Abstract
Invadopodia are specialized structures of cancer cells which aid in cancer cell invasion and metastasis. Therefore, studying the early steps of invadopodium assembly and its life cycle at the subcellular level by using high spatiotemporal resolution imaging provides an opportunity for understanding the signaling mechanisms involved in this very important process. In this chapter, we describe the design of a custom-built high-resolution fluorescence microscope which makes this challenging imaging possible. We also describe an ImageJ plugin that we have developed for tracking of invadopodia and lifetime analysis.
Keywords: Live-cell imaging, Time-lapse microscopy, Autofocus, Invadopodia, Breast carcinoma cells, Gelatin degradation, Particle tracking
1 Introduction
Metastasis is the leading cause of death in most cancer patients, as opposed to the growth of the primary tumor, which can be managed clinically to a large extent by surgery and chemotherapy. Invadopodia are specialized protrusive structures found in cancer cells, with a diameter around 0.5–1 µm and length of a couple of microns [1]. These structures are proteolytically active and degrade extracellular matrix (ECM) which aids in cancer cell invasion into the surrounding tissue and blood vessels leading to metastasis [2–4]. Since their first discovery in src-transformed fibroblasts [5], invadopodia have been found in many types of cancer cells including: breast [3, 6–8], melanoma [9, 10], head and neck [11], glioma [12], colon [13], pancreatic [14], and prostate [15].
By utilizing a 2D model in which cancer cells are plated on ECM coated surfaces, the mechanism of invadopodium formation and regulation has been studied in great detail. Although many invadopodium-associated proteins (e.g., cortactin, N-WASp, Tks5, Cofilin, Nck1, p190RhoGAP [7, 16–18]) have been identified, the molecular interaction(s) during invadopodium initiation is not well understood. We were interested in imaging various invadopodium proteins during invadopodium precursor assembly at high spatial and temporal resolution (on the order of seconds). To achieve these challenging imaging requirements, we have developed a robust custom-built wide-field fluorescence microscope. In this chapter, we provide technical details on the microscope design and data acquisition. To analyze the arrival of different invadopodium proteins, invadopodia lifetimes and trajectories, we have also developed an ImageJ plugin called “Invadopodia tracker.” This plugin tracks multiple invadopodia in a cell throughout the whole time-lapse sequence. A step-by-step invadopodia tracking analysis procedure is also described.
2 Materials
2.1 Microscope Design
Excitation, emission, and ND filters (see Table 1).
Mercury arc lamp and controller (Chiu Technical Corporation, Mercury-100 W).
Dual filter wheel with six positions each for Excitation and ND filters (Ludl Electronics, Dual Filter Wheel).
8-position filter wheel for emission filters (Applied Scientific Instrumentation, FW1000).
Continuous Reflective-Interface Feedback Focus unit (Applied Scientific Instrumentation, CRIFF).
Z-focus Stepper Motor (Applied Scientific Instrumentation, MFC2000Z Axis Drive).
Fast piezo controlled Z-focus drive (Physik Instrumente, PIFoc).
Linear encoded XY Stage (Applied Scientific Instrumentation, MFC2000).
Glass reflector, 6 % reflection/94 % transmission (Chroma, 6/94bs).
60× 1.42 NA oil, Plan Apo N objective (Olympus, part# 1-U2B933).
Two channel image splitter (Optical Insights, Dual-View).
High Performance 12 bit CCD camera (Cooke, SensiCamQE).
Table 1.
List of excitation, emission, ND, Turret, and Dual-View filters
| Location | Use | Filter | Vendor |
|---|---|---|---|
| Filter Turret Pos. A | Dichroic | FF01-444/520/590 | Semrock |
| Emission | FF01-465/537/623 | Semrock | |
| Filter Turret Pos. B | Dichroic | FF493/574-Di01 | Semrock |
| Emission | FF01-512/630-25 | Semrock | |
| Filter Turret Pos. C | Dichroic | FF660-Di01 | Semrock |
| Emission | FF01-685/40-25 | Semrock | |
| Filter Turret Pos. D | 6 % Reflector | 6/94bs | Chroma |
| Excitation Wheel Pos. 1 | CFP Excitation | FF01-434/17-25 | Semrock |
| Excitation Wheel Pos. 2 | GFP Excitation | FF01-472/30-25 | Semrock |
| Excitation Wheel Pos. 3 | YFP Excitation | FF01-500/24-25 | Semrock |
| Excitation Wheel Pos. 4 | RFP Excitation | FF01-550/32-25 | Semrock |
| Excitation Wheel Pos. 5 | RFP Excitation | FF01-575/25-25 | Semrock |
| Excitation Wheel Pos. 6 | Cy5 Excitation | FF01-617/73-25 | Semrock |
| Emission Wheel Pos. 1 | Dual View Emission | Open | - |
| Emission Wheel Pos. 2 | CFP Emission | FF01-475/50-25 | Semrock |
| Emission Wheel Pos. 3 | GFP Emission | FF01-514/30-25 | Semrock |
| Emission Wheel Pos. 4 | YFP Emission | FF01-519-LP | Semrock |
| Emission Wheel Pos. 5 | RFP Emission | FF01-607/36-25 | Semrock |
| Emission Wheel Pos. 6 | Cy5 Emission | FF01-685/40-25 | Semrock |
| CFP-YFP DualView Cube | Dichroic | t505lpxr | Chroma |
| Short Pass | FF01-475/50-25 | Semrock | |
| Long Pass | FF01-500LP-25 | Semrock | |
| GFP-RFP DualView Cube | Dichroic | t540lpxr | Chroma |
| Short Pass | FF01-514/30-25 | Semrock | |
| Long Pass | BLP01-532R-25 | Semrock | |
| ND Filter Wheel | 0.1, 0.2, 0.3, 0.5, 0.6, 1.0, 2.0 | nd filter set | Chroma |
2.2 Labeling Gelatin with Alexa 405 Dye
Bio-Gel P-30 Gel (Bio-Rad cat#150-4154).
PBS with 2 mM sodium azide: Add 65 mg sodium azide in 500 mL PBS. Use this PBS for all the steps described in Subheadings 2.2 and 3.2.
Glass column (Bio-Rad, cat# 737-1021).
Alexa Fluor 405 dye (Life Technologies, cat#A30000).
0.2 % gelatin solution: Add 50 mg gelatin from porcine skin (Sigma-Aldrich, cat# G2500) into 25 mL PBS. Vortex briefly to mix and leave in the 37 °C water bath for ½–1 h. During the incubation period, take the tube out two times and vortex briefly.
1 M sodium bicarbonate solution: 840.1 mg sodium bicarbonate powder in 10 mL ddH2O; prepare fresh every time.
DMSO.
UV lamp.
2.3 Preparation of Gelatin-Coated MatTek Dishes
MatTek dish (MatTek corporation, cat# P35G-1.5-14-C).
1 N HCl.
2 mg/mL Poly-l-lysine solution: Add 12.5 mL ddH2O to 25 mg poly-l-lysine powder (Sigma-Aldrich, cat# P1399). The solution can be stored at 4 °C for up to 1 year.
Prepare 0.2 % gelatin solution as described in Subheading 2.2, item 5.
0.2 % glutaraldehyde solution: Add 160 µL of 25 % glutaraldehyde stock (Sigma-Aldrich, cat#G5882) into 20 mL PBS, leave on ice.
Sodium borohydride (Sigma-Aldrich, cat#452882).
70 % ethanol solution.
10× penicillin/streptomycin solution: Add 2 mL, 100× penicillin/treptomycin stock (Life Technologies, cat# 15140-122) into 18 mL PBS.
2.4 Transfection of MTLn3 Cells and Preparing Cells for Live Imaging
MTLn3 cells [19].
Culture medium: MEM Alpha (Life Technologies, cat#12561-056), 5 % fetal bovine serum (FBS) (Gemini Bio-products, cat#100106) and 1× penicillin/streptomycin.
0.05 % trypsin–EDTA (Life Technologies).
Lipofectamine 2000 (Life Technologies).
Opti-MEM medium (Life Technologies).
DNA constructs (e.g., TagRFP-cortactin and GFP-Cofilin [17, 20]).
Serum starvation medium: Dissolve 0.345 g bovine serum albumin (BSA; Fisher Scientific, cat# BP1600-100) in 5 mL ddH2O. Syringe filter through a 0.2 µm size filter. Add 2.5 mL of this solution into 47.5 mL L-15 medium (Life Technologies, cat# 21083). Place at 37 °C before use.
Steady state imaging medium: L-15 + 5 % FBS.
2.5 Live Imaging of Invadopodia
10 nM EGF: Dilute 5 µL EGF stock (50 µM, Life Technologies, cat# 53003-018) into 245 µL serum starvation medium. Add 25 µL of this solution into 2 mL serum starvation medium.
3 Methods
3.1 Microscope Design
The microscope system (Fig. 1a) is built upon an Olympus IX-70 microscope stand with the following custom modifications:
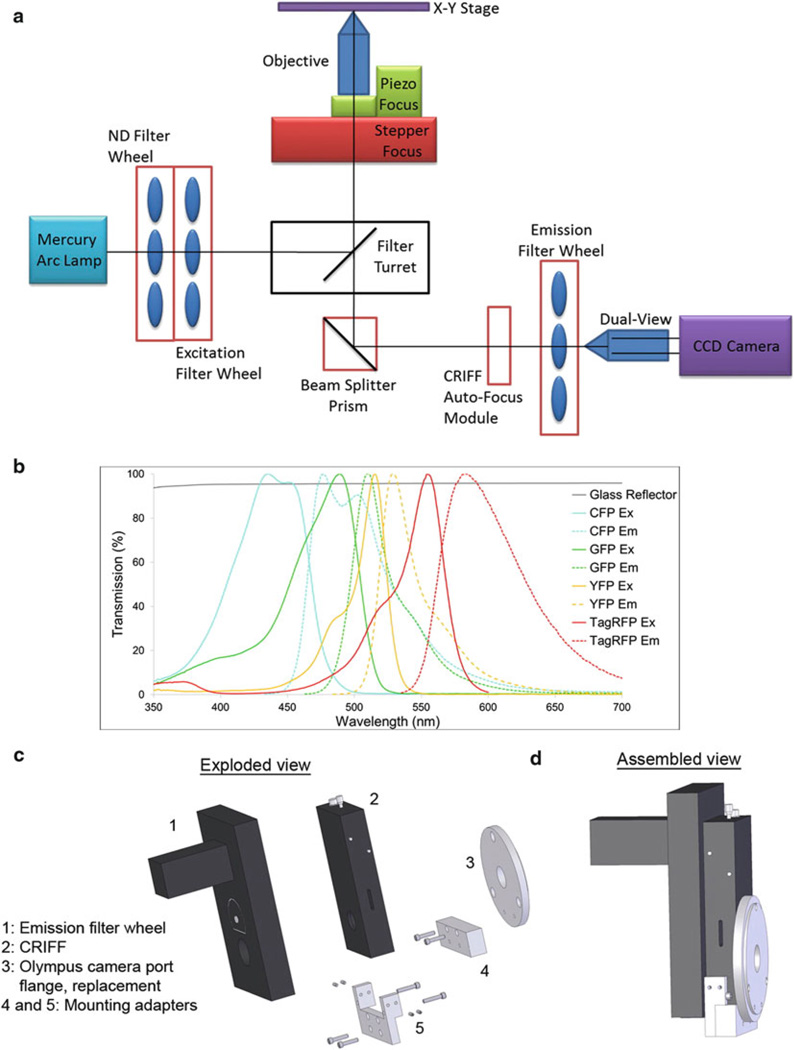
Fig. 1.
Design of custom-built high-resolution wide-field fluorescence microscope. (a) Optical layout showing different parts of the microscope. For clarity, the environmental heat chamber, which surrounds the microscope, is not shown. (b) Transmission properties of the glass reflector (6 % reflection/94 % transmission) and excitation and emission spectra of commonly used fluorophores in live cell imaging. (c) Exploded view of CRIFF, emission filter wheel, flange and the mounting adapters. (d) Assembled view of the microscope parts shown in (c)
The use of a glass reflector in place of a dichroic mirror in the filter turret of the microscope enables complete flexibility in the choice of fluorophores and efficient collection of emitted photons (Fig. 1b). However, as only 6 % of the excitation light makes it to the sample, a bright light source is required. For this purpose we have chosen a mercury arc lamp over other options (e.g., xenon or LED source). In cases where the fluorophores chosen were not bright enough for use with the glass reflector, other filter turret positions were loaded with multi-band dichroics specific for particular applications (e.g., CFP-YFP-RFP, GFP-RFP and Cy5 imaging).
Control of the excitation intensity is accomplished with ND filters in one wheel of a dual filter wheel system. The second wheel of this system is used to house excitation filters which allow separate and individual control of excitation wavelengths.
Emission wavelengths were individually controlled with a separate filter wheel mounted in front of the CCD camera. The sensitivity of the measurements to drifts in focus necessitates an active autofocus module. For this purpose, a CRIFF unit utilizing a stepper motor z-focus drive was employed. To accommodate both the emission filter wheel and the CRIFF unit in the emission path of the microscope, a custom mount was designed and machined (Fig. 1c, d, see Note 1).
Additional fast z-scanning capabilities are attained by use of a piezo controlled focus mount. Mosaicing and multiple sample position acquisition are facilitated by a motorized xy stage equipped with linear encoders for accurate stage repositioning.
In some experiments, performing sequential imaging would be too slow to capture the rapid dynamics of the biological process of interest. In these cases, the emission filter wheel is put into an “open” position and an image splitter device (Dual-View) is placed in the emission path. This device splits the image into two halves, passing one through a long pass filter and the other through a short pass filter, and then projects the two images onto each half of the CCD chip.
The microscope utilizes a high numerical aperture objective lens (60×, 1.42 NA) for high resolution imaging.
For a list of fluorophores (and dyes) which can be imaged sequentially or simultaneously on the microscope, see Table 2.
Table 2.
Microscope imaging modes
| Use # | Turret position | Dual-view | Usage |
|---|---|---|---|
| 1 | A, B, C, D | OFF | Trans-illumination |
| 2 | A | OFF | CFP, Cerulean |
| 3 | A | OFF | YFP, Venus |
| 4 | A | OFF | RFP, mCherry, TRITC, Alexa 555 |
| 5 | A | OFF | CFP-YFP FRET, Cerulean-Venus FRET |
| 6 | B | OFF | GFP, FITC, Alexa 488 |
| 7 | B | OFF | RFP, mCherry, TRITC, Alexa 555 |
| 8 | B | OFF | GFP-RFP FRET |
| 9 | C | OFF | Cy5, Alexa 647 |
| 10 | D | OFF | CFP, Cerulean |
| 11 | D | OFF | YFP, Venus |
| 12 | D | OFF | CFP-YFP FRET, Cerulean-Venus FRET |
| 13 | D | OFF | GFP, FITC, Alexa 488 |
| 14 | D | OFF | RFP, mCherry, TRITC, Alexa 555 |
| 15 | D | OFF | GFP-RFP FRET |
| 16 | D | OFF | Cy5, Alexa 647 |
| 17 | D | CFP-YFP DualView cube | CFP (Left half) + YFP-FRET (right half) |
| 18 | D | CFP-YFP DualView cube | Blank (Left half) + YFP (right half) |
| 19 | D | CFP-YFP DualView cube | Blank (Left half) + RFP (right half) |
| 20 | D | CFP-YFP DualView cube | Blank (Left half) + Cy5 (right half) |
| 21 | D | GFP-RFP DualView cube | GFP (Left half) + RFP-FRET (right half) |
| 22 | D | GFP-RFP DualView cube | Blank (Left half) + RFP (right half) |
| 23 | D | GFP-RFP DualView cube | Blank (Left half) + Cy5 (right half) |
3.2 Labeling Gelatin with Alexa 405 Dye
A day before labeling the gelatin, take 2 g Bio-Gel P-30 powder and add 36 mL PBS, mix by gently inverting the tube 5–6 times and leave at room temperature (RT). Hold the glass column vertical by clamping on a stand. Mix Bio-Gel slurry from the previous day by pipetting up and down and transfer it to the column until it is full. The column will start draining PBS from the bottom. As the top level of packed column drops, add mixed Bio-Gel slurry to the column. Leave approximately a 3 cm space with PBS above the packed column. The column can be stored at 4 °C for up to 1 week (see Notes 2 and 3).
While the column is draining in step 1 above, bring 0.2% gelatin solution to RT. Transfer 2 mL gelatin solution into a 15 mL conical tube. Add 200 µL 1 M sodium bicarbonate solution.
Briefly centrifuge Alexa 405 dye containing tube to dislodge any dye stuck in the cap. Add 100 µL DMSO to 1 mg Alexa 405 dye, mix by pipetting up and down 3–4 times. Add the dye/DMSO solution into 2.2 mL gelatin solution from step 2. Cover the conical tube with aluminum foil and rotate at RT for 1 h.
After the column in step 1 has stopped draining, transfer the dye–gelatin solution from step 3 to the top of the column. After all the dye–gelatin solution enters the column, add PBS gently to the top.
After 15 min, check the dye–gelatin solution in the column with a UV lamp. It should look like a bright blue smear through the column. As the dye–gelatin solution reaches the bottom of the column, start collecting the dye labeled gelatin solution into eppendorf tubes. Collect about 2–2.5 mL (see Note 4). This solution can be stored at 4 °C for up to 2 months.
3.3 Preparation of Gelatin-Coated MatTek Dishes (For Ten Dishes)
Add 300 µL 1 N HCl into the well of each MatTek dish. Incubate for 10 min at RT. Wash three times with PBS in 5 min intervals.
Dilute poly-l-lysine solution to 50 µg/mL (75 µL stock solution in 3 mL PBS). Put 300 µL into each well and incubate for 20 min at RT. Wash three times with PBS in 5 min intervals.
Take out 50 µL of Alexa 405 dye-labeled gelatin (prepared above in Subheading 3.2) into an eppendorf tube, cover it with aluminum foil and leave it on the bench for it to reach RT.
Once gelatin is completely dissolved, take it out of the water bath and leave it at RT to cool. Add 50 µL of Alexa 405 dye-labeled gelatin from step 3 into 2 mL 0.2 % gelatin solution (final concentration 1:40). Mix by pipetting 3–4 times and leave the mixture in a 37 °C water bath for 5–10 min. Take the tube out and let it cool at RT for 5 min before placing 200 µL of the solution into each well of the MatTek dish. Leave for 10 min at RT (see Note 5).
For making unlabeled gelatin dishes, skip steps 3 and 4 above. Place 200 µL of 0.2 % RT gelatin solution into each well of the MatTek dish and leave for 10 min at RT.
Wash three times with PBS in 5 min intervals. Transfer dishes on a tray filled with ice. Add 2 mL cold 0.2 % glutaraldehyde solution into each dish and incubate on ice for 15 min. Remove the dishes from ice and wash three times with PBS in 5 min intervals.
Prepare 5 mg/mL sodium borohydride solution (100 mg powder in 20 mL PBS) and immediately add 2 mL of this solution into each dish. Incubate for 15 min at RT. Wash three times with PBS in 5 min intervals.
Add 2 mL 70 % ethanol into each dish and incubate at RT for 15 min. Wash three times with PBS in 5 min intervals.
Add 2 mL 10× penicillin/streptomycin solution into each dish. Store dishes at 4 °C for up to 10 days.
3.4 Transfection of MTLn3 Cells and Preparing Cells for Live Imaging
The day before the transfection plate MTLn3 cells in 35 mm dishes at 1.2 × 105 cells/dish in 2 mL culture medium.
For each dish take two eppendorf tubes. Add 250 µL Opti-MEM into each tube. In the first tube add 0.5–1 µg of DNA and mix. In the second tube, add 4 µL of Lipofectamine 2000 and mix. Leave both tubes in the culture hood for 5 min.
Mix DNA and Lipofectamine 2000 solutions together and leave in the hood for 20 min.
Wash MTLn3 cells with pre-warmed Opti-MEM solution two times and leave 500 µL Opti-MEM. Add 500 µL DNA–Lipofectamine mixture from step 3 into each dish. Transfer cells into the cell culture incubator for 45 min.
Wash cells two times with cell culture medium and leave 2 mL culture media on cells. Transfer cells to the cell culture incubator and let the gene(s) of interest express for 6–8 h.
Wash gelatin coated MatTek dishes two times with the culture medium and leave 1 mL culture medium each dish. Transfer dishes in the incubator for at least 30 min. Trypsinize transfected cells and plate cells from each 35 mm dish into two gelatin coated MatTek dishes. Leave cells in the incubator overnight.
3.5 Live Imaging of Invadopodia
For EGF stimulation experiments: Wash cells two times with serum starvation medium, leave 2 mL serum starvation medium on cells. Incubate cells for 3–4 h in a 37 °C incubator (no CO2). Transfer cells from the incubator to the microscope stage and take the cover off the MatTek dish. Depending on the number of fluorophores you want to image, set the imaging parameters. For two fluorophores (e.g., GFP and RFP), we typically image every 3 s for up to 5 min (Fig. 2a, b). After capturing 1–2 frames in each channel, pause imaging, add 2 mL 10 nM EGF (final concentration 5 nM) in the dish and resume imaging.
For steady state imaging: Wash cells two times with steady state imaging medium, leave 2 mL steady state imaging medium on cells. Transfer cells to the microscope stage, start imaging.
For both EGF stimulation and steady state imaging, using the autofocus unit helps in maintaining focus throughout the imaging period (Fig. 2c, d). To use the autofocus unit, before starting imaging, turn the CRIFF laser ON and calibrate the focus by following the vendor’s protocol.
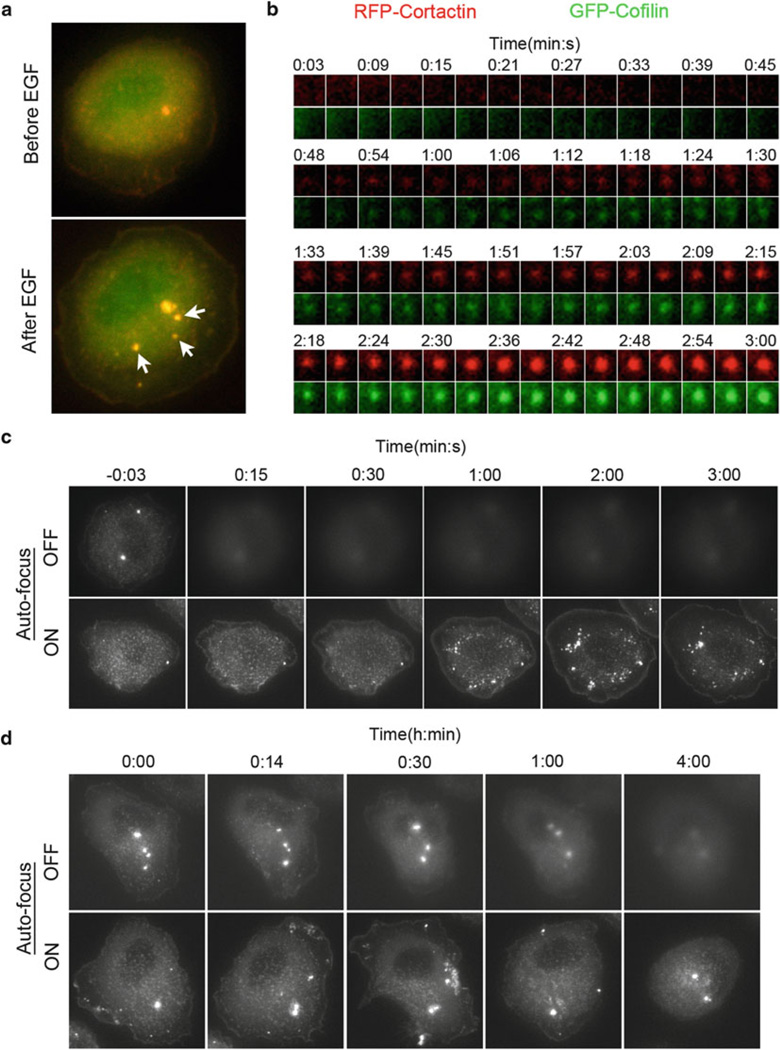
Fig. 2.
Live-cell image acquisition. (a) TagRFP-Cortactin and GFP-Cofilin transfected MTLn3 cells were stimulated with EGF and imaged every 3 s for up to 5 min. Images show an MTLn3 cell before and after EGF stimulation. White arrows indicate newly forming invadopodium precursors after EGF stimulation. (b) Time-lapse sequence of a single invadopodium precursor in GFP and RFP channels is depicted as montage. Time is in min:s. (c) Demonstration of the advantage of using autofocus (with CRIFF unit) in maintaining focus during EGF stimulation experiment. Cells expressing TagRFP-Cortactin were imaged with (lower panel) or without (upper panel) autofocus every 3 s for up to 5 min. Only selected time points are shown. Time is in min:s and time 0:00 corresponds to EGF addition. (d) Demonstration of the advantage of using autofocus (with CRIFF unit) in maintaining focus during steady state long time-lapse imaging. Cells expressing TagRFP-Cortactin were imaged with (lower panel) or without (upper panel) autofocus every 2 min for up to 4 h. Only selected time points are shown. Time is in h:min. (a–d) In all experiments, an environmental heat chamber enclosing the microscope was preheated to 37 °C for > 3 h, before the start of imaging
3.6 Invadopodia Tracking with “Invadopodia Tracker” Plugin
Put the plugin file Invadopodia_tracker.java (available upon request) under the plugins folder in ImageJ. Run “Plugins - > Compile and Run…,” select Invadopodia_tracker.java file from the plugins folder. Ignore any error(s). Check to see that there is a new file named “Invadopodia_tracker.class” in the plugins folder. Restart ImageJ, “Invadopodia tracker” command should appear under Plugins menu in ImageJ.
Open the 16-bit time-lapse stack of a single fluorescence channel (Fig. 3a).
Go through the whole stack and choose the frame where invadopodia are clearly visible (typically after 1–2 min in EGF stimulation experiments). Using multi-point tool, select invadopodia- of-interest (Fig. 3b). Alternatively, invadopodia-of-interest can be automatically selected by “Process - > Find Maxima…” command and adjusting the noise tolerance.
Run “Invadopodia tracker” from the Plugins menu. A GUI will open asking for maximum invadopodium displacement from one frame to the next (a typical value is 3–5 pixels) and estimates of minimum and maximum number of particles considered to be invadopodia in the whole field per frame (Fig. 3c). A typical range for minimum and maximum number of invadopodia is 25–50 and 100–300, respectively. The user needs to optimize these numbers for correct invadopodia tracking (see Notes 6 and 7). For the next three parameters—Noise tolerance, Delta noise tolerance and maximum iterations, the user should start with default values—500, 20, 200 respectively (see Note 8).
After tracking is over, tracked invadopodia are marked in red circles and added as overlays (Fig. 3d, f; see Note 9). A log file, containing invadopodia centroids in each frame, is also generated (Fig. 3e).
Copy and paste invadopodia centroids into Excel. It will show up as three columns—first column is the frame number, second and third columns are the x and y coordinates of invadopodium centroid (Fig. 3g).
From the centroid data, invadopodium trajectories can be easily plotted in Excel (Fig. 3h).
Fig. 3.
Flow diagram of invadopodia tracking with the “Invadopodia tracker” plugin in ImageJ. Step-by-step analysis procedure is described in Subheading 3.6.
Acknowledgments
We thank Dr. Louis Hodgson, and members of the Analytical Imaging Facility and Gruss-Lipper Biophotonics Center for helping in the microscope design. We also thank people from Condeelis, Segall, and Cox laboratories for helpful discussions. This work was supported by a postdoctoral fellowship to Ved Sharma from Susan G. Komen for the Cure© (KG111405), the Integrated Imaging Program and CA150344.
Footnotes
The use of CRIFF unit requires a high numerical aperture objective e.g., 60×, 1.42 NA, because it works on the total internal reflection (TIR) principle.
While packing the column make sure that the top of the packed column does not dry up. As soon as the PBS level falls off, replenish the top with more PBS.
If the column is packed properly then it will flow approximately 2–3 drops per min. If not, then transfer the column packing into a tube, add some PBS, mix, and transfer it to a thoroughly cleaned column.
The Alexa 405 dye–gelatin solution runs as a bright blue smear in the column. As the leading front reaches the bottom of the column, a slightly gray zone separating the Alexa 405 dye labeled gelatin (fast running fraction) with the Alexa 405 dye alone (slower running fraction) will become visible.
If you start seeing many aggregated bright Alexa 405 dye particles in your Alexa 405 labeled gelatin MatTek dishes (which might interfere with imaging and affect degradation area calculation), then the pre-warmed dye–gelatin solution in Subheading 3.3 step 4 can be centrifuged at 10,000 × g for 5 min before adding it to the wells of the MatTek dish.
In the case that “Invadopodia tracker” plugin does track invadopodia through only some frames but misses others, the sensitivity of the invadopodia detection can be enhanced by increasing the values for the estimates of minimum and maximum number of invadopodia. Conversely, if the tracker identifies faint particles (i.e., background noise) as invadopodia, the sensitivity of the invadopodia detection can be decreased by lowering the values for the estimates of minimum and maximum number of invadopodia.
If the “Invadopodia tracker” fails to track invadopodia due to a narrow range between the estimates of minimum and maximum number of invadopodia, then either the value for estimate of minimum number of invadopodia should be decreased or the value for estimate of maximum number of invadopodia should be increased, or both.
The last three parameters in the “Invadopodia tracker” plugin are automatically adjusted according to user-selected values for maximum invadopodium displacement and the estimates of minimum and maximum number of invadopodia. In the case where plugin is not tracking invadopodia properly after adjusting the parameters described in Notes 6 and 7 the last three parameters may be changed to help converge on a solution.
After running the “Invadopodia tracker” plugin, tracked invadopodia are shown in red circles as overlays. In the case a user wants to run the plugin again with different parameters, these overlays can be removed by selecting “Image - > Overlay - > Remove Overlay” command in ImageJ.
References
- 1.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert MA, Yang J. Targeting invadopodia to block breast cancer metastasis. Oncotarget. 2011;2:562–568. doi: 10.18632/oncotarget.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen WT, Chen JM, Parsons SJ, Parsons JT. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- 6.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASPArp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 2009;69:8594–8602. doi: 10.1158/0008-5472.CAN-09-2305. [DOI] [PubMed] [Google Scholar]
- 9.Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- 11.Ammer AG, Kelley LC, Hayes KE, Evans JV, Lopez-Skinner LA, Martin KH, Frederick B, Rothschild BL, Raben D, Elvin P, Green TP, Weed SA. Saracatinib impairs head and neck squamous cell carcinoma invasion by disrupting invadopodia function. J Cancer Sci Ther. 2009;1:52–61. doi: 10.4172/1948-5956.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–8275. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- 13.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–4298. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neel NF, Rossman KL, Martin TD, Hayes TK, Yeh JJ, Der CJ. The RalB small GTPase mediates formation of invadopodia through a GTPase-activating protein-independent function of the RalBP1/RLIP76 effector. Mol Cell Biol. 2012;32:1374–1386. doi: 10.1128/MCB.06291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai B, Ma T, Chellaiah MA. Invadopodia and matrix degradation, a new property of prostate cancer cells during migration and invasion. J Biol Chem. 2008;283:13856–13866. doi: 10.1074/jbc.M709401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stylli SS, Stacey TT, Verhagen AM, Xu SS, Pass I, Courtneidge SA, Lock P. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J Cell Sci. 2009;122:2727–2740. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segall JE, Tyerech S, Boselli L, Masseling S, Helft J, Chan A, Jones J, Condeelis J. EGF stimulates lamellipod extension in metastatic mammary adenocarcinoma cells by an actin-dependent mechanism. Clin Exp Metastasis. 1996;14:61–72. doi: 10.1007/BF00157687. [DOI] [PubMed] [Google Scholar]
- 20.Sharma VP, Beaty BT, Patsialou A, Liu H, Clarke M, Cox D, Condeelis JS, Eddy RJ. Reconstitution of in vivo macrophagetumor cell pairing and streaming motility on one-dimensional micro-patterned substrates. IntraVital. 2012;1:77–85. doi: 10.4161/intv.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]