (n = 30) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Week 0 | Week 8 | P*** | Week 0 | Week 8 | P*** | Week 0 | Week 8 | P*** | |
| Hs-CRP (mg/l) | 5.08 ± 5.02** | 5.03 ± 4.46 | 0.90 | 7.48 ± 5.64 | 5.95 ± 5.87 | 0.010 | 4.43±4.13 | 1.60±1.41 | 0.010 |
| IL-6 (pg/ml) | 12.88 ± 9.13 | 13.51 ± 8.86 | 0.70 | 16.13 ± 10.21 | 12.95 ± 8.10 | 0.060 | 18.59±11.12 | 13.37±9.44 | 0.040 |
| MDA (mol/l) | 4.46 ± 2.52 | 3.64 ± 1.32 | 0.09 | 3.7 ± 1.77 | 2.4 ± 0.80 | < 0.001 | 3.98±1.50 | 2.87±1.55 | 0.001 |
| GPx (nmol/ml/min) | 172.06 ± 55.84 | 194.13 ± 105.42 | 0.14 | 125 ± 46.06 | 171.4 ± 68.90 | < 0.001 | 144.57±56.89 | 174.61±62.80 | 0.001 |
| BMI (kg/m2) | 24.66 ± 2.34 | 24.70 ± 2.26 | 0.31 | 24.02 ± 2.76 | 23.98 ± 2.78 | 0.370 | 24.30±2.34 | 24.40±2.34 | 0.450 |
CLA: Conjugated linoleic acids; Hs-CRP: High-sensitivity C-reactive protein; IL-6: Interleukin-6; MDA: Malondialdehyde; GPx: Glutathione peroxidase; BMI: Body mass index
N refers to number of participant in each group
Values are mean ± SD
Significance was determined using paired t-test
In both CLA and omega-3 groups, MDA and GPx reduced significantly as compared to the baseline (Table 2). As shown in table 4, there were no significant changes in mean differences of MDA and GPx between the groups. Although hs-CRP and IL-6 differed significantly in the ù-3 group relative to the placebo group, there was no significant change in mean differences of hs-CRP and IL-6 in CLA groups in comparison to the placebo (Table 4).
Table 4.
Mean differences† and changes in biochemical indices
| Control group (n = 28)* | CLA group (n = 29) | Omega-3 group (n = 30) | P | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean difference | Changes** (%) | Mean difference | Changes** (%) | Mean difference | Changes** (%) | ||
| hs-CRP (mg/l) | -0.05 ± 0.78*** | -0.98 | -1.52 ± 0.77 | -20.00 | -2.80‡ ± 0.76 | -63.88 | 0.04 |
| IL-6 (pg/ml) | 0.62 ± 1.66 | 4.89 | -3.17 ± 1.63 | -19.71 | -5.18 ± 1.60 | -28.07 | 0.41 |
| MDA (mol/l) | -0.81 ± 0.36 | -18.16 | -1.30 ± 0.36 | -35.13 | -1.10 ± 0.35 | -27.88 | 0.62 |
| GPx (nmol/ml/min) | 22.07 ± 12.76 | 12.82 | 45.45 ± 12.54 | 36.80 | 30.04 ± 12.33 | 20.77 | 0.41 |
CLA: Conjugated linoleic acids; Hs-CRP: High-sensitivity C-reactive protein; IL-6: Interleukin-6; MDA: Malondialdehyde; GPx: Glutathione peroxidase
Difference between values after and before the study
N refers to number of participant in each group
The percent of changes in biochemical before and after study
Values are mean ± SD
Significance was determined using ANOVA
Discussion
The present study determined the effect of CLA and omega-3 fatty acid supplementation on some key atherosclerosis risk factors in a group of atherosclerosis patients. Considering the association between inflammation and atherosclerosis, we evaluated several plasma markers of inflammation, such as hs-CRP, as potential tools for prediction of the risk of coronary disease.19 Evidence indicated that oxidative stress may cause pro-inflammatory effects.20,21 Some reports demonstrated that oxidative stress is necessary for NF-κB pathway.5 Peroxisome proliferator-activated receptors (PPAR) are ligand-activated transcription factors whose activation suppresses the production of pro-inflammatory cytokines by inhibiting NF-κB pathway.5 CLA and ù-3 fatty acids increase the peroxisome proliferator-activated receptor (PPAR).20,21
A few studies have investigated the effect of CLA isomers on inflammation in the human population. As a report by Steck et al. showed, CLA isomers increase the CRP level.22 However, t10,c12 CLA (albeit at a high dose) had more significant effects on increased CRP in recent studies.23 Raff et al. reported the non-significant effect of CLA supplementation on CRP concentration.24 In the present study, supplementation with CLA for 2 months reduced the hs-CRP level in this group during the study. The dose of t10,c12 CLA used in this study (1.27 g/d for 8 weeks) was lower than that used in the studies by Steck et al.22 (3.2 g/d for 12 weeks) and Raff et al.24 (2.1 g/d for 5 weeks), which may account for the effect of CLA on CRP in our study. As reported by LaRosa et al. t10,c12 CLA supplementation increases IL-6 level in rats.25 Although, in the current study, CLA had no effect on IL-6, which is consistent with the study of Raff et al.24
Our data suggest that supplementation with ω-3 decreases hs-CRP and IL-6 measurement. A similar result was gained by Rallidis et al. who reported that 3 months of ل-linolenic acid supplementation in dyslipidaemic patients decreases CRP and IL-6 levels.26 In a study by Chan et al. 6 weeks of ω-3 supplementation did not cause any significant change in CRP levels.27 Several studies indicated that ω-3 fatty acid anti-inflammatory effect may result from activation of PPAR-γ. This fatty acid also directly decreases the inflammatory cytokine production. However, the mechanism is unclear.28
Extensive evidence from studies in animal models and data from human studies have indicated the role of oxidative stress in cardiovascular disease. In this study, we showed the significant effect of CLA and ω-3 on oxidative stress. CLA and ω-3 increase the levels of GSH with over-expression of gamma-glutamylcysteine ligase which was accepted as an antioxidant response.29 In the study of Choi et al. CLA supplementation increased GPx activity.30 Glutathione peroxidase (GPx) is an antioxidant enzyme that reduces hydrogen peroxide by reduced glutathione.31
On the other hand, the study by Taylor et al. demonstrated that CLA increase oxidative stress.23 There is conflicting evidence about the effects of CLA supplementation on oxidative stress. According to a previous study, CLA increases the oxidative stability of the liver which suggests CLA supplementation enhances the protection to oxidative stress.32 Park et al. indicated that CLA supplementation reduces oxidative stress in mice.32
Other studies have shown that supplementation with ω-3 fatty acids slows the progression of oxidative stress.33 As reported by Tayyebi-Khosroshahi et al. ω-3 fatty acids increase the level of glutathione peroxidase and decrease the level of MDA in hemodialysis patients.33 In the study by Bhattacharya et al. ω-3 supplementation increased GPx activity.34 In another study by Iraz et al. ω-3 supplementation decreased MDA levels.35 However, the study by Oarada et al. has revealed increased lipid peroxidation due to ω-3 supplementation in mice.36 Shidfar et al. in their study, suggest that these contradictory results of plasma MDA and lipid peroxidation with ù-3 supplementation may be due to the level of antioxidants in the plasma or supplement content to suppress free radical production, differences in the population of studies, and the duration of the study.37
Conclusion
In conclusion, this study showed the beneficial properties of CLA and the many more of ω-3 fatty acids on inflammatory and oxidative stress markers in atherosclerosis. However, more research, particularly on CLA supplementation, is necessary in order to give definite comments in this regard.
To determine the food intake of participants the FFQ was completed for each patient at the beginning of the study. However, the limitation of this study was that we did not assess dietary intake and physical activity during the study, although the randomized design should have clearly lowered the risk of such bias, and all subjects were instructed to maintain their usual lifestyle habits. On the other hand, patients had normal BMI, so this might have affected our results.
Acknowledgments
The present study was funded with the grant number 89-5407 by Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank Dr. Ahmadinia, the director of ARMAN SETAD Company, for supplying ù-3 fatty acid supplements. We gratefully acknowledge all patients who participated in this study. The authors would like to hereby declare that the investigation undertaken and described in this article has been extracted from the thesis of our MSc student of nutrition sciences, Ms. Fereshte Aliasghari, the second author of this article. The authors would also like to thank Dr. Nasrin Shokrpour, at the Center for Development of Clinical Research of Nemazee Hospital, for editorial assistance.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112(23):3547–53. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 2.Hatmi ZN, Tahvildari S, Gafarzadeh MA, Sabouri KA. Prevalence of coronary artery disease risk factors in Iran: a population based survey. BMC Cardiovasc Disord. 2007;7:32. doi: 10.1186/1471-2261-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83(2):456S–60S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 4.Inoue N. Vascular C-reactive protein in the pathogenesis of coronary artery disease: role of vascular inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets. 2006;6(4):227–31. doi: 10.2174/187152906779010719. [DOI] [PubMed] [Google Scholar]
- 5.Shibata N, Kobayashi M. The role for oxidative stress in neurodegenerative diseases. Brain Nerve. 2008;60(2):157–70. [PubMed] [Google Scholar]
- 6.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol (1985) 2008;105(4):1333–41. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(5):904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 8.Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, et al. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr. 2007;86(5):1392–8. doi: 10.1093/ajcn/86.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, et al. Effect of a conjugated linoleic acid and omega-3 fatty acid mixture on body composition and adiponectin. Obesity (Silver Spring) 2008;16(5):1019–24. doi: 10.1038/oby.2008.41. [DOI] [PubMed] [Google Scholar]
- 10.Rist L, Mueller A, Barthel C, Snijders B, Jansen M, Simoes-Wust AP, et al. Influence of organic diet on the amount of conjugated linoleic acids in breast milk of lactating women in the Netherlands. Br J Nutr. 2007;97(4):735–43. doi: 10.1017/S0007114507433074. [DOI] [PubMed] [Google Scholar]
- 11.Nagao K, Yanagita T. Conjugated fatty acids in food and their health benefits. J Biosci Bioeng. 2005;100(2):152–7. doi: 10.1263/jbb.100.152. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura YK, Flintoff-Dye N, Omaye ST. Conjugated linoleic acid modulation of risk factors associated with atherosclerosis. Nutr Metab (Lond) 2008;5:22. doi: 10.1186/1743-7075-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjula B, Gowda KP, Ahamed SM, Nagarjan T, Gaurav G. Role of omega fatty acids in human body. Asian Journal of Research in Chemistry. 2009;2(2):93–9. [Google Scholar]
- 14.Holub BJ. Docosahexaenoic acid (DHA) and cardiovascular disease risk factors. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2-3):199–204. doi: 10.1016/j.plefa.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26(5):995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 16.Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, et al. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5). Environ Health Perspect. 2008;116(9):1237–42. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omrani GH, Mazloom Z, Savid M, Ashraf Rashidi A. Effect of omega-3 fatty acids on glycemic control and lipid profile in patients with type 2 diabetes. Iran J Diabetes Lipid Disord. 2001;2(1):11–6. [Google Scholar]
- 18.Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E, Mendoza-Nunez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis. 2005;178(1):115–21. doi: 10.1016/j.atherosclerosis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Jaudszus A, Krokowski M, Mockel P, Darcan Y, Avagyan A, Matricardi P, et al. Cis-9,trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J Nutr. 2008;138(7):1336–42. doi: 10.1093/jn/138.7.1336. [DOI] [PubMed] [Google Scholar]
- 21.Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM, et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90(2):415–24. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- 22.Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, et al. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr. 2007;137(5):1188–93. doi: 10.1093/jn/137.5.1188. [DOI] [PubMed] [Google Scholar]
- 23.Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP. Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol. 2006;26(2):307–12. doi: 10.1161/01.ATV.0000199679.40501.ac. [DOI] [PubMed] [Google Scholar]
- 24.Raff M, Tholstrup T, Basu S, Nonboe P, Sorensen MT, Straarup EM. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J Nutr. 2008;138(3):509–14. doi: 10.1093/jn/138.3.509. [DOI] [PubMed] [Google Scholar]
- 25.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics. 2006;27(3):282–94. doi: 10.1152/physiolgenomics.00076.2006. [DOI] [PubMed] [Google Scholar]
- 26.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. . Atherosclerosis. 2003;167(2):237–42. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 27.Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. . Clin Chem. 2002;48(6 Pt 1):877–83. [PubMed] [Google Scholar]
- 28.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67(3):867–74. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 29.Arab K, Rossary A, Soulere L, Steghens JP. Conjugated linoleic acid, unlike other unsaturated fatty acids, strongly induces glutathione synthesis without any lipoperoxidation. Br J Nutr. 2006;96(5):811–9. doi: 10.1017/bjn20061910. [DOI] [PubMed] [Google Scholar]
- 30.Choi JS, Koh IU, Jung MH, Song J. Effects of three different conjugated linoleic acid preparations on insulin signalling, fat oxidation and mitochondrial function in rats fed a high-fat diet. Br J Nutr. 2007;98(2):264–75. doi: 10.1017/S000711450770497X. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa KB, Volp AC, Hermsdorff HH, Navarro-Blasco I, Zulet MA, Martinez JA, et al. Relationship of oxidized low density lipoprotein with lipid profile and oxidative stress markers in healthy young adults: a translational study. Lipids Health Dis. 2011;10:61. doi: 10.1186/1476-511X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park NY, Valacchi G, Lim Y. Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/342328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tayyebi-Khosroshahi H, Houshyar J, Tabrizi A, Vatankhah AM, Razzagi ZN, Dehghan-Hesari R. Effect of omega-3 fatty acid on oxidative stress in patients on hemodialysis. Iran J Kidney Dis. 2010;4(4):322–6. [PubMed] [Google Scholar]
- 34.Bhattacharya A, Ghosal S, Bhattacharya SK. Effect of fish oil on offensive and defensive factors in gastric ulceration in rats. Prostaglandins Leukot Essent Fatty Acids. 2006;74(2):109–16. doi: 10.1016/j.plefa.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Iraz M, Erdogan H, Ozyurt B, Ozugurlu F, Ozgocmen S, Fadillioglu E. Brief communication: omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Ann Clin Lab Sci. 2005;35(2):169–73. [PubMed] [Google Scholar]
- 36.Oarada M, Tsuzuki T, Gonoi T, Igarashi M, Kamei K, Nikawa T, et al. Effects of dietary fish oil on lipid peroxidation and serum triacylglycerol levels in psychologically stressed mice. Nutrition. 2008;24(1):67–75. doi: 10.1016/j.nut.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterr Health J. 2008;14(2):305–13. [PubMed] [Google Scholar]