Abstract
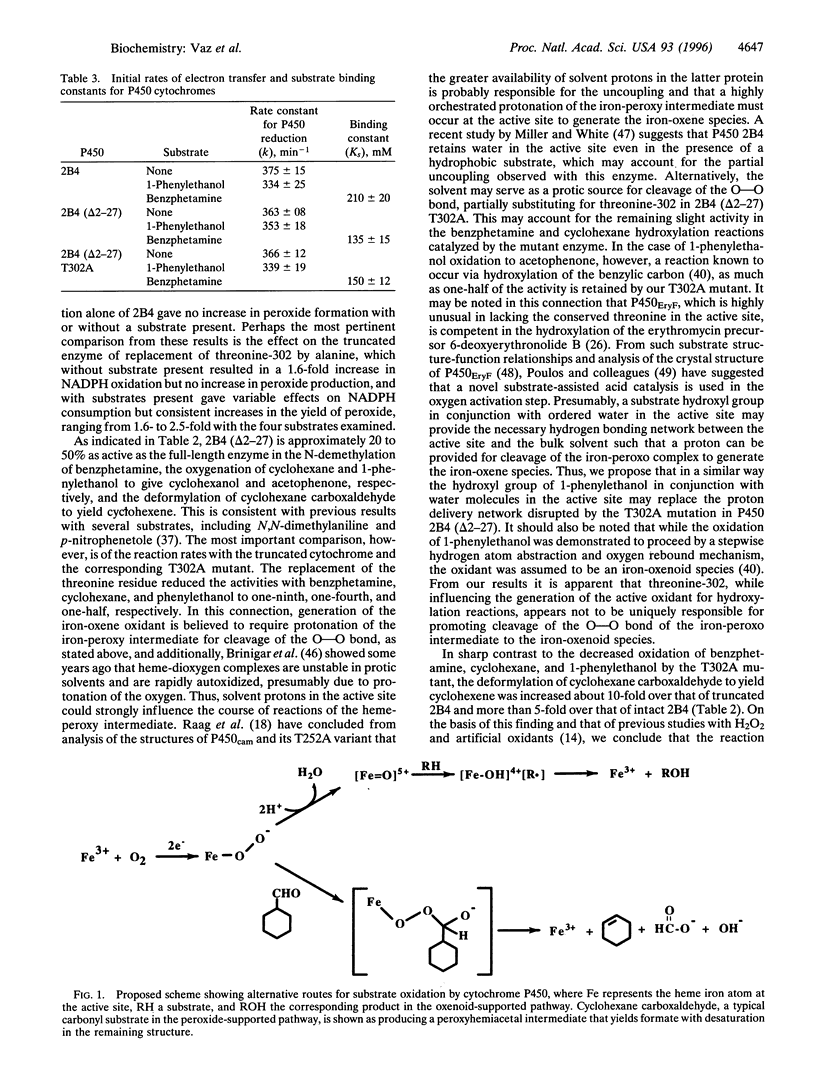
Among biological catalysts, cytochrome P450 is unmatched in its multiplicity of isoforms, inducers, substrates, and types of chemical reactions catalyzed. In the present study, evidence is given that this versatility extends to the nature of the active oxidant. Although mechanistic evidence from several laboratories points to a hypervalent iron-oxenoid species in P450-catalyzed oxygenation reactions, Akhtar and colleagues [Akhtar, M., Calder, M. R., Corina, D. L. & Wright, J. N. (1982) Biochem. J. 201, 569-580] proposed that in steroid deformylation effected by P450 aromatase an iron-peroxo species is involved. We have shown more recently that purified liver microsomal P450 cytochromes, including phenobarbital-induced P450 2B4, catalyze the analogous deformylation of a series of xenobiotic aldehydes with olefin formation. The investigation presented here on the effect of site-directed mutagenesis of threonine-302 to alanine on the activities of recombinant P450 2B4 with N-terminal amino acids 2-27 deleted [2B4 (delta2-27)] makes use of evidence from other laboratories that the corresponding mutation in bacterial P450s interferes with the activation of dioxygen to the oxenoid species by blocking proton delivery to the active site. The rates of NADPH oxidation, hydrogen peroxide production, and product formation from four substrates, including formaldehyde from benzphetamine N-demethylation, acetophenone from 1-phenylethanol oxidation, cyclohexanol from cyclohexane hydroxylation, and cyclohexene from cyclohexane carboxaldehyde deformylation, were determined with P450s 2B4, 2B4 (delta2-27), and 2B4 (delta2-27) T302A. Replacement of the threonine residue in the truncated cytochrome gave a 1.6- to 2.5-fold increase in peroxide formation in the presence of a substrate, but resulted in decreased product formation from benzphetamine (9-fold), cyclohexane (4-fold), and 1-phenylethanol (2-fold). In sharp contrast, the deformylation of cyclohexane carboxaldehyde by the T302A mutant was increased about 10-fold. On the basis of these findings and our previous evidence that aldehyde deformylation is supported by added H202, but not by artificial oxidants, we conclude that the iron-peroxy species is the direct oxygen donor. It remains to be established which of the many other oxidative reactions involving P450 utilize this species and the extent to which peroxo-iron and oxenoid-iron function as alternative oxygenating agents with the numerous isoforms of this versatile catalyst.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Calder M. R., Corina D. L., Wright J. N. Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem J. 1982 Mar 1;201(3):569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. F., Tatsuta K., Gunji H., Ishiyama T., Hutchinson C. R. Substrate specificity of 6-deoxyerythronolide B hydroxylase, a bacterial cytochrome P450 of erythromycin A biosynthesis. Biochemistry. 1993 Mar 2;32(8):1905–1913. doi: 10.1021/bi00059a004. [DOI] [PubMed] [Google Scholar]
- Brinigar W. S., Chang C. K., Geibel J., Traylor T. G. Solvent effects on reversible formation and oxidative stability of heme-oxygen complexes. J Am Chem Soc. 1974 Aug 21;96(17):5597–5599. doi: 10.1021/ja00824a061. [DOI] [PubMed] [Google Scholar]
- Cole P. A., Robinson C. H. Mechanism and inhibition of cytochrome P-450 aromatase. J Med Chem. 1990 Nov;33(11):2933–2942. doi: 10.1021/jm00173a001. [DOI] [PubMed] [Google Scholar]
- Coon M. J., van der Hoeven T. A., Dahl S. B., Haugen D. A. Two forms of liver microsomal cytochrome P-450, P-450lm2 and P-450LM4 (rabbit liver). Methods Enzymol. 1978;52:109–117. doi: 10.1016/s0076-6879(78)52012-0. [DOI] [PubMed] [Google Scholar]
- Cupp-Vickery J. R., Poulos T. L. Structure of cytochrome P450eryF involved in erythromycin biosynthesis. Nat Struct Biol. 1995 Feb;2(2):144–153. doi: 10.1038/nsb0295-144. [DOI] [PubMed] [Google Scholar]
- Fischer R. T., Trzaskos J. M., Magolda R. L., Ko S. S., Brosz C. S., Larsen B. Lanosterol 14 alpha-methyl demethylase. Isolation and characterization of the third metabolically generated oxidative demethylation intermediate. J Biol Chem. 1991 Apr 5;266(10):6124–6132. [PubMed] [Google Scholar]
- French J. S., Coon M. J. Properties of NADPH-cytochrome P-450 reductase purified from rabbit liver microsomes. Arch Biochem Biophys. 1979 Jul;195(2):565–577. doi: 10.1016/0003-9861(79)90383-7. [DOI] [PubMed] [Google Scholar]
- French J. S., Guengerich F. P., Coon M. J. Interactions of cytochrome P-450, NADPH-cytochrome P-450 reductase, phospholipid, and substrate in the reconstituted liver microsomal enzyme system. J Biol Chem. 1980 May 10;255(9):4112–4119. [PubMed] [Google Scholar]
- Furuya H., Shimizu T., Hirano K., Hatano M., Fujii-Kuriyama Y., Raag R., Poulos T. L. Site-directed mutageneses of rat liver cytochrome P-450d: catalytic activities toward benzphetamine and 7-ethoxycoumarin. Biochemistry. 1989 Aug 22;28(17):6848–6857. doi: 10.1021/bi00443a011. [DOI] [PubMed] [Google Scholar]
- Gasser R., Negishi M., Philpot R. M. Primary structures of multiple forms of cytochrome P-450 isozyme 2 derived from rabbit pulmonary and hepatic cDNAs. Mol Pharmacol. 1988 Jan;33(1):22–30. [PubMed] [Google Scholar]
- Groves J. T. Biological strategies for the manipulation of dioxygen. The chemistry of cytochrome P-450. Ann N Y Acad Sci. 1986;471:99–107. doi: 10.1111/j.1749-6632.1986.tb48029.x. [DOI] [PubMed] [Google Scholar]
- Groves J. T., McClusky G. A. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. Evidence for a carbon radical intermediate. Biochem Biophys Res Commun. 1978 Mar 15;81(1):154–160. doi: 10.1016/0006-291x(78)91643-1. [DOI] [PubMed] [Google Scholar]
- Hasemann C. A., Kurumbail R. G., Boddupalli S. S., Peterson J. A., Deisenhofer J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure. 1995 Jan 15;3(1):41–62. doi: 10.1016/s0969-2126(01)00134-4. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G., Roots I., Tjoe M., Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- Hiroya K., Murakami Y., Shimizu T., Hatano M., Ortiz de Montellano P. R. Differential roles of Glu318 and Thr319 in cytochrome P450 1A2 catalysis supported by NADPH-cytochrome P450 reductase and tert-butyl hydroperoxide. Arch Biochem Biophys. 1994 May 1;310(2):397–401. doi: 10.1006/abbi.1994.1184. [DOI] [PubMed] [Google Scholar]
- Imai M., Shimada H., Watanabe Y., Matsushima-Hibiya Y., Makino R., Koga H., Horiuchi T., Ishimura Y. Uncoupling of the cytochrome P-450cam monooxygenase reaction by a single mutation, threonine-252 to alanine or valine: possible role of the hydroxy amino acid in oxygen activation. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7823–7827. doi: 10.1073/pnas.86.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase). Biochem Biophys Res Commun. 1989 Feb 15;158(3):717–722. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- Imai Y., Nakamura M. The importance of threonine-301 from cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase) in substrate binding as demonstrated by site-directed mutagenesis. FEBS Lett. 1988 Jul 18;234(2):313–315. doi: 10.1016/0014-5793(88)80106-6. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Shimada H., Hirose T., Ishimura Y. Role of Thr-252 in cytochrome P450cam: a study with unnatural amino acid mutagenesis. Biochem Biophys Res Commun. 1995 Mar 8;208(1):96–102. doi: 10.1006/bbrc.1995.1310. [DOI] [PubMed] [Google Scholar]
- Larson J. R., Coon M. J., Porter T. D. Alcohol-inducible cytochrome P-450IIE1 lacking the hydrophobic NH2-terminal segment retains catalytic activity and is membrane-bound when expressed in Escherichia coli. J Biol Chem. 1991 Apr 25;266(12):7321–7324. [PubMed] [Google Scholar]
- Larson J. R., Coon M. J., Porter T. D. Purification and properties of a shortened form of cytochrome P-450 2E1: deletion of the NH2-terminal membrane-insertion signal peptide does not alter the catalytic activities. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9141–9145. doi: 10.1073/pnas.88.20.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., White R. E. Photoaffinity labeling of cytochrome P450 2B4: capture of active site heme ligands by a photocarbene. Biochemistry. 1994 Jan 25;33(3):807–817. doi: 10.1021/bi00169a023. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Strobel H. W. On the membrane topology of vertebrate cytochrome P-450 proteins. J Biol Chem. 1988 May 5;263(13):6038–6050. [PubMed] [Google Scholar]
- Nelson D. R., Strobel H. W. Secondary structure prediction of 52 membrane-bound cytochromes P450 shows a strong structural similarity to P450cam. Biochemistry. 1989 Jan 24;28(2):656–660. doi: 10.1021/bi00428a036. [DOI] [PubMed] [Google Scholar]
- Oprian D. D., Vatsis K. P., Coon M. J. Kinetics of reduction of cytochrome P-450LM4 in a reconstituted liver microsomal enzyme system. J Biol Chem. 1979 Sep 25;254(18):8895–8902. [PubMed] [Google Scholar]
- Pernecky S. J., Olken N. M., Bestervelt L. L., Coon M. J. Subcellular localization, aggregation state, and catalytic activity of microsomal P450 cytochromes modified in the NH2-terminal region and expressed in Escherichia coli. Arch Biochem Biophys. 1995 Apr 20;318(2):446–456. doi: 10.1006/abbi.1995.1253. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Howard A. J. High-resolution crystal structure of cytochrome P450cam. J Mol Biol. 1987 Jun 5;195(3):687–700. doi: 10.1016/0022-2836(87)90190-2. [DOI] [PubMed] [Google Scholar]
- Pufahl R. A., Wishnok J. S., Marletta M. A. Hydrogen peroxide-supported oxidation of NG-hydroxy-L-arginine by nitric oxide synthase. Biochemistry. 1995 Feb 14;34(6):1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- Raag R., Martinis S. A., Sligar S. G., Poulos T. L. Crystal structure of the cytochrome P-450CAM active site mutant Thr252Ala. Biochemistry. 1991 Dec 3;30(48):11420–11429. doi: 10.1021/bi00112a008. [DOI] [PubMed] [Google Scholar]
- Roberts E. S., Vaz A. D., Coon M. J. Catalysis by cytochrome P-450 of an oxidative reaction in xenobiotic aldehyde metabolism: deformylation with olefin formation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8963–8966. doi: 10.1073/pnas.88.20.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney D. C., Mak A. Y. Androgen formation by cytochrome P450 CYP17. Solvent isotope effect and pL studies suggest a role for protons in the regulation of oxene versus peroxide chemistry. Biochemistry. 1994 Mar 1;33(8):2185–2190. doi: 10.1021/bi00174a027. [DOI] [PubMed] [Google Scholar]
- Vaz A. D., Coon M. J. On the mechanism of action of cytochrome P450: evaluation of hydrogen abstraction in oxygen-dependent alcohol oxidation. Biochemistry. 1994 May 31;33(21):6442–6449. doi: 10.1021/bi00187a008. [DOI] [PubMed] [Google Scholar]
- Vaz A. D., Kessell K. J., Coon M. J. Aromatization of a bicyclic steroid analog, 3-oxodecalin-4-ene-10-carboxaldehyde, by liver microsomal cytochrome P450 2B4. Biochemistry. 1994 Nov 22;33(46):13651–13661. doi: 10.1021/bi00250a015. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Yeom H., Sligar S. G., Li H., Poulos T. L., Fulco A. J. The role of Thr268 in oxygen activation of cytochrome P450BM-3. Biochemistry. 1995 Nov 14;34(45):14733–14740. doi: 10.1021/bi00045a014. [DOI] [PubMed] [Google Scholar]



