Abstract
The epithelial-mesenchymal transition (EMT) enhances cellular invasiveness and confers tumor cells with cancer stem cell like characteristics, through transcriptional and translational mechanisms. The mechanisms maintaining transcriptional and translational repression of EMT and cellular invasion are poorly understood. Herein, the cell fate-determination factor Dachshund (DACH1), suppressed EMT via repression of cytoplasmic translational induction of Snail by inactivating the Y box-binding protein (YB-1). In the nucleus, DACH1 antagonized YB-1-mediated oncogenic transcriptional modules governing cell invasion. DACH1 blocked YB-1-induced mammary tumor growth and EMT in mice. In basal-like breast cancer (BLBC) the reduced expression of DACH1 and increased YB-1, correlated with poor metastasis free survival. The loss of DACH1 suppression of both cytoplasmic translational and nuclear transcriptional events governing EMT and tumor invasion may contribute to poor prognosis in basal-like forms of breast cancer, a relatively aggressive disease subtype.
Keywords: DACH1, YB-1, breast cancer, epithelial-mesenchymal transition (EMT)
INTRODUCTION
Dedifferentiation through activation of the embryonic epithelial mesenchymal transition (EMT) program strongly enhances cancer cell motility and dissemination. EMT, a characteristic of embryonic development, wound healing and tissue remodeling, involves a molecular program inducing abundance of transducer proteins (SNAIL, TWIST, ZEB2), which coordinate the reduction in epithelial (E-cadherin) and induction of mesenchymal (N-cadherin, Fibronectin, Vimentin) marker proteins (1, 2). A variety of extracellular signals induce EMT including transforming growth factor beta (TGFβ), and extracellular matrix components such as collagen and hyaluronic acid. Together these pathways converge on EMT-inducing transcription factors, including SNAIL, Slug, ZEB1/2, Twist, FoxC2, ID1 and Goosecoid. EMT induction of tumor cells introduces cancer stem cell (CSC)- like properties with consequent therapeutic resistance and tumor recurrence. Induction of the EMT program involves both transcriptional and translational activities, although the mechanisms coordinating these activities are not well understood.
Y box-binding protein 1 (YB-1) is an oncogenic factor associated with the induction of EMT. In normal murine and human tissues YB-1 resides in the cytoplasm found in complexes with translationally inactive mRNA. The cap-independent translational activation of Snail and other developmentally regulated transcription factors occurs via a cytoplasmic pool of YB-1 (3–6). PI3K-AKT activation induces nuclear translocation of YB-1, wherein growth promoting function occurs via binding to target sequences within genes governing tumorigenesis. Diverse target genes including the EGFR, Her2 and c-Myc contain regulatory regions bound by YB-1. In transgenic mice, YB-1 is sufficient for the induction of mammary tumors characterized by a high degree of chromosomal instability (7). YB-1 up regulates the transcription of cyclin A (8), Topoisomerase IIa (9) and a variety of proteins involved in promoting migration and cellular invasion. YB-1 is overexpressed in hormone-dependent cancers arising in the breast, ovary and prostate, and is not amplified. Rather induction of YB-1 expression likely occurs due to activation of the YB-1 promotor by transcription induction and/or loss of transcriptional repression (10).
The Drosophila homologue of DACH1, the Dac gene is a key member of the retinal determination gene network that specifies organismal development. The dachshund (dac), eya1, eyes-absent (eya), twin of eyeless (toy), teashirt (tsh) and sinocules (so) are expressed in progenitor cells, contributing to development of the eye and genitalia. Loss of DACH1 expression contributes to the expansion of neural progenitors, muscle satellite cell differentiation and breast cancer stem cells (11–13). In recent studies Dachshund repressed breast cancer stem cell expansion (11). DACH1 expression is reduced in a variety of human cancers including prostate, ovarian and human breast cancer (14, 15). In order to understand at a high level of resolution the molecular mechanisms by which the cell fate determination factor DACH1 coordinates breast cancer cellular invasiveness and TIC properties, we sought to identify DACH1 interactive proteins that may participate in these distinct functions. Through proteomic analysis we identified YB-1 as a DACH1 binding protein. DACH1 repressed the translational induction of EMT by inactivating the cytoplasmic YB-1 mediated induction of Snail translation and in the nucleus DACH1 repressed a transcriptional program promoting cellular invasion. DACH1 suppresses mammary tumor EMT and invasion through the coordination of cytoplasmic translational and nuclear transcriptional activities.
MATERIALS AND METHODS
Cell culture, plasmid construction, reporter genes, expression vectors, DNA transfection, luciferase assays and proteomic analysis
Cell culture, DNA transfection, and luciferase assays using the Snail 3′UTR luciferase reporter gene, were performed as previously described (3–6). Proteomic analysis of DACH1 associated proteins was conducted as recently described (16).
RNA isolation, RT-PCR and quantitative real-time PCR
Total RNA was isolated from MDA-MB-231 cells infected with the DACH1 expression vector system, using Trizol (17).
Microarray and cluster analysis
DNA-free total RNA isolated from MDA-MB-231 cells expressing GFP or DACH1 were used to probe Affymetrix Gene 1.0 arrays (Affymetrix, Santa Clara, CA). RNA quality was determined by gel electrophoresis. Probe synthesis and hybridization were performed as previously described (18).
Immunohistochemistry and Immunofluorescence
Immunohistochemical analysis of human breast cancer was conducted using a polyclonal DACH1 antibody (19) polyclonal KLF4 antibody (SC-20691) and monoclonal antibody to YB-1 (cell signaling 4202S). Human breast cancer tissue arrays were from Biomax, US.
Western blot and Immunoprecipitation Study
Whole cells were analyzed in RIPA Buffer (150M NaCl, 20mM Tris-HCI, 1mM EDTA, 1mM EGTA, 1mM Na3VO3, 2.5 mM sodium Pyrophosphate, 1mM β-glycerophosphate, 1% Triton x-100), supplied with proteinase inhibitors. Protein was separated by a 9% SDS PAGE. Antibodies used in Western blot were from Santa Cruz and included cyclin A (sc-596), cyclin B1 (sc-595), cdc25A (sc-97), YB-1 (sc-101198), HA-epitope (sc-7392), c-Myc (sc-40), EZRIN (sc-20773), cyclin D1 (sc-20044) and β-actin (sc-47778). Antibodies from Cell Signaling were YB-1(D299), ZO-1 (5406p), SNAIL (3879p), N-cadherin (4061p), ZEB1 (3396p) and SLUG (9585p). Immunoprecipitation Western blot analysis study was performed as described previously (19).
Mammosphere Formation
Mammosphere formation assays were conducted as previously described (20). ALDEFLOUR staining and immunostaining of cell surface markers by FACS analysis for breast cancer stem cells, was based on prior publications (21, 22).
Migration and Invasion Analysis
Transwell migration assays were performed as described previously (23).
Tumor Implantation Study
2×105 MDA-MB-231 cells expressing GFP control or DACH1 or shYB-1 were implanted subcutaneously into 5–6 week old ethymic female nude mice purchased from NCI-Fredrick. The tumor growth was measured weekly for 4–5 weeks using a digital caliper. Tumor weight was measured when mice were sacrificed.
RESULTS
DACH1 binds YB-1 in breast cancer cell lines
DACH1 has been implicated in the regulation of stem cells of several tissue types, including muscle, neural and breast (12, 13, 11). We conducted a proteomic analysis in order to identify candidate DACH1-binding partners that may govern this function. DACH1 protein complexes were prepared from HEK293T cells transfected with the FLAG-DACH1 expression vector and DACH1-associated proteins were resolved on a 6 – 10% percent hydrochloride gel and silver stained (S. 1A). Analysis of the proteins recovered from the gel through in-gel trypsin digestion and in sequential ms/ms identified a ~50 kDa protein identical to YB-1 (S. 1B). HEK293T cells expressing FLAG-tagged DACH1 and Myc-tagged YB-1, demonstrated the association of YB-1 with DACH1 in immunoprecipitation-Western blotting (S. 1C). MDA-MB-231 cells were used to examine the binding of endogenous YB-1 to DACH1. The MDA-MB-231 cells were stably transduced with an expression vector encoding DACH1 and immunoprecipitation, conducted with a FLAG antibody directed to the amino-terminus of DACH1, co-precipitated endogenous YB-1 (S. 1D).
The carboxyl domain of YB-1 binds to the carboxyl terminus of DACH1
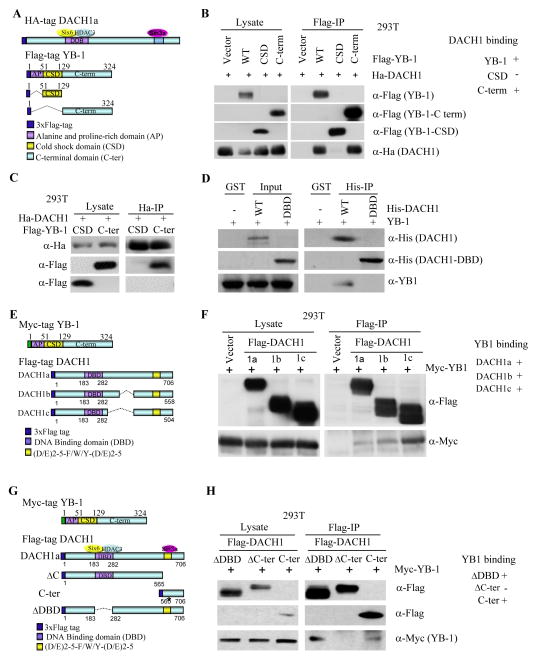
The YB-1 protein consists of three key domains; a C-terminal domain (CTD), which contributes to DNA/RNA binding (24), a highly conserved cold shock domain (CSD) which forms an antiparallel β barrel to facilitate binding to nucleic acids as a chaperone protein, and an nonconserved variable N-terminal domain characterized by four alternating clusters of basic and acidic amino acids, which is both alanine and proline rich and is thought to have been involved in transactivation (25). Nuclear cytoplasmic shuffling of YB-1 is controlled by both a distinct nuclear localization signal and cytoplasmic retention signals in the CTD (26). HEK293T cells were transfected with a series of FLAG-tagged YB-1 mutant expression vectors and HA-tagged DACH1 (Fig. 1A). The FLAG-IP precipitated YB-1 fragment. Western blotting for DACH1 using the HA antibody demonstrated the association of DACH1 with each YB-1 fragment but a failure to bind the CSD alone (Fig. 1B). Immunoprecipitation of DACH1 with the HA antibody demonstrated DACH1 binding to the YB-1 C-terminus (Fig. 1C). In GST pulldown 6-His DACH1 bound GST-expressed YB-1. The DACH1 DBD (DNA Binding Domain) did not bind YB-1 (Fig. 1D). Thus, the C-terminus of YB-1 was sufficient for binding DACH1 and the YB-1 CSD domain was incapable of binding DACH1 (Fig. 1A, B).
Figure 1. DACH1 binding requires the YB-1 Cold Shock Domain (CSD).
A) Schematic representation of DACH1, YB-1 and YB-1 mutant expression vectors. B) IP-Western blot was conducted of HEK293T cells transfected with expression vectors encoding either N-terminal 3x FLAG-tagged YB-1 or HA-tagged DACH1 expression vectors. In (B) the IP was conducted with an antibody to the FLAG-tag of YB-1 and in (C) the IP was directed to the HA tag of DACH1. DACH1 binding to YB-1 was derived from N=3 separate experiments. (D) DACH1-YB-1 binding characterized in pulldown using 6-His-tagged DACH1 or DACH1 DBD region and GST-YB-1. (E–G) Schematic representation of YB-1 and DACH1 mutant expression vectors. (F–H) IP-Western blot of Myc-tagged YB-1 and associated DACH1 expression vectors transfected into HEK293T cells. Antibodies were directed for IP to the 3x FLAG tag of DACH1 and Western blot to the Myc epitope of YB-1. Antibodies are directed to the proteins as indicated. Data are representative of N=3 separate experiments. The DACH1 isoforms are indicated as DACH1a, b and c.
DACH1 exists in three isoforms (DACH1a, b, c) and each of the DACH1 isoforms were capable of binding YB-1 with similar affinity (Fig. 1E, F). DACH1 mutant expression vectors were co-expressed with YB-1 WT in order to define the domains of DACH1 governing association with YB-1 (Fig. 1G). Deletion of the DNA-binding domain (ΔDBD) of DACH1 did not affect binding to YB-1, however deletion of the C-terminus (DACH1 ΔC) abolished YB-1 binding and the C-terminus was sufficient for YB-1 binding (Fig. 1H).
Phosphorylation determines DACH1 nuclear-cytoplasmic translocation
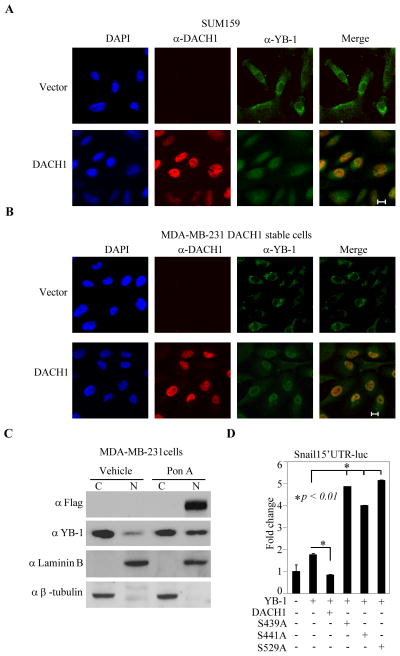
In order to determine whether DACH1 is phosphorylated, cellular extracts from HEK293T cells were examined before and after treatment with calf intestinal phosphatase (CIP). The treatment of cells with CIP reduced the mobility of DACH1 (S. 2A). Mass spectrometry was used to identify the phosphorylation sites and point mutation of the residues with highest Mascot Scores was conducted (S. 2B). The electrophoretic mobility of the DACH1 protein expressing a mutation of S439 was similar to the CIP treated cells expressing DACH1 wt (S. 2C). By IHC, the DACH1 protein was located primarily in the nucleus (S. 2D). The S439A S441 and S529 mutants were cytoplasmic. These findings suggest DACH1 phosphorylation determines the cytoplasmic vs. nuclear location.
DACH1 inhibits and YB-1 induces breast cancer cellular EMT in cell lines
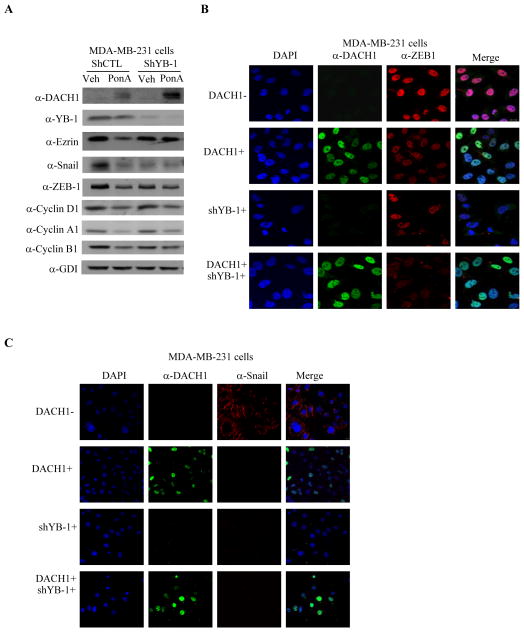
As YB-1 located in the cytoplasmic pool regulates translational control to induce EMT and DACH1 expression reduced cytoplasmic YB-1, we examined the possibility that DACH1 expression may reduce YB-1-dependent EMT. The MBA-MB-231 breast cancer cell line stably transduced with a ponasterone-inducible DACH1 expression vector demonstrated the expression of FLAG-tagged DACH1 upon the addition of Ponasterone A, which reduced the abundance of Ezrin, SNAIL and ZEB-1 (Fig. 2A). Vinculin and GDI, two loading controls for protein abundance were unchanged. Consistent with a role for YB-1 to induce EMT, shRNA to YB-1, reduced the abundance of SNAIL and ZEB-1 (Fig. 2A). Immunohistochemical staining demonstrated a reduction in ZEB-1 upon expression of DACH1 (Fig. 2B). Similarly, shRNA to YB-1 reduced ZEB-1 abundance (Fig. 2B). The abundance of SNAIL was reduced by either DACH1 expression (Fig. 2C) or shRNA to YB-1 (Fig. 2C). The reduction in SNAIL abundance by YB-1 shRNA in MDA-MB-231 cells is consistent with prior findings in MCF10A-Ras breast cancer cells (6). Together these studies suggest DACH1 inhibits YB-1 induced EMT.
Figure 2. DACH1 inhibits YB-1 induced EMT.
A) MDA-MB-231 cells stably transduced with a Ponasterone A-inducible FLAG-tagged DACH1 expression vector were sequentially transduced with shRNA to YB-1. Pon A treatment (2 μM) was used to induce DACH1. Western blot was conducted with the antibodies as indicated. (B–D) Immunohistochemistry for markers of mesenchymal cells include, (B) ZEB1, (C) Snail.
DACH1 antagonizes YB-1 mediated gene expression
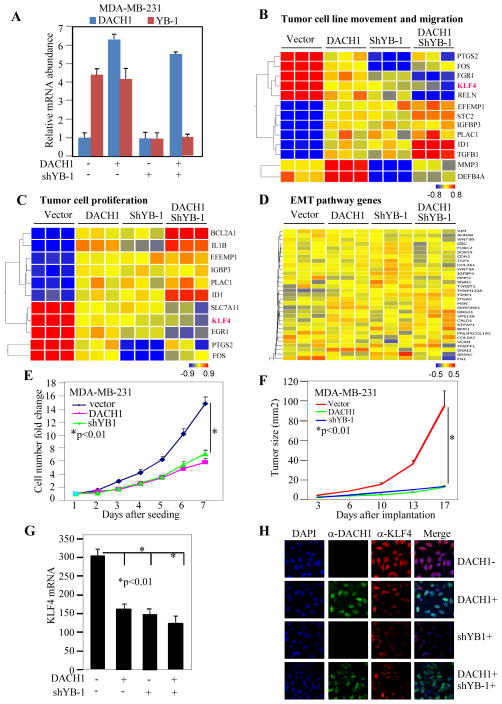
In order to examine further the mechanisms by which DACH1 antagonized YB-1-mediated EMT, we considered the possibility that DACH1 may antagonize the transcriptional function of YB-1. In order to determine the gene expression pathway regulated by either DACH1 expression or YB-1 shRNA reflecting endogenous YB-1, MDA-MB-231 cells transduced with either a ponasterone inducible DACH1 cDNA, or shRNA to YB-1, were subjected to micro-array gene expression analysis (Fig. 3). The relative mRNA abundance of DACH1 and YB-1 was determined in the cell lines after treatment with Ponasterone A for 24 hours (Fig. 3A). DACH1 mRNA was induced 6-fold. YB-1 mRNA levels were not affected by expression of DACH1. YB-1 shRNA reduced YB-1 abundance by 75% without affecting DACH1 levels.
Figure 3. DAHC1 inhibits YB-1 nuclear transcriptional signaling pathways.
(A) MDA-MB-231 cells expressing a DACH1 expression vector and/or YB-1 shRNA were analyzed by (A, E) RT-PCR or (B–D) genome wide microarray analysis. The relative changes in gene expression are shown. The -fold change or as a color scale of relative change in gene expression. The genes within DAVID signaling pathways for (B) “tumor cell movement” (C) “tumor cell proliferation” and (D) “EMT pathways” are shown for N=3 separate experiments. Note in (B) and (C) KLF-4 is a component of both DAVID pathways. Note in (D), no significant alterations in gene expression of the EMT pathway were detected. (E) Cellular proliferation and (F) tumor growth rates of MDA-MB-231 cells, expressing Ponasterone A inducible DACH1 with or without shRNA to YB-1, implanted into athymic nude mice. (G) Relative mRNA expression of KLF4 in MDA-MB-231 cells with etopic expression of DACH1 or shYB-1. (H) Immunohistochemistry of MDA-MB-231 cells shows relative KLF-4 abundance in ponasterone-inducible DACH1 stable MDA-MB-231 cells transduced with shRNA for YB-1. Cells were treated with Ponasterone A (2 μM) for 48 hours and shYB-1 for 48 hours. Antibodies are to proteins as indicated in the figure.
We next examined the functional pathways and the gene expression modules governed by DACH1 and YB-1 by interrogating the mRNA derived from MDA-MB-231 cells expressing inducible DACH1, either with or without YB-1 shRNA (Fig. 3B–D). DACH1 expression repressed gene expression associated with “tumor cell movement”, “cell proliferation” but not “EMT” (Fig. 3B–D). shRNA to YB-1 or expression of DACH1 had similar effects on the expression of genes in the tumor cell proliferation and tumor migration and invasion pathways. KLF4 was a member of each DAVID functional pathway that was repressed by DACH1 and shRNA to YB-1. Functional analysis was conducted in vivo using MDA-MB-231 cells expressing a Ponasterone A inducible DACH1 and/or YB-1 shRNA. Cells were introduced into immune-deficient mice. DACH1 expression inhibited cell proliferation and tumor growth, as did YB-1 shRNA (Fig. 3E. F). DACH1 expression or YB-1 shRNA, identified KLF4 as a common gene target associated with inhibition of tumor cell migration and tumor cell proliferation. Quantitation of KLF4 mRNA in multiplicate experiments demonstrated that DACH1 expression reduced KLF4 levels by >50% as did shRNA to YB-1 (Fig. 3G). Immunohistochemical staining of the Ponasterone-inducible DACH1 MDA-MB-231 cells transduced with shRNA to YB-1 demonstrated that DACH1 or YB-1 shRNA reduced KLF4 abundance (Fig. 3H). These studies demonstrate there is substantial overlap in GEO-terms and gene expression regulated by either DACH1 expression or YB-1 shRNA.
YB-1 induction of breast cancer invasiveness is antagonized by DACH1
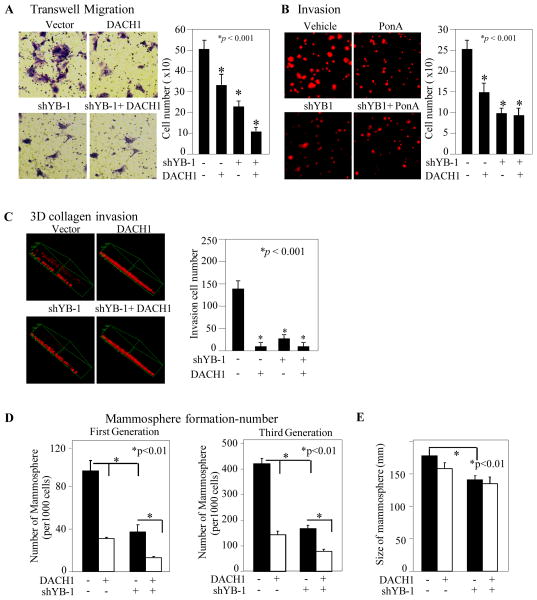
Ectopic expression of YB-1 confers invasiveness of MCF10A cells (6). Transmigration and cellular invasion may involve distinguishable or common processes. In order to determine whether endogenous YB-1 promotes cellular migration in MDA-MB-231 cells, shRNA to YB-1 was used to transduce cells. In order to determine whether DACH1 could antagonize YB-1 induction of invasion, 3D-matrigel invasion assays were conducted. The expression of DACH1 was sufficient to inhibit MDA-MB-231 cell transwell migration (Fig. 4A), matrigel invasion (Fig. 4B) and collagen invasion (Fig. 4C, D). shRNA to YB-1 similarly reduced migration and invasion (Fig. 4A–C).
Figure 4. DACH1 antagonizes YB-1 induced migration and stem cell function.
MDA-MB-231 cells, encoding Ponasterone A on a inducible DACH1, with or without YB-1 shRNA, were analyzed for migration, invasion and stem cell function using (A) transwell migration assays, (B) matrigel invasion assays and (C) 3D collagen invasion assays with the number of migrating or invading cells throughout shown as mean ±SEM for N=3 separate experiments. In (D), the same cell lines were used to determine mammosphere number and (E) mammosphere size with data shown throughout as mean ±SEM for N=5 separate experiments.
The induction of EMT may enrich gene expression associated with ES and/or TIC. In order to determine whether YB-1 abundance may induce TIC, we deployed a widely accepted model of mammosphere formation in which the number of mammospheres reflects symmetrical division in multi-potential cells. MDA-MB-231 cells were stably transduced with Ponasterone-inducible DACH1 and/or shRNA to YB-1. Increased DACH1 expression or reduced YB-1 abundance via shRNA, reduced the number of mammospheres by >60% in multiple generations (Fig. 4D). shRNA to YB-1 reduced mammosphere size by ~15% (Fig. 4E). DACH1 expression did not further reduce the mammosphere number in the presence of YB-1 shRNA (Fig. 4E).
DACH1 expression regulates nuclear cytoplasmic distribution of YB-1 and thereby YB-1-mediated cap-independent translation of Snail
The finding that DACH1 repression of YB-1 shRNA induced EMT, without affecting expression of genes governing EMT, led us to consider alternate mechanisms. Cytoplasmic YB-1 is known to activate cap-independent translation of mRNA encoding Snail and other factors implicated in activators of mesenchymal genes (6). As cytoplasmic, not nuclear YB-1, governs EMT, we considered the possibility that DACH1 expression may regulate the relative abundance of cytoplasmic vs. nuclear pools of YB-1. To examine the effects of DACH1 expression, on YB-1 sub-cellular localization, IHC for DACH1 and YB-1 was conducted on postasterone-inducible DACH1 expressing breast cancer cell lines. Expression of DACH1 in SUM159 cells was readily detectable upon Ponasterone A treatment in a nuclear compartment, detected by the FLAG antibody to the N-terminal tag of DACH1. YB-1 was predominately cytoplasmic in location. Upon expression of DACH1, YB-1 became predominantly nuclear (Fig. 5A). Similar findings were made in MDA-MB-231 cells in which expression of DACH1 was associated with increased nuclear abundance of YB-1, detected either by immunohistochemistry (Fig. 5B) or by Western blot of nuclear vs. cytoplasmic fractions (Fig. 5C). As the DACH1 phosphorylation sites determined nuclear localization, we assessed whether DACH1 induction of YB-1 nuclear translocation involved the DACH1 phosphorylation sites (S. 3A). DACH1 WT was located in nucleus, identified by α-FLAG immunohistochemistry (S. 3A). The DACH1 phosphorylation mutant was predominantly cytoplasmic. YB-1, detected by the myc epitope was cytoplasmic, and co-transfection with DACH1 WT induced nuclear translocation of YB-1. In contrast, co-transfection with the DACH1 phosphorylation mutant (DACH1 S439, 441, 529A) resulted in predominantly cytoplasmic YB-1 immunostaining (S. 3A). Several pathways have been shown to influence YB-1 location, including the PI3K-AKT pathway (27), which phosphorylated YB-1 at Ser102. In order to determine whether DACH1-mediated YB1 nuclear translocation involued AKT, we deployed an expression vector encoding a point mutation of YB-1 (Ser102) (S. 3B). Co-transfection of DACH1 induced nuclear translocation of YB-1 or YB-1 (Ser102) to a similar level (S. 3B). These findings suggest DACH1-mediated YB-1 nuclear translocation does not involve the AKT pathway.
Figure 5. DACH1 promotes nuclear translocation of YB-1, inhibiting YB-1 dependent translation.
Confocal microscopic images of SUM159 (CA) and MDA-MB-231 cells (B) with ectopic expression of DACH1 using antibodies as indicated. (C) Western blot analysis of cytoplasmic and nuclear protein as indicated. (D) MCF10A-RAS cell transfected with YB-1, DACH1 and SNAIL-5′UTR Luc reporter were starved and treated with rapamycin (uM). Luciferase activity was measured after 24 hours. The data are mean ±SEM.
We had shown that DACH1 expression or YB-1 shRNA reduced SNAIL abundance. The role of SNAIL1 as an inducer of EMT is well established. The Snail1 mRNA harbors specific motifs in its 5′UTR that are conserved and are responsible for cap-independent translation by YB-1. YB-1 has been shown to induce cap-independent translation of genes enhancing EMT (6) including Snail in MCF10AT cells. (The MCF10AT subclone is derived by transformation of the immortalized benign MCF10A human mammary epithelial cells with oncogenic Ha-Ras and subsequent passaging through mouse xenotransplants). We examined the role of DACH1 in YB-1-dependent regulation of Snail. We deployed a Snail1 5′UTR linked to the luciferase reporter gene. Conditions of starvation, or starvation combined with rapamycin, restricts cap-dependent translation. Consistent with previous observations that YB-1 induces cap-dependent translation of Snail1 5′UTR-Luc activity (6), we also show that YB-1 induced Snail 5′UTR-Luc activity. Ectopic expression of DACH1 repressed YB-1 activation (Fig. 5D). The induction of Snail 5′UTR-Luc activity by YB-1 requires the cytoplasmic pool. DACH1 expression reduces the cytoplasmic YB-1. The phosphorylation point mutants of DACH1 are localized to the cytoplasm and fail to undergo nuclear translocation. Consistent with a model in which DACH1 inhibits YB-1 cytoplasmic EMT function through inducing nuclear translocation, each of the DACH1 phosphorylation point mutants failed to repress YB-1-mediated induction of Snail 5′UTR-Luc activity; indeed the mutants conveyed a dominant positive effect on Snail1 5′UTR-Luc activity (Fig. 5E).
YB-1 and DACH1 expression is inversely correlated in Luminal A and Basal human breast cancer
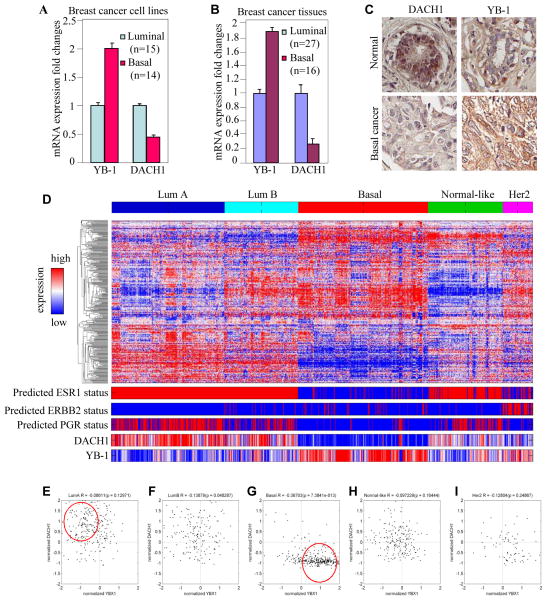
We examined further the relationship between YB-1 and DACH1 expression in breast cancer cell line and tissues. We examined the relative mRNA levels for DACH1 in luminal (n=15) and basal (n=14) breast cancer cell lines. DACH1 relative mRNA levels were reduced and YB-1 levels increased in basal breast cancer cell lines compared with luminal breast cancer cell lines (Fig. 6A). Previous studies had demonstrated reduced DACH1 abundance in human breast cancer correlating with poor prognosis (28). RNA expression of DACH1 was higher in luminal cancer with decreased abundance in human basal cancer. In contrast, YB-1 levels were increased in human basal breast cancer compared with luminal cancer (Fig. 6A). Immunohistochemical staining was conducted on normal human breast tissue, with comparison made to human breast cancer (Fig. 6B). DACH1 was expressed in normal human tissue with reduced expression in basal breast cancer. YB-1 expression was reduced in normal breast tissue but increased in basal breast cancer (Fig. 6C).
Figure 6. YB-1 and DACH1 expression is inversely correlated in human Luminal A and Basal Genetic subtypes.
The mRNA abundance for YB-1 and DACH1 in (A) basal breast cancer cell lines and (B) human breast cancer cell line. C) Immunohistochemistry for DACH1 and YB-1 in normal breast and basal breast cancer (B) mRNA for YB-1 and DACH1 in A) basal like breast cancer cell lines and B) human breast cancer cell lines. D) A combined breast cancer microarray data set assigning the five breast cancer genetic expression subtypes is shown as a heat map. The ESR1, epidermal-growth factor receptor (ERBB2) and progesterone receptor (PGR), is shown together with YB-1 and DACH1 expression. E–I) Normalized DACH1 expression plotted versus normalized YB-1 expression level reveals the inverse relationship between DACH1 and YB-1 expression in luminal A and basal genetic subtype (red circle).
In order to determine the relative abundance of DACH1 and YB-1 in human breast cancer we examined gene expression of 2,550 breast cancer samples (Fig. 6D). The tumors were sub-classified by the genetic sub-types of luminal A, luminal B, basal, normal-like and HER2 (Fig. 6D). Molecular subtyping allowed us to identify an enhancement of DACH1 expression with low YB-1 expressing tumors as luminal A (Fig. 6E) and conversely increased YB-1 expression with low DACH1 levels was identified in the basal phenotype (Fig. 6G). In the other genetic subtypes, there was no specific statistically significant association between YB-1 and DACH1 expression. These studies raised the possibilities that DACH1 may regulate the expression of YB-1 in a subset of breast cancers.
In order to determine whether a reduction of DACH1 abundance in the basal breast cancer subtype correlated independently with clinical outcome, we examined a series of basal, claudin-positive, breast cancer samples and determined the correlation with metastasis free, relapse free and overall survival. A reduction of DACH1 abundance was associated with reduced survival probability, relapse free and metastasis free survival (S. 4A–C). Similar analysis demonstrated that increased levels of YB-1 were associated with reduced metastasis free survival, relapse free survival and overall survival (S. 4D–F).
DISCUSSION
Herein, DACH1 bound to, and inhibited functions of, YB-1 including the induction of cellular proliferation, migration and epithelial mesenchymal transition. The antagonism of YB-1 function by DACH1 involved two distinct mechanisms. First, DACH1 repressed mRNA expression of nuclear YB-1 induced genes that promote tumor cell movement and tumor cell proliferation. Secondly, DACH1 inhibited EMT via repression of cytoplasmic YB-1 EMT-inducing translational activity. The finding in the current studies that DACH1 binds YB-1 extends prior findings that DACH1 associates with auxiliary proteins to conduct its diverse functions. DACH1-associated proteins include members of the RDGN (Six, Eya), the co-activators CBP/p300, c-Jun and CA150.
YB-1 induces EMT at least in part through directly activating cap-independent translation of messenger RNAs encoding SNAIL, Twist and ZEB2/Sip1 and other activators of EMT (6). Herein, DACH1 inhibited YB-1 mediated induction of cap-independent translation of Snail 5′UTR Luc activity. The cytoplasmic pool of YB-1 enhances the translation of EMT genes including SNAIL. DACH1 expression induced the translocation of YB-1 from the cytoplasmic to a nuclear pool. Previous studies have identified mechanism governing YB-1 cytoplasmic-nuclear translocation including AKT and MAPK (27). In the current study, point mutation of the AKT phosphorylation sites of YB-1(Ser102) did not affect DACH1-mediated nuclear translocation of YB-1 suggesting the mechanism is AKT-independent. The role of other factors showed to regulate YB-1 nuclear translocation, including ΔNP63α (29) remain to be determined. The current studies are the first to define the importance of specific DACH1 phosphorylation sites in regulating function, as DACH1 S439, S441 and S529 were required for nuclear translocation and repression of the Snail 5′UTR Luc reporter. Together these studies are consistent with a model in which DACH1 promotes nuclear translocation of YB-1, thereby reducing the cytoplasmic translation inducing pool of YB-1.
DACH1 expression, or YB-1 shRNA, inhibited breast cancer cellular transwell migration, invasion and 3D matrigel invasion. Analysis of the transcriptional modules regulated by DACH1 expression or YB-1 shRNA identified KLF4 mRNA abundance as a common target of YB-1 and DACH1 governing cell proliferation and migration. Kruppel-like factor 4 (KLF4) is highly expressed in approximately 70% of breast cancers functioning as an oncogene and serving as a distal target of stem or progenitor cell expansion in MDA-MB-231 and MCF7 cells (30, 11). It will be of interest to determine whether KLF4 serves as a common functional distal target in both DACH1 and YB1 shRNA-mediated transcriptional repression of tumor cell migration and growth in vivo.
Herein DACH1 inhibited YB-1-mediated induction of the breast cancer stem cell phenotype. Mammosphere number, which serves as a useful surrogate marker of breast cancer stem cells, were maintained by endogenous YB-1 and repressed by DACH1. YB-1 up-regulates markers of stem cells, including p63, CD44 and CD10 and appears to link the acquisition of the mesenchymal phenotype and the stem cell associated gene expression. DACH1 regulates gene transcription both directly and indirectly. DACH1 binds a Forkhead-like consensus sequence and ChIP-Seq analysis identified DACH1 bound ES-cell inducing target genes (Sox2, Nanong, and EKLF). In addition, DACH1 binds to several transcription factors (c-jun, Six) (31), and thereby regulates gene expression through indirect mechanisms. The current findings are consistent with recent studies demonstrating the importance of endogenous DACH1 in reprogramming mammary breast cancer stem cells via transcriptional induction (32) and herein through indirect mechanisms via binding of YB-1. It will be of interest to determine whether genetic deletion of DACH1 is capable of affecting murine mammary gland stem cells in vivo and thereby mammary gland development.
Extending the previous studies showing reduced expression of DACH1 in human breast cancer (28) and the ability of DACH1 to block breast cancer metastasis (23), herein, DACH1 and YB-1 abundance were inversely correlated in luminal A and basal breast cancer. Although prior analysis established YB-1 upregulation correlated with tumor aggressiveness and loss of DACH1 expression across all breast cancer types demonstrated a modest but significant increase in cumulative months of survival (n=2,125 patients), the precise mechanisms remained largely unknown. Analysis of ~2250 human breast cancer samples demonstrated that features of EMT gene expression in a subset of basal-like human breast cancers.
The current studies are the first to identify genetic subtypes in which reduced DACH1 expression and increased YB-1 expression correlate and show a significant adverse outcome for DACH1 loss or YB-1 overexpression in the basal claudin positive subtype. The clinical outcome of this subset of patients reinforces the critical functional significance of the balance between YB-1 and DACH1 in governing the EMT switch and proliferative/invasive features of tumors. The loss of DACH1 and increased YB-1 levels may contribute to the EMT phenotype of basal-like breast cancer. The current study may have broad implications for understanding the process of EMT, and tumor stem cell expansion in other tumor types. The abundance of YB-1 is increased in a variety of malignancies including breast, prostate, liver, lung, and brain and experimental data suggests an oncogenic role for YB-1 in tissue culture and mouse models (33). Conversely DACH1 abundance is reduced in these same malignancies, with a growing body of evidence to suggest DACH1 may function as a new type of tumor suppressor (14, 34–36, 15). It will be of interest to determine in future studies whether the interaction between DACH1 and YB-1 plays a broad role in other malignancies.
Supplementary Material
Acknowledgments
This work was supported in part, by the National Institutes of Health Grants R01CA132115, R01CA70896, R01CA75503 and R01CA86072 (R.G.P.) and grant P30CA56036 (Kimmel Cancer Center Core Grant to R.G.P.). This work was also supported by grants from the Breast Cancer Research Foundation (R.G.P.), the Pennsylvania Department of Health Grant (R.G.P.) from the Dr. Ralph and Marian C. Falk Medical Research Trust (to R.G.P.), Margaret Q. Landenberger Research Foundation (to K.W.), Department of Defense Concept Award W81XWH-11-1-0303 (K.W.). Dr. Kongming Wu’s research is supported from China NSFC grant No.81072169, 81172422 and 81261120395. The Pennsylvania Department of Health specifically disclaims responsibility for any analysis, interpretations or conclusions. Michael P. Lisanti and his laboratory were supported via resources of the Thomas Jefferson University. We thank Peter Vogt for the expression plasmids and helpful discussion.
Footnotes
Conflicts of Interest: R.G.P. holds major (> $10,000) ownership interests in, and serves as CSO/Founder of the biopharmaceutical companies ProstaGene, LLC and AAA Phoenix, Inc. R.G.P. additionally holds ownership interests (value unknown) for several submitted patent applications.
References
- 1.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 2.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu M, Wang C, Wang J, Zhang X, Sakamaki T, Yeung YG, et al. Androgen receptor acetylation governs trans activation and MEKK1-induced apoptosis without affecting in vitro sumoylation and trans-repression function. Mol Cell Biol. 2002:3373–3388. doi: 10.1128/MCB.22.10.3373-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu M, Wang C, Wang J, Sakamaki T, Di Vizio D, Zhang X, et al. Acetylation of the androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol. 2003:8563–8575. doi: 10.1128/MCB.23.23.8563-8575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu M, Wang C, Reutens AT, Wang J, Angeletti RH, Siconolfi-Baez L, et al. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000:20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 6.Evdokimova V, Tognon C, Ng T, Ruzanov P, Melnyk N, Fink D, et al. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell. 2009:402–415. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann S, Royer-Pokora B, Fietze E, Jurchott K, Hildebrandt B, Trost D, et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005:4078–4087. doi: 10.1158/0008-5472.CAN-04-4056. [DOI] [PubMed] [Google Scholar]
- 8.Jurchott K, Bergmann S, Stein U, Walther W, Janz M, Manni I, et al. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J Biol Chem. 2003:27988–27996. doi: 10.1074/jbc.M212966200. [DOI] [PubMed] [Google Scholar]
- 9.Shibao K, Takano H, Nakayama Y, Okazaki K, Nagata N, Izumi H, et al. Enhanced coexpression of YB-1 and DNA topoisomerase II alpha genes in human colorectal carcinomas. Int J Cancer. 1999:732–737. doi: 10.1002/(sici)1097-0215(19991210)83:6<732::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Stratford AL, Astanehe A, Dunn SE. YB-1 is a Transcription/Translation Factor that Orchestrates the Oncogenome by Hardwiring Signal Transduction to Gene Expression. Translational Oncogenomics. 2007;49 [PMC free article] [PubMed] [Google Scholar]
- 11.Wu K, Jiao X, Li Z, Katiyar S, Casimiro MC, Yang W, et al. Cell fate determination factor Dachshund reprograms breast cancer stem cell function. The Journal of Biological Chemistry. 2011:2132–2142. doi: 10.1074/jbc.M110.148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurusu M, Nagao T, Walldorf U, Flister S, Gehring WJ, Furukubo-Tokunaga K. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and Dachshund genes. Proc Natl Acad Sci USA. 2000:2140–2144. doi: 10.1073/pnas.040564497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, et al. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2010;4:77–91. doi: 10.1016/j.scr.2009.10.003. 2009/12/08. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, Katiyar S, Witkiewicz A, Li A, McCue P, Song LN, et al. The cell fate determination factor dachshund inhibits androgen receptor signaling and prostate cancer cellular growth. Cancer Res. 2009:3347–3355. doi: 10.1158/0008-5472.CAN-08-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popov VM, Zhou J, Shirley LA, Quong J, Yeow WS, Wright JA, et al. The cell fate determination factor DACH1 is expressed in estrogen receptor-alpha-positive breast cancer and represses estrogen receptor-alpha signaling. Cancer Res. 2009:5752–5760. doi: 10.1158/0008-5472.CAN-08-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Liu Y, Zhang W, Popov VM, Wang M, Pattabiraman N, et al. Transcription elongation regulator 1 is a co-integrator of the cell fate determination factor Dachshund homolog 1. The Journal of Biological chemistry. 2010:40342–40350. doi: 10.1074/jbc.M110.156141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, et al. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol. 2006:5449–5469. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Wang C, Jiao X, Katiyar S, Casimiro MC, Prendergast GC, et al. Alternate cyclin D1 mRNA splicing modulates p27KIP1 binding and cell migration. J Biol Chem. 2008:7007–7015. doi: 10.1074/jbc.M706992200. [DOI] [PubMed] [Google Scholar]
- 19.Wu K, Li A, Rao M, Liu M, Dailey V, Yang Y, et al. DACH1 is a cell fate determination factor that inhibits cyclin D1 and breast tumor growth. Molecular and cellular biology. 2006:7116–7129. doi: 10.1128/MCB.00268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao X, Katiyar S, Willmarth NE, Liu M, Ma X, Flomenberg N, et al. c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion. J Biol Chem. 2010:8218–8226. doi: 10.1074/jbc.M110.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay J, Jiao X, Sakamaki T, Casimiro MC, Shirley LA, Tran TH, et al. ErbB2 induces Notch1 activity and function in breast cancer cells. Clin Transl Sci. 2008:107–115. doi: 10.1111/j.1752-8062.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K, Katiyar S, Li A, Liu M, Ju X, Popov VM, et al. Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8. Proc Natl Acad Sci USA. 2008:6924–6929. doi: 10.1073/pnas.0802085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommerville J, Ladomery M. Masking of mRNA by Y-box proteins. FASEB J. 1996:435–443. doi: 10.1096/fasebj.10.4.8647342. [DOI] [PubMed] [Google Scholar]
- 25.Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays. 2003:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- 26.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland BW, Kucab J, Wu J, Lee C, Cheang MC, Yorida E, et al. Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene. 2005:4281–4292. doi: 10.1038/sj.onc.1208590. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Li A, Rao M, Liu M, Dailey V, Yang Y, et al. DACH1 is a cell fate determination factor that inhibits Cyclin D1 and breast tumor growth. Mol Cell Biol. 2006:7116–7129. doi: 10.1128/MCB.00268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Costanzo A, Troiano A, di Martino O, Cacace A, Natale CF, Ventre M, et al. The p63 protein isoform DeltaNp63alpha modulates Y-box binding protein 1 in its subcellular distribution and regulation of cell survival and motility genes. J Biol Chem. 2012:30170–30180. doi: 10.1074/jbc.M112.349951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu K, Liu M, Li A, Donninger H, Rao M, Jiao X, et al. Cell fate determination factor DACH1 inhibits c-Jun-induced contact-independent growth. Mol Biol Cell. 2007:755–767. doi: 10.1091/mbc.E06-09-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu K, Jiao X, Li Z, Katiyar S, Casimiro MC, Yang W, et al. Cell fate determination factor Dachshund reprograms breast cancer stem cell function. J Biol Chem. 2011:2132–2142. doi: 10.1074/jbc.M110.148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasham A, Print CG, Woolley AG, Dunn SE, Braithwaite AW. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 34.Chen K, Wu K, Gormley M, Ertel A, Zhang W, Zhou J, et al. Acetylation of the Cell-Fate Factor Dachshund Determines P53 Binding and Signaling Module in Breast Cancer. Oncotarget. 2013:923–935. doi: 10.18632/oncotarget.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen K, Wu K, Cai S, Zhang W, Zhou J, Wang J, et al. Dachshund Binds p53 to Block the Growth of Lung Adenocarcinoma Cells. Cancer Res. 2013:3262–3274. doi: 10.1158/0008-5472.CAN-12-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popov VM, Wu K, Zhou J, Powell MJ, Mardon G, Wang C, et al. The Dachshund gene in development and hormone-responsive tumorigenesis. Trends Endocrinol Metab. 2010:41–49. doi: 10.1016/j.tem.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.