Abstract
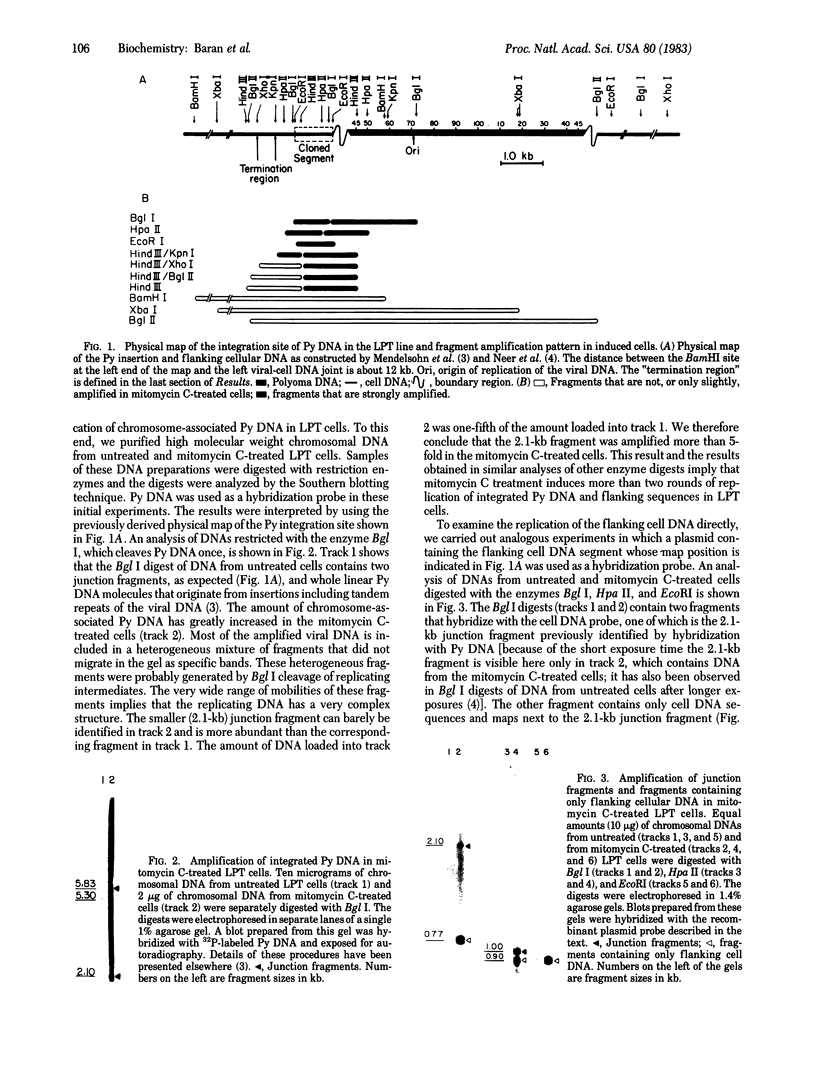
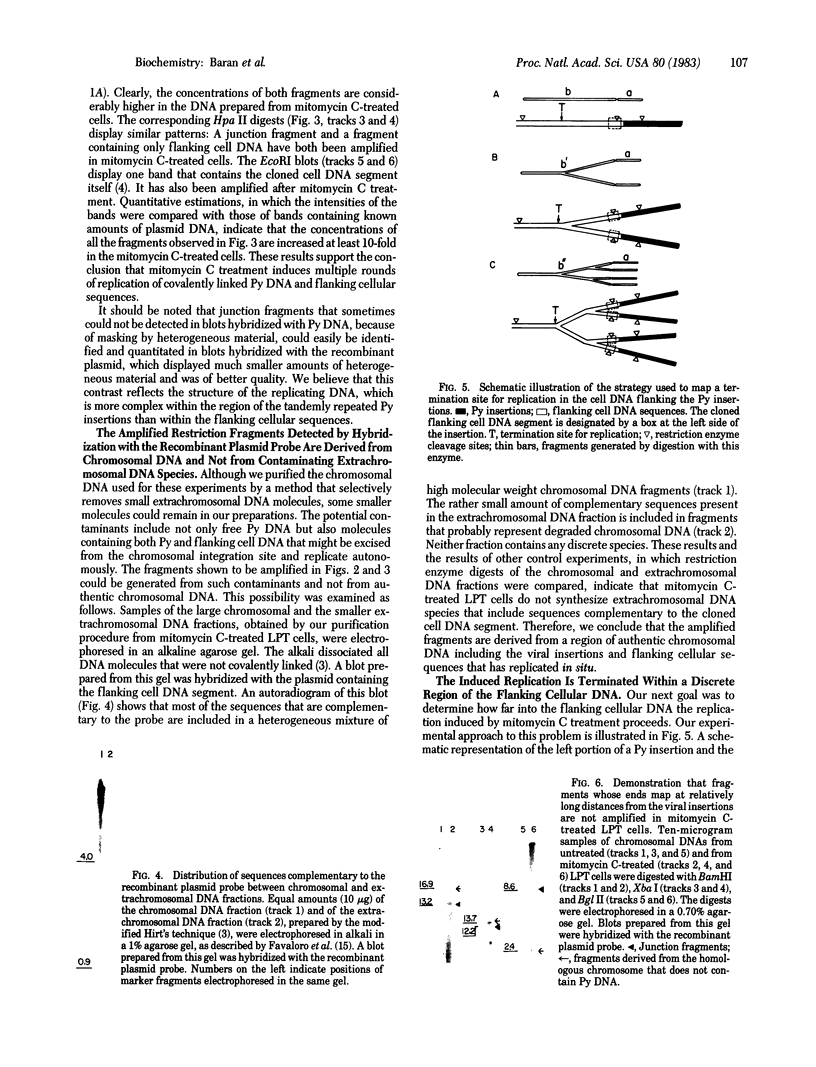
Replication of integrated polyoma virus DNA and flanking cellular sequences was studied in an inducible line of polyoma-transformed rat cells, designated the LPT line, that contains a single viral integration site. Chromosomal DNAs were purified from LPT cells treated with the virus-inducing agent mitomycin C and from untreated cells and were digested with restriction enzymes. The digests were analyzed by the Southern blotting technique. The virus DNA and a recombinant plasmid containing flanking cell DNA were used as hybridization probes. The analysis showed that mitomycin C treatment caused a more than 10-fold amplification of restriction fragments extending up to about 2.0 kilobase pairs into the cellular DNA flanking one end of the viral insertions, defined as the left joint. Fragments extending beyond this region were not amplified. These results showed that (i) integrated polyoma virus DNA undergoes multiple rounds of replication in mitomycin C-treated LPT cells and (ii) the replication extends into the flanking sequences and is arrested within a 0.40-kilobase-pair cellular DNA segment located about 2.0 kilobase pairs beyond the left joint. This segment may include a terminator of a normal cellular replicon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication and degradation of bromouracil-substituted DNA in mammalian cells after irradiation with ultraviolet light. Biophys J. 1968 Jul;8(7):775–791. doi: 10.1016/S0006-3495(68)86520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fogel M. Induction of virus synthesis in polyoma-transformed cells by DNA antimetabolites and by irradiation after pretreatment with 5-bromodeoxyuridine. Virology. 1972 Jul;49(1):12–22. doi: 10.1016/s0042-6822(72)80003-5. [DOI] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Fogel M., Sachs L. The activation of virus synthesis in polyoma-transformed cells. Virology. 1969 Mar;37(3):327–334. doi: 10.1016/0042-6822(69)90216-5. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Bancuk J. E. Polyoma genome in hamster BHK-21-C13 cells: integration into cellular DNA and induction of the viral replication. J Virol. 1976 Oct;20(1):133–141. doi: 10.1128/jvi.20.1.133-141.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderud S., Andreassen R., Haugli F. DNA replication in Physarum polycephalum: electron microscopic and autoradiographic analysis of replicating DNA from defined stages of the S-period. Nucleic Acids Res. 1979 Apr;6(4):1417–1431. doi: 10.1093/nar/6.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Termination of DNA replication in vitro at a sequence-specific replication terminus. Cell. 1981 Mar;23(3):681–687. doi: 10.1016/0092-8674(81)90431-1. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huberman E., Fogel M. Activation of carcinogenic polycyclic hydrocarbons in polyoma-virus-transformed cells as a prerequisite for polyoma virus induction. Int J Cancer. 1975 Jan 15;15(1):91–98. doi: 10.1002/ijc.2910150111. [DOI] [PubMed] [Google Scholar]
- Kowalski J., Cheevers W. P. Synthesis of high molecular weight DNA strands during S phase. J Mol Biol. 1976 Jul 5;104(3):603–615. doi: 10.1016/0022-2836(76)90123-6. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A., Seeley N. R. Terminus region of the chromosome in Escherichia coli inhibits replication forks. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3927–3931. doi: 10.1073/pnas.74.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanotte M., Moerman C., Panijel J. DNA synthesis in mouse thymocytes. Exp Cell Res. 1977 Oct 1;109(1):191–200. doi: 10.1016/0014-4827(77)90057-x. [DOI] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Evidence for a fixed termination site of chromosome replication in Escherichia coli K12. J Mol Biol. 1977 Sep 25;115(3):295–314. doi: 10.1016/0022-2836(77)90156-5. [DOI] [PubMed] [Google Scholar]
- Manor H., Fogel M., Sachs L. Integration of viral into chromosomal deoxyribonucleic acid in an inducible line of polyoma-transformed cells. Virology. 1973 May;53(1):174–185. doi: 10.1016/0042-6822(73)90476-5. [DOI] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Mendelsohn E., Baran N., Neer A., Manor H. Integration site of polyoma virus DNA in the inducible LPT line of polyoma-transformed rat cells. J Virol. 1982 Jan;41(1):192–209. doi: 10.1128/jvi.41.1.192-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer A., Baran N., Manor H. In situ hybridization analysis of polyoma DNA replication in an inducible line of polyoma-transformed cells. Cell. 1977 May;11(1):65–71. doi: 10.1016/0092-8674(77)90317-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Mercado C. M., Olson J., Chatterjie N. The mode of interaction of mitomycin C with deoxyribonucleic acid and other polynucleotides in vitro. Biochemistry. 1974 Nov 19;13(24):4878–4887. doi: 10.1021/bi00721a002. [DOI] [PubMed] [Google Scholar]