Abstract
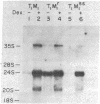
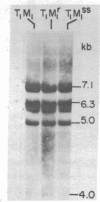
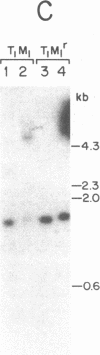
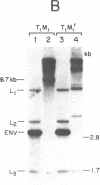
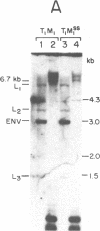
Murine mammary tumor virus (MMTV) expression is analyzed in a T-lymphoid cell line (T1M1) sensitive to the killing effect of glucocorticoids and in two of its variants, one resistant (T1M1r) and one supersensitive (T1M1ss) to glucocorticoid-induced lymphocytolysis. In the T1M1 line, MMTV is expressed and induced approximately 10-fold by short treatment with dexamethasone. Southern blot analyses of restriction enzyme digests of DNA from T1M1 cells reveal three proviruses similar to those of normal C57BL mouse tissue. In the T1M1ss line, which has retained functional glucocorticoid receptors, MMTV mRNA is inducible by glucocorticoids, while induction is reduced in the T1M1r line defective in glucocorticoid receptors. Moreover, the T1M1r line expresses a strikingly elevated basal level of MMTV mRNA in the absence of hormone. No rearrangements or superinfection have occurred in the variants, but all the regions containing 5'-long terminal repeats are demethylated in the T1M1r variant although other sites of the provirus remain methylated. Because this variant was selected by prolonged treatment with dexamethasone, these observations raise the possibility that the continuous transcription of MMTV that occurred during this selection can result in glucocorticoid-induced demethylation of long-terminal-repeat sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Crepin M. In vitro mouse mammary tumor virus transcription from chromatin. A system to study the mechanism of action of glucocorticoid hormones. FEBS Lett. 1977 Dec 15;84(2):266–270. doi: 10.1016/0014-5793(77)80703-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Dieckmann B. S., Schroer T. A., Ringold G. M. Isolation of glucocorticoid-unresponsive rat hepatoma cells by fluorescence-activated cell sorting. Cell. 1980 Aug;21(1):47–56. doi: 10.1016/0092-8674(80)90113-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Mermod J. J., Intrière L., MacLeod C. L., Bourgeois S. Characterization of a new type of thymoma variants supersensitive to dexamethasone. J Steroid Biochem. 1981 Dec;15:25–34. doi: 10.1016/0022-4731(81)90254-5. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Pfahl M., Kelleher R. J., Jr, Bourgeois S. General features of steroid resistance on lymphoid cell lines. Mol Cell Endocrinol. 1978 Apr;10(2):193–207. doi: 10.1016/0303-7207(78)90125-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F., Cozens P. J., Mattaj I. W., Jost J. P. Estrogen induces a demethylation at the 5' end region of the chicken vitellogenin gene. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4252–4255. doi: 10.1073/pnas.79.14.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Studies of X chromosome DNA methylation in normal human cells. Nature. 1982 Feb 25;295(5851):667–671. doi: 10.1038/295667a0. [DOI] [PubMed] [Google Scholar]