Abstract
Background
Hypoandrogenemia is associated with an increased risk of ischemic diseases. Since actions of androgens are exerted through androgen receptor (AR) activation, we studied hind limb ischemia in AR knockout (KO) mice to elucidate the role of AR in response to ischemia.
Methods and Results
Both male and female ARKO mice exhibited impaired blood flow recovery, more cellular apoptosis and a higher incidence of autoamputation after ischemia. In ex vivo and in vivo angiogenesis studies, AR-deficient vascular endothelial cells showed reduced angiogenic capability. In ischemic limbs of ARKO mice, reductions in the phosphorylation of the Akt protein kinase and endothelial nitric oxide synthase (eNOS) were observed despite a robust increase in hypoxia-inducible factor 1α and vascular endothelial cell growth factor (VEGF) gene expression. In in vitro studies, siRNA-mediated ablation of AR in vascular endothelial cells blunted VEGF-stimulated phosphorylation of Akt and eNOS. Immunoprecipitation experiments documented an association between AR and kinase insert domain protein receptor (KDR) that promoted the recruitment of downstream signaling components.
Conclusion
These results document a physiological role of AR in gender-independent angiogenic potency and provide evidence for a novel cross-talk between androgen/AR signaling and VEGF/KDR signaling pathways.
Keywords: androgen receptor, angiogenesis, KDR, Akt, eNOS
Introduction
Cardiovascular disease (CVD) remains a major cause of human deaths, especially in males and gender differences in its onset and severity make it a key contributor to the life span gap between genders1. Although the gender disparity in cardiovascular disease has been considered primarily as a reflection of estrogen-mediated protection against atherogenesis, conclusive clinical evidence for support of this hypothesis is lacking. In fact, two large-scale prospective randomized clinical trials showed no cardiovascular benefits of combined estrogen plus progestin therapy in postmenopausal women 2, 3. On the other hand, increased understanding of the biological effects of androgens in the cardiovascular system has revealed a close relationship between a fall in male testosterone levels, called “andropause”, and an increase in cardiovascular events with aging. Many clinical studies have shown that hypoandrogenemia is associated with CVD 4, cardiovascular death 5, atherosclerosis 6, metabolic syndrome, visceral fat obesity, type 2 diabetes 7, 8. Testosterone replacement therapy has been shown to have beneficial effects on CVD risk factors, including visceral abdominal fat mass, insulin sensitivity, glycemic control, and hyperlipidemia 9. We have also reported that serum levels of dehydroepiandrosterone sulfate, a major active androgen precursor, are inversely associated with carotid atherosclerosis 10. However, the role of androgens in cardiovascular physiology and pathophysiology remains controversial, and some epidemiological studies have shown no obvious association of hypoandrogenemia with CVD 11, 12. In addition, one study showed that testosterone administration to older men with a prevalence of chronic disease led to an increased risk of adverse cardiovascular events 13. Thus, a better understanding of the molecular links between androgens and cardiovascular disease is warranted.
Androgen exerts various actions in its target organs, including the male genitalia, brain, bone, skeletal muscles, and endothelial cells, smooth muscle cells and fibroblasts in the vascular system 14. Biological activities of androgen are predominantly mediated through the transcriptional control of target genes by genomic actions of the nuclear androgen receptor (AR) 15. Androgen also exerts nongenomic actions by interacting with multiple signaling pathways, independent of transcriptional control 16, 17. Such nongenomic effects of androgen may occur through AR in the cytoplasm to induce the rapid activation of kinase-signaling cascades 17.
In order to clarify the pathophysiological role of AR in various target organs in vivo, we examined AR knockout (KO) mice generated by a Cre-loxP system. Male ARKO mice manifest late-onset obesity 18, high-turnover osteopenia 18, impaired brain masculinization 19, and aberrant adiponectin expression 20. In addition, we have demonstrated that AR activation plays a pivotal role in not physiological cardiac growth, as well as protection from pathological cardiovascular remodeling induced by angiotensin II 21, 22 and cardiotoxicity induced by the anticancer agent doxorubicin 23.
Although skeletal muscle ischemia caused by peripheral arterial disease (PAD) and/or thrombotic disorders is also a critical problem in elderly subjects with andropause 24, it has been unclear whether AR plays a distinct pathophysiologic role in response to skeletal muscle ischemia. In order to clarify this issue, we studied the role of AR in response to ischemic injury using a hind limb ischemia model in ARKO mice. The results indicated gender-independent novel protective mechanisms of AR actions against ischemic injury.
Methods
Animals and Experimental Protocol
Male and female ARKO mice were generated by targeted disruption of the AR gene using the Cre-loxP system as previously described 18, 19, 25, 26. Male ARKO mice exhibited testicular feminization, and reduction in levels of serum gonadal androgens (testosterone and dihydrotestosterone), whereas serum adrenal androgen and estrogen levels remained normal 18. Female ARKO mice have normal serum hormone levels, including 17β-estradiol, progesterone, testosterone, luteinizing hormone and follicle-stimulating hormone 26. In the present study, we used 25-week-old male and female ARKO mice and age-matched littermate male and female wild-type (WT) mice. Mice were backcrossed for 10 generations with the C57BL/6J strain. To create hind limb ischemia in these mice, the proximal portion of the femoral artery and the distal portion of the saphenous artery were ligated and then excised 27, 28. We performed the following experimental procedures: macroscopic evaluation of ischemic severity, laser speckle blood flow analysis, immunohistochemistry, real-time PCR analysis, Western blot analysis, aortic ring assay, in vivo angiogenesis assay and bone marrow transplantation, SiRNA experiments, immunoprecipitation, in situ proximity ligation assay. All experimental procedures were performed in accordance with the guidelines of the Animal Research Committee, The University of Tokushima Graduate School of Health Biosciences. Details of the experimental procedures can be found in the online supplement.
Statistical analysis
Values for each parameter within a group are expressed as dot plots with mean bars. For comparisons of quantitative data among groups, statistical significance was assessed by the Kruskal-Wallis test. The Bonferroni-corrected Mann-Whitney U test or Dunn's test was used for multiple comparisons. For comparison of time-dependent changes among groups, statistical significance was assessed by linear mixed effects regression analysis. Limb survival rate was assessed by the log-rank test. These analyses were performed by using Excel (Microsoft Office Excel 2007; Microsoft, Richmond, CA), PASW Statistics 18.0 (IBM SPSS Japan Inc., Tokyo, Japan), GraphPad Prism6 (GraphPad Software, San Diego, CA) and JMP (SAS Institute Japan Ltd., Tokyo, Japan). Statistical significance was set at P <0.05.
Results
Increased incidence of autoamputation in ARKO mice after hind limb ischemia
In order to elucidate the role of AR in ischemic response, we created a model of hind limb ischemia induced by surgical arteriectomy of the right femoral artery in ARKO and control mice. Three weeks after surgery, necrotic changes were macroscopically evaluated. Both male and female WT mice showed limited necrosis of toes. In contrast, hind limb autoamputations occurred in more than half (53%) of male ARKO mice at day 21 after ischemic surgery (Figure 1A and B). Interestingly, female ARKO mice also demonstrated extensive necrosis, leading to autoamputation at nearly the same frequency as male ARKO mice (42%) (Figure 1C).
Figure 1. Increased hind limb autoamputation in both male and female ARKO mice after femoral arteriectomy.
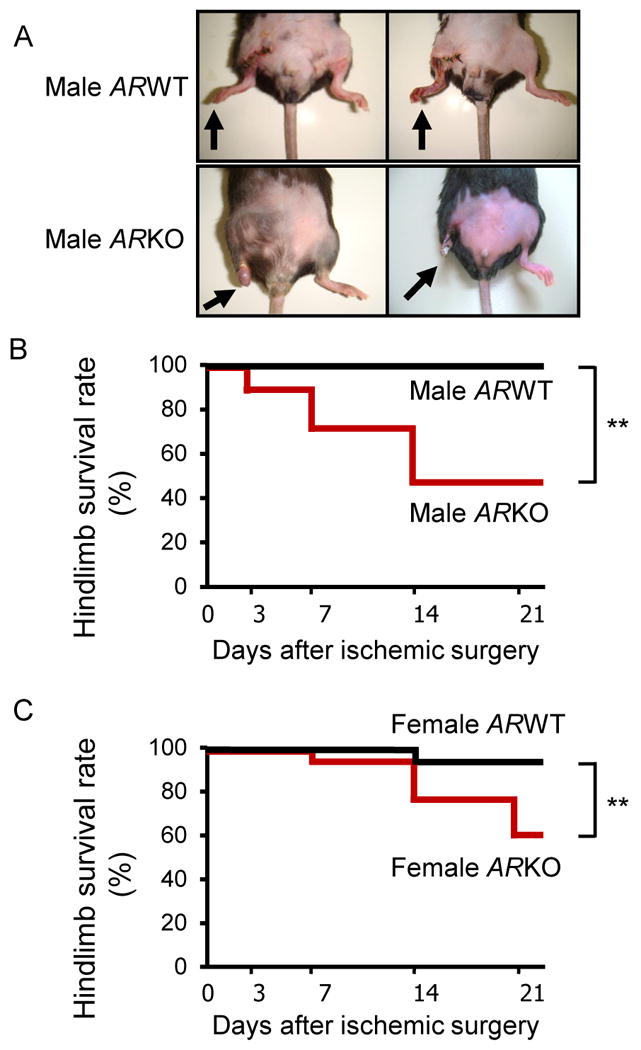
(A) Representative photographs of hind limbs of male WT and male ARKO mice at day 21 after ischemic surgery. (B) Limb survival rate after ischemic surgery in male WT and ARKO mice. Autoamputation of the hind limb was defined as extended necrosis above the knee. n=17 in each male mice group. **P<0.01, male ARWT vs. male ARKO using Log-rank test. (C) Limb survival rate after ischemic surgery in female WT and ARKO mice. n=25 in the female WT mouse group and n=12 in the female ARKO mouse group. **P<0.01, female ARWT vs. female ARKO using Log-rank test.
Accelerated cellular apoptosis of ischemic skeletal muscle in ARKO mice
Ischemia-induced cellular apoptosis of skeletal muscle was examined in both genders of WT and ARKO mice by TUNEL staining. The number of TUNEL-positive cells in ischemic skeletal muscle was significantly increased in both male and female ARKO mice compared to those in respective male and female WT mice (Figure 2A-D). Immunohistochemical examination confirmed that the majority of TUNEL-positive cells were skeletal myocytes (black arrows), but apoptotic endothelial cells were also observed (blue arrows) (Figure 2E). Next, the expression ratio of Bcl-2-to-Bax, key factors of the apoptotic signal pathway was analyzed in ischemic muscle. Real-time PCR analysis and Western blot analysis using tissue homogenates of ischemic adductor muscles obtained from male and female ARKO mice at 1 day after ischemia showed a lower Bcl-2 (Bcl-2)-to-Bax (BAX) expression ratio than those in respective male and female WT mice (Figure 2F-K). In male mice, the Bcl-2-to-Bax ratio at day 1 decreased on mRNA level, though the protein ratio of those proteins was different result. These results indicate the possibility that there is a gender difference in the stability of Bax and Bcl2 mRNAs. Taken together, these findings indicate that the severity of ischemia-induced cellular apoptosis leading to autoamputation of the hind limb is more accelerated in ARKO mice than those in WT mice regardless of gender.
Figure 2. Accelerated cellular apoptosis in ischemic skeletal muscles of both male and female ARKO mice.
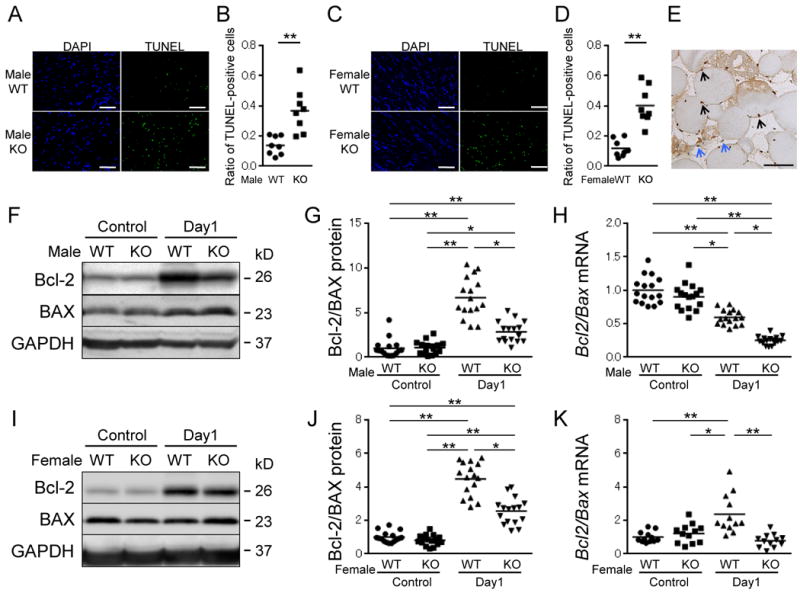
(A and C) Immunofluorescence of TUNEL staining in ischemic adductor muscle of male WT and ARKO mice (A) or female WT and ARKO mice (C) at day 1 after ischemia. Scale bar indicates 200 μm. (B and D) Ratio of TUNEL-positive cells to total nuclei in ischemic muscle of male mice (B) and female mice (D). n=8 in each group. (E) TUNEL staining by an enzyme antibody technique in ischemic muscle of male ARKO mice at day 1. Black arrows denote nuclei of skeletal muscle cells. White arrows denote nuclei of endothelial cells. Scale bar indicates 50 μm. (F, G, I and J) Protein levels of Bcl-2, BAX and Bcl-2-to-BAX ratio by Western blot analysis in muscle of both sexes of WT and ARKO mice at before surgery (control) and day1 after ischemia. The value of WT control was set as 1. (H and K) mRNA expression levels of Bcl-2-to-Bax ratio by quantitative real-time PCR in ischemic muscle of both sexes of WT and ARKO mice before surgery (control) and at day 1 after ischemic surgery. n= 16 in each group in Figures G, H and J, n= 12 in each group in Figure K. *P<0.05, **P<0.01 using the Bonferroni-corrected Mann-Whitney U test following the Kruskal-Wallis test. Bars represent mean values in each group.
Impaired blood flow recovery with reduced capillary density in surviving skeletal muscle of ARKO mice after ischemia
Blood flow recovery of ischemic skeletal muscle in both male and female WT mice and that of surviving ischemic skeletal muscle in both male and female ARKO mice were evaluated for 3 weeks by laser speckle blood flow (LSBF) imager. In male and female WT mice, blood flow fell precipitously until one day after ischemic surgery; however, blood flow recovered promptly and had returned to almost non-ischemic hind limb levels at day 21. In contrast, the recovery of blood perfusion in surviving ischemic hind limbs of male and female ARKO mice was poor throughout the follow-up period, and the ratio of ischemic to non-ischemic laser speckle blood flow was persistently lower in ARKO mice than in WT mice regardless of sex (Figure 3A, B, E and F). At 3 weeks after surgery, capillary vessels identified by CD31 staining were markedly increased in ischemic muscle of male and female WT mice, whereas this compensatory response against ischemia was almost completely abrogated in male and female ARKO mice (Figure 3C, D, G and H). In addition, smaller numbers of α smooth muscle actin (αSMA)-stained cells indicating vascular pericytes, were observed in male and female ARKO mice after ischemia (supplemental figure 1).
Figure 3. Impaired blood flow recovery and reduced capillary density in both male and female ARKO mice after ischemia.
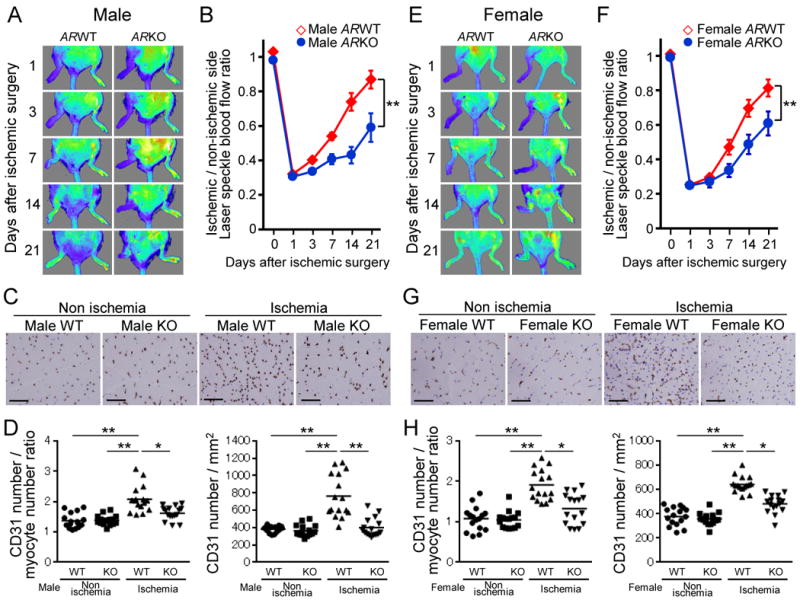
(A and E) Representative laser speckle blood flow (LSBF) images of the ischemic hind limb in WT and ARKO mice during the experimental period. A low perfusion signal is indicated as dark blue and according to increase in perfusion signals, the colors are indicated as green, yellow and red in series. (A) Male WT mice (n=17) and male ARKO mice (surviving hind limbs, n=8). (E) Female WT mice (surviving hind limbs, n=16) and female ARKO mice (surviving hind limbs, n=7). (B and F) Cumulative results are shown as ratio of blood flow in the ischemic limb to that in the nonischemic limb at each time point (B: male mice, F: female mice). **P<0.01 using a linear mixed effects regression analysis. Error bars represent ± SEM. (C and G) CD31 immunohistochemical staining to determine capillaries in ischemic and nonischemic thigh adductor muscles in WT and ARKO mice at day 21 after surgery (C: male mice, G: female mice). Scale bar indicates 100 μm. (D and H) Quantification of capillary density is expressed as CD31-positive number per myocyte number or per square millimeter in WT and ARKO mice (D: male mice, H: female mice). n=16 in each group. *P<0.05, **P<0.01 using Dunn's test following the Kruskal-Wallis test. Bars represent mean values in each group.
Attenuated angiogenic potency in AR-deficient vascular endothelial cells
In order to clarify the influence of AR deficiency on angiogenic potency of vascular endothelial cells, aortic ring assay and in vivo angiogenesis assay were performed (Figure 4A and B). Figure 4A shows representative photos and quantitative results of microvascular sprouting at day 7 after aortic ring implantation. We found that the number of sprouting microvessels and length of microvessels were significantly reduced in aortas from male ARKO mice compared to those in aortas from male WT mice. Finally, the angiogenesis assay involving the implantation of tubes containing basement membrane extract with the angiogenic factors also showed reduced vessel invasion when the tubes were implanted in male ARKO mice compared to that in tubes implanted in male WT mice (Figure 4B). These results are consistent with the notion that AR-deficient vascular endothelial cells have reduced angiogenic potency.
Figure 4. Attenuated angiogenic potency in endothelial cells but not in bone marrow progenitor cells of male ARKO mice.
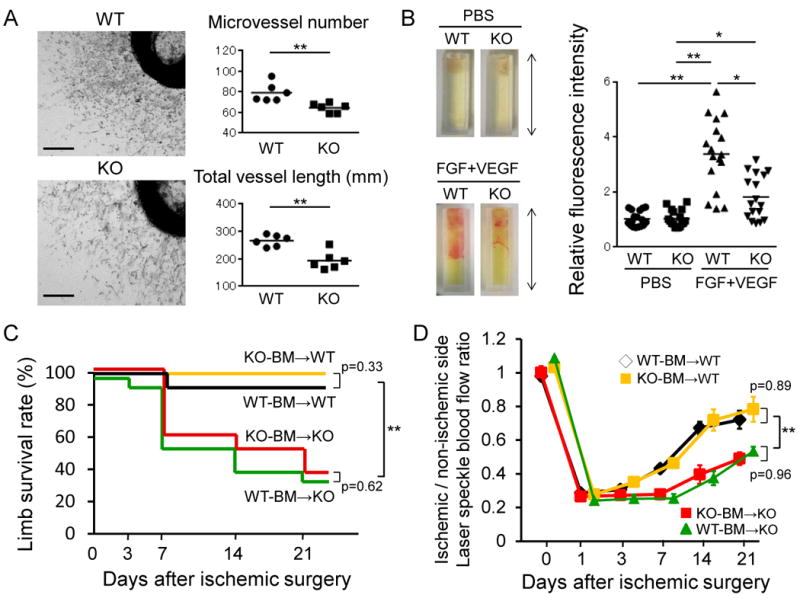
(A) Aortic ring assay. (A, left) Micrographs of representative aortic ring microvessels from male WT and male ARKO mice grown in the presence of growth factors at day 7. Scale bar indicates 0.5 mm. (A, right) Microvessel number and total length of microvessel were quantified. n=6 in each group. (B) In vivo angiogenesis using a Directed In Vivo Angiogenesis Assay kit. (B, left) Photographs of silicone cylinders at 15 days after implantation in male WT and male ARKO mice, the capillary vessels migrated into and proliferated in silicone cylinders with angiogenic factors (FGF+VEGF) from subcutaneous vessels. The value of WT control was set as 1. n=17 in each group, 2 cylinders being implanted in each mouse. Arrowed line indicates 1.0 cm. (B, right) Relative invasion to male WT mice without angiogenic factors (PBS) was determined by fluorescence of cell pellets labeled with FITC-Lectin. *P<0.05, **P<0.01 using Dunn's test following the Kruskal-Wallis test. Bars represent mean values in each group. (C) Bone marrow (BM) transplantation model. Limb survival rate after ischemic surgery in male WT mice receiving male WT BM (WT-BM→WT) or male ARKO BM (KO-BM→WT), and male ARKO mice receiving male WT BM (WT-BM→KO) or male ARKO BM (KO-BM→KO). n=10 in each recipient group. **P<0.01 vs. male ARKO mice receiving the same genotype of BM at day 21 after ischemia using the log-rank test with Bonferroni-corrected pairwise comparisons. (D) Ratio of blood flow in ischemic to nonischemic limb by LSBF at each time point after ischemic surgery in male WT and male ARKO mice with BM transplantation treatment between BM of male WT mice and BM of male ARKO mice. n=10 in each recipient group. **P<0.01 vs. male ARKO mice receiving the same genotype of BM at days 3 to 21 after ischemia using a linear mixed effects regression analysis. Error bars represent SEM.
Bone marrow cells are not involved in impaired angiogenesis of male ARKO mice after ischemia
Bone marrow (BM)-derived progenitor cells have been shown to participate in revascularization in ischemic tissues 29. In this regard, it has been proposed that the mobilization of angiogenic progenitors contributes to the effects of androgens on the revascularization process 30. To determine whether BM-derived cells are involved in impaired angiogenesis of ARKO mice, we performed BM transplantation between male WT and male ARKO mice. As shown in Figure 4C and D, limb survival rate and blood flow recovery from ischemia were not decreased in male WT mice receiving male ARKO BM cells. In addition, male WT BM cells transplanted into male ARKO mice did not prevent autoamputation in these mice after hind limb ischemia (Figure 4C). Laser speckle imaging also demonstrated that male WT BM cell transplantation failed to restore the impaired blood flow recovery in surviving skeletal muscle of male ARKO mice after ischemia (Figure 4D). These results indicate that the impaired blood flow recovery from ischemic stress in male ARKO mice is not due to an impaired angiogenic potency of BM-derived progenitor cells of these mice.
Aberrant gene expression levels of angiogenesis-related factors in ARKO mice after hind limb ischemia
Gene expression levels of angiogenesis-related factors were analyzed in the adductor muscle by real-time PCR analysis. Under non-ischemic conditions, there were no significant differences in gene expression levels of proangiogenic factors, including hypoxia inducible factor 1 α (Hif1a), vascular endothelial growth factor A (Vegfa), fibroblast growth factor 2 (Fgf2) and the VEGF receptor, Kdr between both sexes of WT and ARKO mice (Figure 5A and B). However, following surgery-induced ischemia, expression levels of Hif1a, Vegfa and Fgf2 were prominently augmented in male and female ARKO mice compared to those in respective male and female WT mice. The ischemia-reduced elevation of Kdr mRNA levels was attenuated in male ARKO mice but not female ARKO mice (Figure 5A and 5B).
Figure 5. Aberrant expression and activation of angiogenesis related factors in ischemic muscles of male and female ARKO mice.
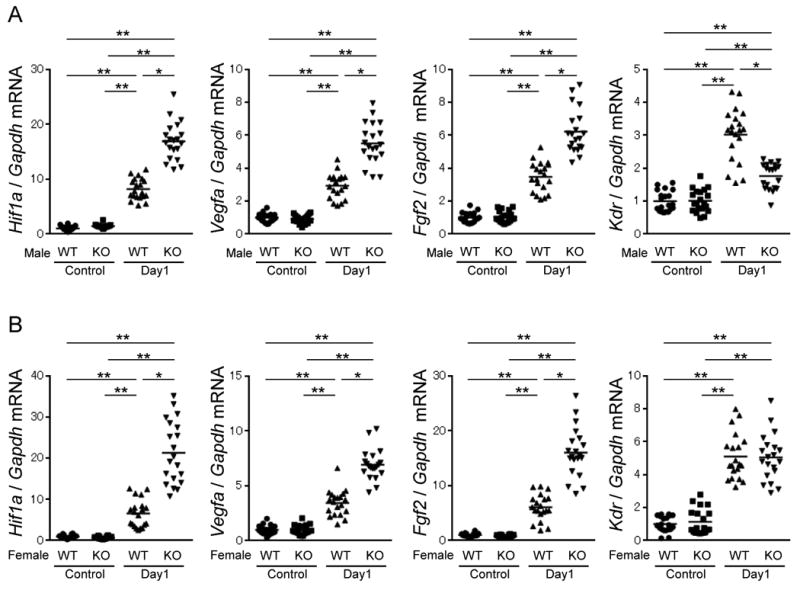
Quantitative PCR analysis for angiogenesis-related factors in skeletal muscles of male WT mice and male ARKO mice (A) or female WT mice and female ARKO mice (B) before surgery (control) and at day 1 after ischemic surgery. mRNA level of Gapdh served as an internal control. The value of WT control was set as 1. n= 20 in each group. *P<0.05, **P<0.01 using Dunn's test following the Kruskal-Wallis test. Bars represent mean values in each group.
Reduced regulation of cell survival and proangiogenic factors in ARKO mice
Our previous studies demonstrated that extracellular signal-regulated kinases 1/2 (ERK1/2) and Akt-eNOS signaling pathways are activated by the non-genomic actions of AR stimulation 21-23. Therefore, we investigated the role of the androgen/AR system in the regulation of these pathways in ischemic limbs because they are involved in cell survival and angiogenesis. Ischemic muscles of male and female ARKO mice showed significant attenuation of ERK1/2, Akt and eNOS phosphorylation compared with those in respective male and female WT mice (Figure 6A and 6B). Since both Akt and ERK1/2 activation promote cell survival and since activation of Akt and eNOS enhances angiogenesis 31, 32, the acceleration of ischemia-induced cellular apoptosis and impaired angiogenesis in both male and female ARKO mice may be explained, at least in part, by the reduced activation of these factors.
Figure 6. Reduced phosphorylation of factors involved in cell survival and angiogenesis in ischemic muscles of male and female ARKO mice.
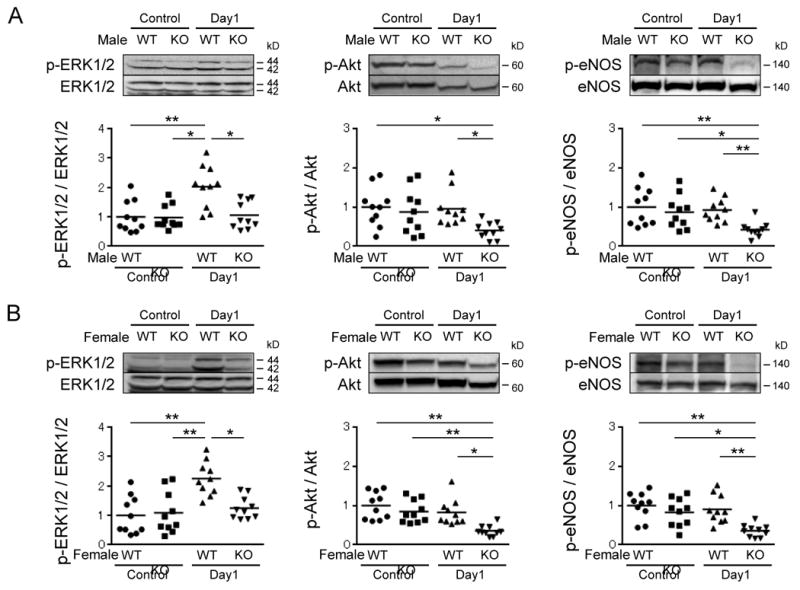
Western blot analysis of total and phosphorylated ERK1/2, Akt and eNOS in skeletal muscles of male WT mice and male ARKO mice (A) or female WT mice and female ARKO mice (B) before surgery (control) and at day 1. The value of WT control was set as 1. n=10 in each group. *P<0.05, **P<0.01 using Dunn's test following the Kruskal-Wallis test. Bars represent mean values in each group.
AR knockdown in HUVECs blunts activation of the VEGF receptor signaling pathway
In order to determine whether reduced activation of the Akt-eNOS pathway in ischemic muscles of ARKO mice was associated with a blunted responsiveness of endothelial cells to VEGF stimulation, we next examined VEGF-induced activation of the Akt-eNOS signaling pathway in vascular endothelial cells with or without siRNA-mediated AR knockdown. (In this study, the siRNA reduced AR mRNA levels to 17.0 ± 1.8% of the control and reduced AR protein levels to 20.8 ± 2.0% of the control.) VEGF stimulation in the presence of 5alpha-dihydrotestosterone (DHT) enhanced Akt and eNOS phosphorylation in control HUVEC cultures. In contrast, VEGF-stimulated Akt and eNOS phosphorylation was blunted in HUVECs with AR knockdown (Figure 7A). These results indicate that AR-mediated signaling potentiates VEGF-mediated activation of the Akt-eNOS pathway in vascular endothelial cells.
Figure 7. Association between AR and VEGF receptor signaling pathway in HUVECs.
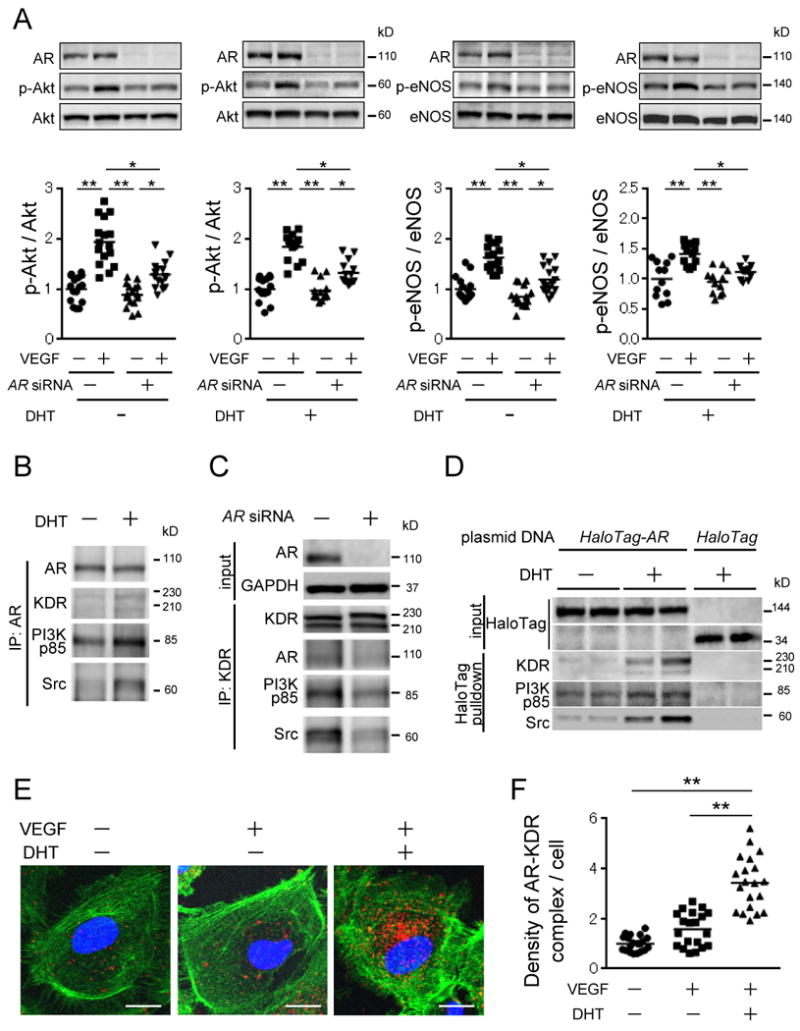
(A) VEGF-stimulated phosphorylation of Akt and eNOS in HUVECs with or without AR knockdown. The value of WT control was set as 1. n=12-16 in each experiment. (B) Immunoprecipitation experiments using AR antibody with or without DHT. (C) Immunoprecipitation experiments using KDR antibody with or without AR knockdown. (D) HaloTag pull-down assay in HUVECs expressing HaloTag-AR fusion protein. (E and F) In site proximity ligation assay using AR and KDR antibodies in HUVECs with or without ligands. (E) Confocal microscopy images indicate endogenous AR-KDR complexes (red dots), nuclear (blue) and filamentous actin (green). Scale bar indicates 20 μm. (F) Quantified results showing fold increase in fluorescent signal density per cell for each condition. Five different visual fields were randomly selected for counting red dots in four independent samples of each group, and the values of control cells (VEGF (-) and DHT (-)) were set at 1.0. **P<0.01 using Dunn's test following the Kruskal-Wallis test. Bars represent mean values in each group.
Ligand-bound AR promotes complex formation with KDR, Src and PI3K
Stimulation by VEGFs rapidly induces KDR dimerization and autophosphorylation, followed by recruitment and activation of Src and phosphoinositide-3-kinase (PI3K) 33. AR is also shown to recruit Src and activate the mitogen-activated protein kinase (MAPK) pathway 34, and activate the PI3K-Akt cascade 35, leading to cell survival and proliferation. Since we found that VEGF-stimulated Akt and eNOS phosphorylation was blunted by AR deficiency, we examined whether AR associates with VEGF receptor and affects its downstream signaling pathway in endothelial cells. Immunoprecipitation of HUVEC lysates using an anti-AR antibody showed an association of AR with KDR, PI3Kp85, and Src that was augmented by DHT supplementation (Figure 7B). Moreover, immunoprecipitation experiments using an anti-KDR antibody in the presence of DHT and VEGF revealed that AR, PI3Kp85 and Src were associated with KDR, whereas AR knockdown attenuated the associations between PI3Kp85, Src and KDR (Figure 7C). In order to clarify the association between KDR and AR more clearly, we employed the HaloTag technology 36. Exogenous AR-HaloTag fusion proteins and HaloTag control proteins were expressed in endothelial cells, and then a HaloTag pull-down assay was performed on cell lysates to identify candidate AR-bound proteins by Western blot analysis. These results showed that DHT supplementation promotes the associations between AR-HaloTag fusion protein and KDR, PI3Kp85 and Src, while those protein associations are diminished in the absence of DHT (Figure 7D). Taken together, these results are consistent with the notion that AR associates with KDR and promotes associations between KDR, Src and PI3K.
Ligand-bound AR associates with KDR at the plasma membrane and cytoplasm of vascular endothelial cells in situ
In order to visualize the association between AR and KDR in vascular endothelial cells, we used an in situ proximity ligation assay (PLA), a method that is used for detecting and imaging protein associations in both tissue sections and cultured cells in vitro 37. The in situ PLA revealed modest AR/KDR proximity signal in HUVECs under baseline conditions (Figure 7E, left panel). VEGF stimulation alone led to a slightly increased the AR/KDR proximity signal (Figure 7E, middle panel). However, the combination of VEGF and DHT led to markedly greater AR/KDR complex formation (Figure 7E, right panel and 7F). These results indicate that DHT promotes the association between AR and KDR outside the nucleus in vascular endothelial cells, consistent with the findings of enhanced VEGF actions under these conditions.
Discussion
In the present study, we showed that AR is essential for robust revascularization in response to ischemia in both male and female mice. Male and female ARKO mice manifested hindlimb autoamputation after ischemic surgery, and AR-deficiency was associated with greater of ischemia-induced cellular apoptosis of myofibers and endothelial cells. Our study also documents a hitherto unrecognized cross-talk mechanism between androgen/AR and VEGF/KDR signaling pathways. Collectively, these data provide a molecular rationale for why “andropause” individuals are at greater risk for ischemic disease.
Pathophysiological roles of AR in females are largely unknown. Shiina et al. have demonstrated that eight-week-old female ARKO mice manifest reduced ovarian follicle numbers, impaired mammary development, and reduced pups numbers 26. In addition, they have shown that forty-week-old ARKO mice have no ovarian follicles leading to infertility with aberrant gene expression levels of folliculogenesis related factors 26. these results and our present study suggest that liganded and/or unliganded AR activation might partially participate in biological homeostasis of multiple organs dependent and/or independent of sex and/or estrogen.
Aspects of our study are consistent with a prior report by Sieveking et al. showing that androgens have a positive effect on angiogenesis 30. For example, they reported that castration led to reduction in ischemia-induced angiogenesis. However, utilization of the ARKO mouse in the current study has enabled us to reach additional conclusions. For example, Sieveking et al. concluded that androgens promote angiogenesis in male but not female mice after ischemia 30. In contrast, we show that AR-deficiency impairs ischemia-induced angiogenesis in both male and female mice. There are a number of possible reasons for this apparent discrepancy. One possibility is the presence (ARKO mice) or absence (ovariectomised WT mice) of estrogen in female mice, which can influence a revascularization response 38, 39. Alternatively, the presence (ovariectomised WT mice) or absence (ARKO mice) of AR in female mice could account for the difference in these findings. t has been reported that unliganded nuclear receptors, including thyroid hormone receptor, estrogen receptor, progesterone receptor and vitamin D receptor have considerable biological actions 40-43, we speculate that unliganded AR also plays a role in angiogenesis after ischemia regardless of gender. Further experiments are required to clarify these issues.
Sieveking et al. 30 also reported that treatment with androgen promoted the mobilization of bone marrow progenitor cells that could theoretically promote revascularization via paracrine secretion of angiogenic factors. Similarly, it has been reported that estrogen can mobilize bone marrow-derived angiogenic cells that can contribute to the revascularization process 44. However, as reported here, limb reperfusion and protection from necrosis were not impaired in male WT mice receiving male ARKO bone marrow cells, nor were these parameters improved when male ARKO mice were transplanted with male WT bone marrow. Thus, while sex hormones can alter the mobilization of cell populations from bone marrow, the transplantation studies conducted in the current study show that AR-deficient bone marrow progenitor cell have little or no impact on the limbs subjected to ischemia.
AR-deficiency led to molecular changes associated with diminished cell viability in the ischemic limb, including a reduction in the Bcl-2-to-Bax ratio and inactivation of ERK1/2 and Akt signaling pathways. The proapoptotic protein BAX plays a critical role in the intrinsic apoptotic pathway, and Bcl-2 forms heterodimers with BAX and prevents its insertion into the mitochondrial membrane. Therefore, the Bcl-2-to-BAX ratio is critical for determination of the apoptotic threshold 45. Lin et al. reported that AR is able to counteract BAX-mediated apoptosis in the prostate gland in an androgen-dependent and independent manner 46. In addition, our previous study showed that the Bcl-2-to-BAX ratio after treatment with doxorubicin, a cytotoxic agent, was lower in male ARKO mice than in male WT mice 23. These results are consistent with the assumption that AR-mediated signaling protects against cellular apoptosis.
The ERK1/2 signaling pathway promotes cell survival by a dual mechanism: inactivation of components of the cell death machinery and enhancement of transcription of pro-survival genes 47. Additionally, Akt has been shown to be a cytoplasmic serine/threonine kinase that contributes to the control of apoptosis machinery and cellular metabolism 48. We previously reported that angiotensin II-induced activation of ERK1/2 is abrogated in aortic tissues and cardiac myocytes of male ARKO mice, leading to aberrant vascular remodeling and left ventricular systolic dysfunction with extended cardiac fibrosis 21, 22. Moreover, we have reported that oxidative stress and apoptosis of cardiomyocytes were enhanced in male ARKO mice by doxorubicin treatment with reduced cardiac mitochondrial transcription factor A expression and Akt phosphorylation 23. Consistent with these observations, the present study demonstrated that AR deficiency accelerates cellular apoptosis through reduced Bcl-2-to-BAX ratio and impaired phosphorylation of ERK1/2 and Akt under the ischemic stress regardless of sex.
The PI3K/Akt pathway is activated by VEGF and is capable of modulating cell survival, migration, tube formation, and nitric oxide (NO) release 49. In fact, studies using mice with Akt 1 gene deletion demonstrated that Akt 1 is essential for promoting ischemic and VEGF-initiated postnatal angiogenesis and vascular maturation 50, 51. It is well known that Akt activates eNOS and enhances NO production 32 and that release of NO from vascular endothelial cells is essential for promoting angiogenesis and collateral vessel remodeling in response to ischemia 28. Therefore, it is reasonable to assume that the reduced Akt-eNOS phosphorylation in ischemic skeletal muscle of ARKO mice is closely associated with not only cellular apoptosis but also suppressed angiogenesis, with disturbed vascular maturation and collateral vessel remodeling. With regard to the relationship between Akt-eNOS signaling and AR signaling pathways, we have previously demonstrated that AR deficiency causes a reduction in eNOS expression and phosphorylation, leading to attenuation of NO bioavailability and acceleration of vascular remodeling under the condition of angiotensin II excess 22. These observations led us to conclude that AR is required for promoting angiogenesis partly through activation of the PI3K/Akt-eNOS signaling pathway in response to ischemic stress.
In the present study, the revascularization impairment in both male and female ARKO mice was associated with a rapid elevation in the levels of angiogenic growth factors (Vegfa and Fgf2), as well as an increase in Hif1a expression. The mechanism whereby the expression of Vegfa and Hif1a genes was rapidly enhanced after ischemia is not clear. However, the observation that ischemic lims from ARKO mice exhibited marked edema and a dusky red-colored skin appearance on day 1 after surgery are consistent with exaggerated ischemia in the operated limbs of ARKO mice. Since previous studies showed that castration rapidly reduces blood flow to the rat ventral prostate gland with acceleration of epithelial cellular apoptosis and tissue hypoxia 53, 54 and since we have reported that AR deficiency reduces nitric oxide bioavailability 22, lack of androgen action may lead to prominent tissue hypoxia due to circulatory insufficiency. Although further investigations are needed to clarify this issue, we speculate that the enhanced Hif1a and Vegfa expression levels reflect a more severe acute ischemic condition in ARKO mice than in WT mice. Interestingly, the enhanced gene expression levels of Kdr as compensatory response against ischemic stress was attenuated in male ARKO mice but not in female ARKO mice. Activation of the VEGF-VEGF receptor axis is a critical step for the initiation and development of angiogenesis. VEGF primarily utilizes its receptor KDR, also known as VEGFR-2 or FLK-1, to induce angiogenic responses mediated by subsequent activation of signaling cascades such as PIK3-Akt and MAPK 52. Given the critical role of KDR signaling in angiogenesis, regulation of KDR is pivotal for the regulation of angiogenesis. Sieveking et al. reported that DHT induced dose-dependent increases in the mRNA expression of KDR in male human vascular endothelial cells 30. Their results as well as our present observations support the notion that AR activation is required for compensatory up-regulation of Kdr gene expression in response to ischemia at least in males. Although the relationship between sex hormones, AR and Kdr gene expression in female mice have remained unclear, the role of AR in female ischemic muscle may be different from that in male ischemic muscle in terms of Kdr expression. Sieveking et al. reported that androgens up-regulate expression levels of Hif1a and VEGF; however, we found that in vivo Hif1a and Vegfa expression levels are augmented more in ARKO mice than in WT mice after ischemia in both males and females. While their experiments were performed using cultured endothelial cells in vitro, we determined gene expression levels of Hif1a and Vegfa in vivo in ischemic tissues including skeletal muscle, vasculature, bone, lymphatic tissue and nerves. Because skeletal muscle is an abundant source of VEGF, the discrepancy between their results and ours may be due to differences in experimental conditions in the two studies.
In the present study, we also found that VEGF-stimulated Akt and eNOS phosphorylation was blunted by AR deficiency and that AR associated with KDR through recruitment of signaling molecules including PI3Kp85 and Src. These results suggest a close interplay between AR and KDR. Further studies will be required to document whether the interaction between AR and KDR is direct, or whether other proteins are required for the formation of this complex. In this regard, Sun et al. showed that a ternary complex formsamong AR, PI3Kp85 and Src that is essential for androgen-stimulated activation of PI3K/Akt and MAPK 35. Therefore, there is a possibility that AR and KDR share these same signaling molecules and that AR activation and association with KDR play an important role in full activation of the KDR signaling pathway in response to ischemia. The present observations provide evidence for a novel cross-talk mechanism between androgen/AR signaling and VEGF/KDR signaling pathways as illustrated in Figure 8 and suggest a therapeutic potential of androgen for ischemic diseases.
Figure 8. Schematic diagram of associations between AR and VEGF receptor signaling pathways.
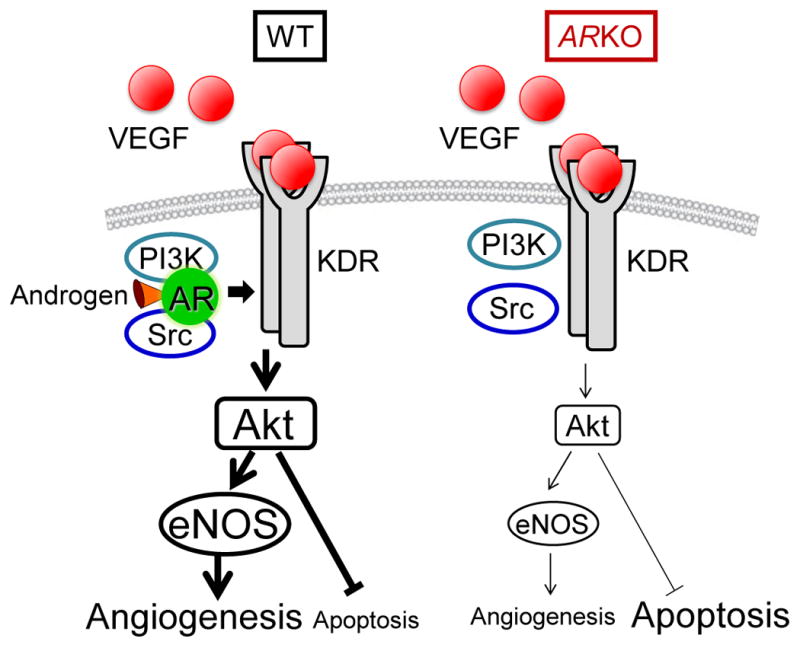
VEGF rapidly induces KDR dimerization and autophosphorylation, followed by the recruitment and activation of Src and PI3K. On the other hand, AR activates the Akt/eNOS cascade depending on association with PI3K and Src. Thus, association between AR and KDR promotes cell survival and angiogenesis via recruitment of Src and PI3K.
Supplementary Material
Clinical Perspectives.
In addition to menopause, andropause has been well recognized as a risk factor for cardiovascular diseases (CVDs). Therefore, gender-specific clinical and experimental data are important in delineating gender-dependent differences in the incidence of and vulnerability to CVDs. Here, we show that androgen receptor (AR) activation plays an important role in angiogenesis after ischemia regardless of genders. This study identifies the function of the AR in the compensatory response to ischemia in vivo, and defines that its mechanism of action involves the association of AR with a VEGF receptor and activation of pro-angiogenic downstream pathways. Although beneficial anabolic effects of androgens on various tissues including bone, skeletal muscles, and central nerves system are well documented, clinical use of androgens has been limited because of their undesirable side effects. These side effects include the increased risk of prostate cancer in men, and hirsutism in women. Recently, selective androgen receptor modulators (SARMs) have been under clinical development, and trials are under way in patients with sarcopenia and cachexia, conditions that are closely associated with cardiovascular complications. Based upon the present study, we speculate that the appropriately targeted regulation of AR function in ischemic tissues may lead to novel therapeutic approaches for the treatment of CVDs in both sexes.
Acknowledgments
We wish to thank Ayako Hashimoto for her technical help.
Grant support: This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant from the Mitsui Life Social Welfare Foundation and a Grant for a Study Group on Aseptic Femoral Neck Necrosis from the Ministry of Health, Labour and Welfare of Japan.
Abbreviations
- Akt
Serine/threonine protein kinase
- AR
androgen receptor
- ARKO
AR knockout
- BM
bone marrow
- CVD
cardiovascular disease
- DHT
dihydrotestosterone
- eNOS
endothelial nitric oxide synthase
- ERK1/2
extracellular signal-regulated kinase1/2
- FGF2
fibroblast growth factor2
- HIF1α
hypoxia-inducible factor 1 α
- KDR
kinase insert domain protein receptor
- LSBF
laser speckle blood flow
- MAPK
mitogen-activated protein kinase
- PAD
peripheral arterial disease
- PI3K
phosphoinositide-3-kinase
- PLA
proximity ligation assay
- VEGF
vascular endothelial cell growth factor
Footnotes
Disclosure summary: The authors have nothing to disclose.
References
- 1.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 2.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (hers) research group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: A review. Atherosclerosis. 1996;125:1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78:539–545. doi: 10.1161/01.cir.78.3.539. [DOI] [PubMed] [Google Scholar]
- 6.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: The rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: Results from the third national health and nutrition examination survey (nhanes iii) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 9.McGrath KC, McRobb LS, Heather AK. Androgen therapy and atherosclerotic cardiovascular disease. Vasc Health Risk Manag. 2008;4:11–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida S, Aihara K, Azuma H, Uemoto R, Sumitomo-Ueda Y, Yagi S, Ikeda Y, Iwase T, Nishio S, Kawano H, Miki J, Yamada H, Hirata Y, Akaike M, Sata M, Matsumoto T. Dehydroepiandrosterone sulfate is inversely associated with sex-dependent diverse carotid atherosclerosis regardless of endothelial function. Atherosclerosis. 2010;212:310–315. doi: 10.1016/j.atherosclerosis.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–2079. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- 12.Contoreggi CS, Blackman MR, Andres R, Muller DC, Lakatta EG, Fleg JL, Harman SM. Plasma levels of estradiol, testosterone, and dheas do not predict risk of coronary artery disease in men. J Androl. 1990;11:460–470. [PubMed] [Google Scholar]
- 13.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-oh kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 17.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 18.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M, Yagi S, Tamaki T, Kawano H, Matsumoto T, Azuma H, Kato S. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin ii-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda Y, Aihara K, Yoshida S, Sato T, Yagi S, Iwase T, Sumitomo Y, Ise T, Ishikawa K, Azuma H, Akaike M, Kato S, Matsumoto T. Androgen-androgen receptor system protects against angiotensin ii-induced vascular remodeling. Endocrinology. 2009;150:2857–2864. doi: 10.1210/en.2008-1254. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda Y, Aihara K, Akaike M, Sato T, Ishikawa K, Ise T, Yagi S, Iwase T, Ueda Y, Yoshida S, Azuma H, Walsh K, Tamaki T, Kato S, Matsumoto T. Androgen receptor counteracts doxorubicin-induced cardiotoxicity in male mice. Mol Endocrinol. 2010;24:1338–1348. doi: 10.1210/me.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tivesten A, Mellstrom D, Jutberger H, Fagerberg B, Lernfelt B, Orwoll E, Karlsson MK, Ljunggren O, Ohlsson C. Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men. The mros study in sweden. J Am Coll Cardiol. 2007;50:1070–1076. doi: 10.1016/j.jacc.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (ar ko) mice. Biochem Biophys Res Commun. 2003;300:167–171. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- 26.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 28.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed MJ, Karres N, Eyman D, Edelberg J. Endothelial precursor cells. Stem Cell Rev. 2007;3:218–225. doi: 10.1007/s12015-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 30.Sieveking DP, Lim P, Chow RW, Dunn LL, Bao S, McGrath KC, Heather AK, Handelsman DJ, Celermajer DS, Ng MK. A sex-specific role for androgens in angiogenesis. J Exp Med. 2010;207:345–352. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlessinger J. New roles for src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 34.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 35.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/akt pathway by androgen through interaction of p85alpha, androgen receptor, and src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 36.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. Halotag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 37.Soderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- 39.Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Sjogren M, Alkemade A, Mittag J, Nordstrom K, Katz A, Rozell B, Westerblad H, Arner A, Vennstrom B. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor alpha1. The EMBO journal. 2007;26:4535–4545. doi: 10.1038/sj.emboj.7601882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merot Y, Ferriere F, Gailhouste L, Huet G, Percevault F, Saligaut C, Flouriot G. Different outcomes of unliganded and liganded estrogen receptor-alpha on neurite outgrowth in pc12 cells. Endocrinology. 2009;150:200–211. doi: 10.1210/en.2008-0449. [DOI] [PubMed] [Google Scholar]
- 42.Alimirah F, Vaishnav A, McCormick M, Echchgadda I, Chatterjee B, Mehta RG, Peng X. Functionality of unliganded vdr in breast cancer cells: Repressive action on cyp24 basal transcription. Molecular and cellular biochemistry. 2010;342:143–150. doi: 10.1007/s11010-010-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng ZY, Lin VC. Anti-estrogenic effect of unliganded progesterone receptor is estrogen-selective in breast cancer cells mcf-7. Cancer letters. 2008;268:202–211. doi: 10.1016/j.canlet.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 44.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 45.Yin XM, Oltvai ZN, Korsmeyer SJ. Bh1 and bh2 domains of bcl-2 are required for inhibition of apoptosis and heterodimerization with bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 46.Lin Y, Kokontis J, Tang F, Godfrey B, Liao S, Lin A, Chen Y, Xiang J. Androgen and its receptor promote bax-mediated apoptosis. Mol Cell Biol. 2006;26:1908–1916. doi: 10.1128/MCB.26.5.1908-1916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolch W. Coordinating erk/mapk signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 48.Amaravadi R, Thompson CB. The survival kinases akt and pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiojima I, Walsh K. Role of akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 50.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase balpha is critical for ischemic and vegf-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. Vegf receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 53.Shabisgh A, Tanji N, D'Agati V, Burchardt M, Rubin M, Goluboff ET, Heitjan D, Kiss A, Buttyan R. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology. 1999;140:1920–1926. doi: 10.1210/endo.140.4.6644. [DOI] [PubMed] [Google Scholar]
- 54.Rudolfsson SH, Bergh A. Testosterone-stimulated growth of the rat prostate may be driven by tissue hypoxia and hypoxia-inducible factor-1alpha. The Journal of endocrinology. 2008;196:11–19. doi: 10.1677/JOE-07-0272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


