Abstract
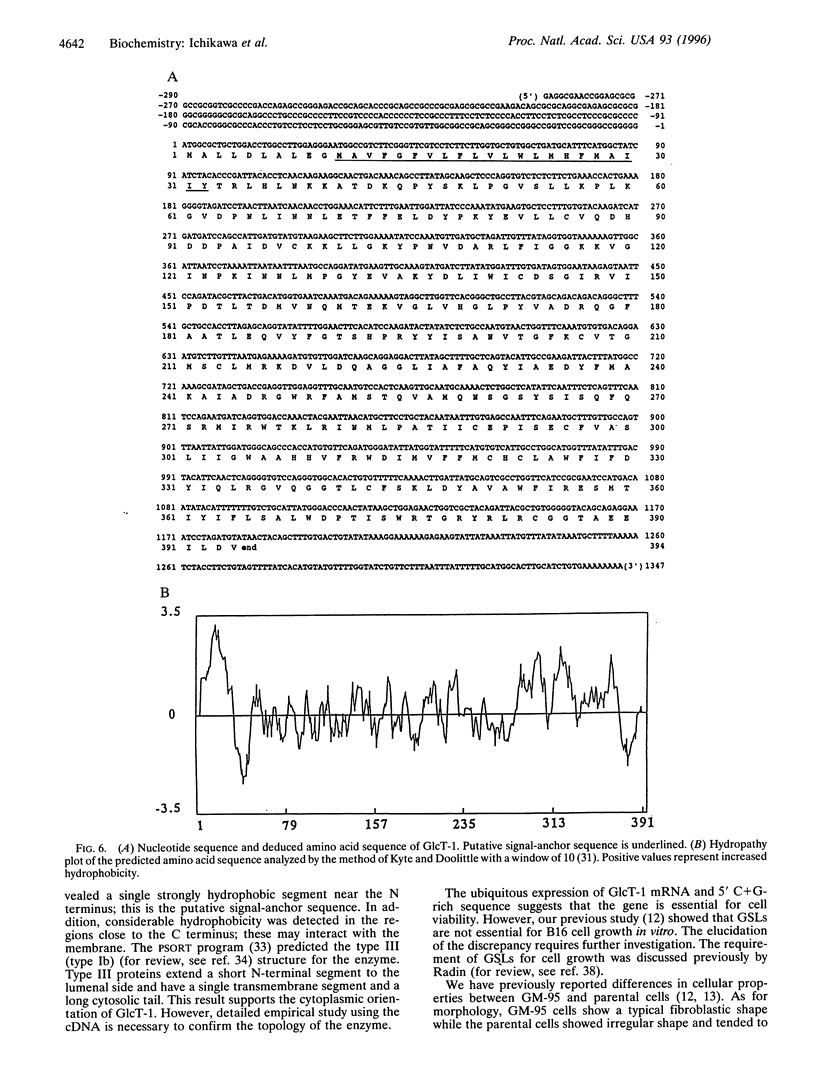
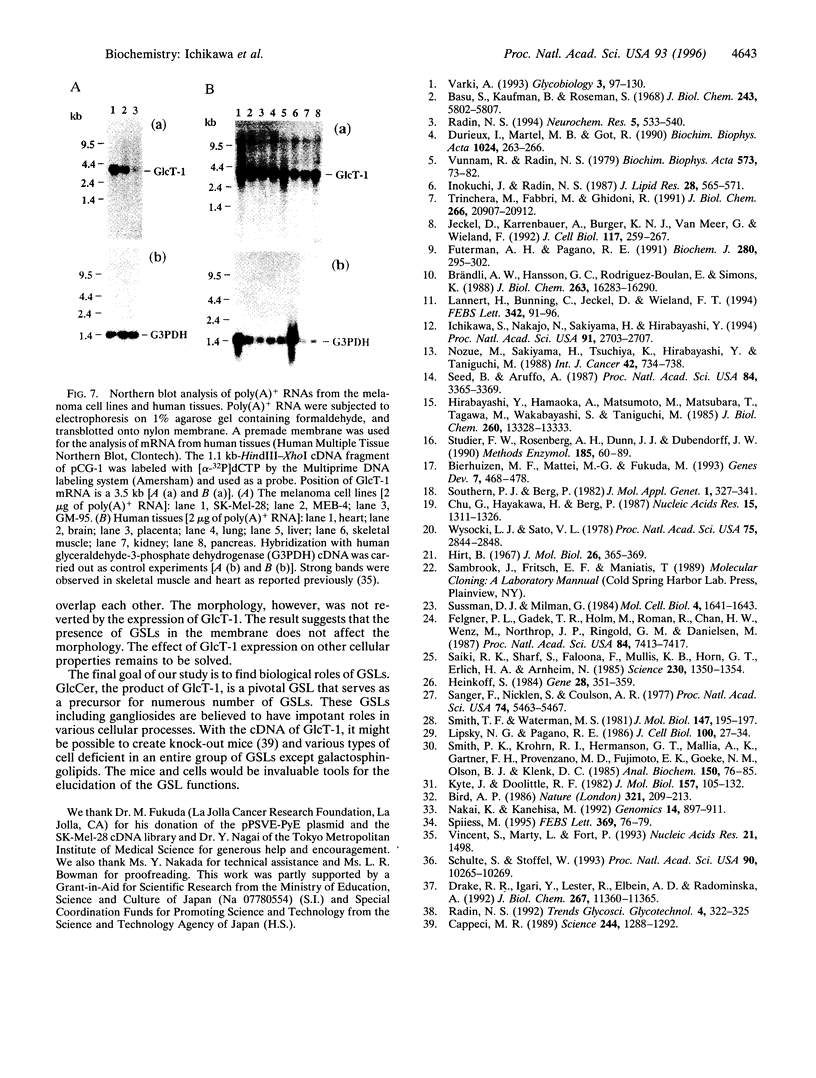
We have isolated a cDNA encoding human ceramide glucosyltransferase (glucosylceramide synthase, UDP-glucose:N-acylsphingosine D-glucosyltransferase, EC 2.4.1.80) by expression cloning using as a recipient GM-95 cells lacking the enzyme. The enzyme catalyzes the first glycosylation step of glycosphingolipid synthesis and the product, glucosylceramide, serves as the core of more than 300 glycosphingolipids. The cDNA has a G+C-rich 5' untranslated region of 290 nucleotides and the open reading frame encodes 394 amino acids (44.9 kDa). A hydrophobic segment was found near the N terminus that is the potential signal-anchor sequence. In addition, considerable hydrophobicity was detected in the regions close to the C terminus, which may interact with the membrane. A catalytically active enzyme was produced from Escherichia coli transfected with the cDNA. Northern blot analysis revealed a single transcript of 3.5 kb, and the mRNA was widely expressed in organs. The amino acid sequence of ceramide glucosyltransferase shows no significant homology to ceramide galactosyltransferase, which indicates different evolutionary origins of these enzymes.
Full text
PDF


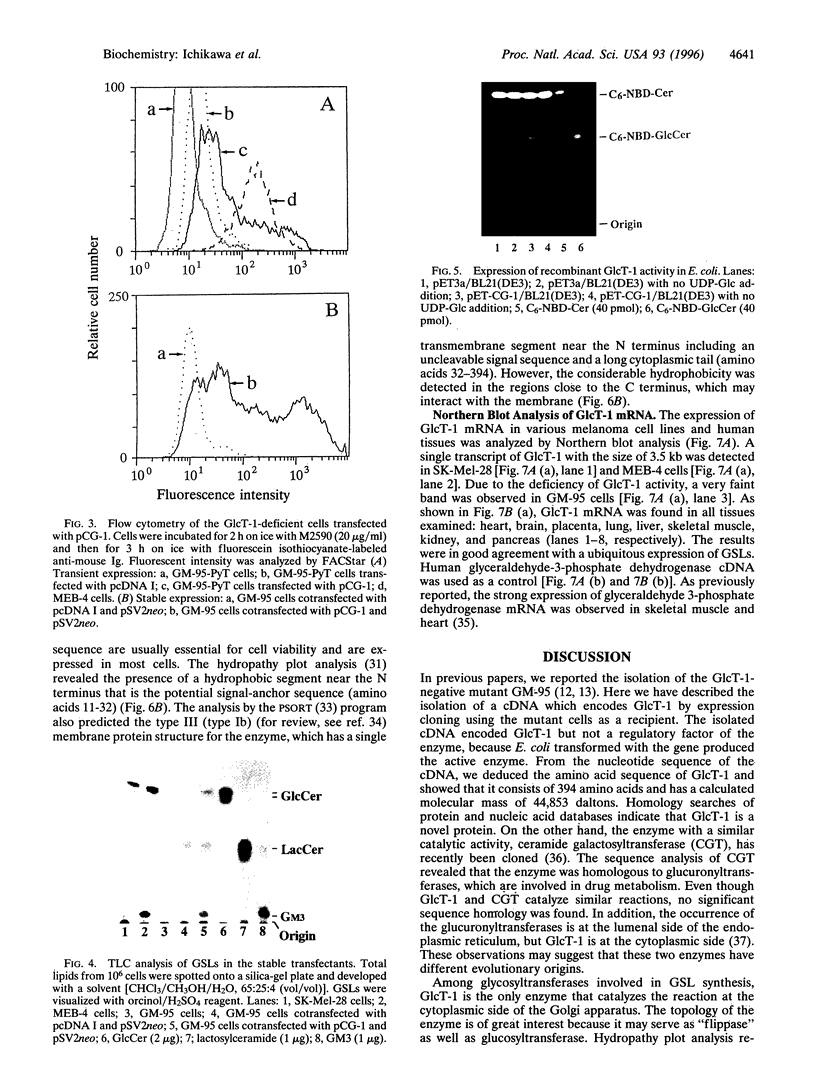
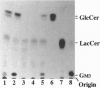
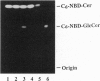
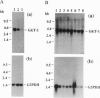
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierhuizen M. F., Mattei M. G., Fukuda M. Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev. 1993 Mar;7(3):468–478. doi: 10.1101/gad.7.3.468. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Brändli A. W., Hansson G. C., Rodriguez-Boulan E., Simons K. A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J Biol Chem. 1988 Nov 5;263(31):16283–16290. [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R. R., Igari Y., Lester R., Elbein A. D., Radominska A. Application of 5-azido-UDP-glucose and 5-azido-UDP-glucuronic acid photoaffinity probes for the determination of the active site orientation of microsomal UDP-glucosyltransferases and UDP-glucuronosyltransferases. J Biol Chem. 1992 Jun 5;267(16):11360–11365. [PubMed] [Google Scholar]
- Durieux I., Martel M. B., Got R. Solubilization of UDPglucose-ceramide glucosyltransferase from the Golgi apparatus. Biochim Biophys Acta. 1990 May 24;1024(2):263–266. doi: 10.1016/0005-2736(90)90352-o. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Pagano R. E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991 Dec 1;280(Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Hamaoka A., Matsumoto M., Matsubara T., Tagawa M., Wakabayashi S., Taniguchi M. Syngeneic monoclonal antibody against melanoma antigen with interspecies cross-reactivity recognizes GM3, a prominent ganglioside of B16 melanoma. J Biol Chem. 1985 Oct 25;260(24):13328–13333. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ichikawa S., Nakajo N., Sakiyama H., Hirabayashi Y. A mouse B16 melanoma mutant deficient in glycolipids. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2703–2707. doi: 10.1073/pnas.91.7.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi J., Radin N. S. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J Lipid Res. 1987 May;28(5):565–571. [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Burger K. N., van Meer G., Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 1992 Apr;117(2):259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B., Basu S., Roseman S. Enzymatic synthesis of disialogangliosides from monosialogangliosides by sialyltransferases from embryonic chicken brain. J Biol Chem. 1968 Nov 10;243(21):5804–5807. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lannert H., Bünning C., Jeckel D., Wieland F. T. Lactosylceramide is synthesized in the lumen of the Golgi apparatus. FEBS Lett. 1994 Mar 28;342(1):91–96. doi: 10.1016/0014-5793(94)80591-1. [DOI] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebroside analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985 Jan;100(1):27–34. doi: 10.1083/jcb.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K., Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992 Dec;14(4):897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue M., Sakiyama H., Tsuchiya K., Hirabayashi Y., Taniguchi M. Melanoma antigen expression and metastatic ability of mutant B16 melanoma clones. Int J Cancer. 1988 Nov 15;42(5):734–738. doi: 10.1002/ijc.2910420518. [DOI] [PubMed] [Google Scholar]
- Radin N. S. Glucosylceramide in the nervous system--a mini-review. Neurochem Res. 1994 May;19(5):533–540. doi: 10.1007/BF00971327. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte S., Stoffel W. Ceramide UDPgalactosyltransferase from myelinating rat brain: purification, cloning, and expression. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10265–10269. doi: 10.1073/pnas.90.21.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spiess M. Heads or tails--what determines the orientation of proteins in the membrane. FEBS Lett. 1995 Aug 1;369(1):76–79. doi: 10.1016/0014-5793(95)00551-j. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchera M., Fabbri M., Ghidoni R. Topography of glycosyltransferases involved in the initial glycosylations of gangliosides. J Biol Chem. 1991 Nov 5;266(31):20907–20912. [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Marty L., Fort P. S26 ribosomal protein RNA: an invariant control for gene regulation experiments in eucaryotic cells and tissues. Nucleic Acids Res. 1993 Mar 25;21(6):1498–1498. doi: 10.1093/nar/21.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunnam R. R., Radin N. S. Short chain ceramides as substrates for glucocerebroside synthetase. Differences between liver and brain enzymes. Biochim Biophys Acta. 1979 Apr 27;573(1):73–82. doi: 10.1016/0005-2760(79)90174-7. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]