Abstract
Background
Temperature extremes represent an important limiting factor to plant growth and productivity. The present study evaluated the effect of hydroponic pretreatment of strawberry (Fragaria x ananassa cv. ‘Camarosa’) roots with an H2S donor, sodium hydrosulfide (NaHS; 100 μM for 48 h), on the response of plants to acute heat shock treatment (42°C, 8 h).
Results
Heat stress-induced phenotypic damage was ameliorated in NaHS-pretreated plants, which managed to preserve higher maximum photochemical PSII quantum yields than stressed plants. Apparent mitigating effects of H2S pretreatment were registered regarding oxidative and nitrosative secondary stress, since malondialdehyde (MDA), H2O2 and nitric oxide (NO) were quantified in lower amounts than in heat-stressed plants. In addition, NaHS pretreatment preserved ascorbate/glutathione homeostasis, as evidenced by lower ASC and GSH pool redox disturbances and enhanced transcription of ASC (GDH) and GSH biosynthetic enzymes (GS, GCS), 8 h after heat stress imposition. Furthermore, NaHS root pretreatment resulted in induction of gene expression levels of an array of protective molecules, such as enzymatic antioxidants (cAPX, CAT, MnSOD, GR), heat shock proteins (HSP70, HSP80, HSP90) and aquaporins (PIP).
Conclusion
Overall, we propose that H2S root pretreatment activates a coordinated network of heat shock defense-related pathways at a transcriptional level and systemically protects strawberry plants from heat shock-induced damage.
Keywords: Ascorbic acid, Heat shock proteins, Hydrogen sulfide, Nitrosative stress, Oxidative stress, Priming, Thermotolerance, Fragaria x ananassa
Background
Threats of climate change and global warming render heat stress a general concern for the agricultural sector worldwide [1]. Plants exposed to high temperatures may experience severe cellular injury that may lead to cell death within a short period [2]. The primary targets of heat shock injury in plants are photosynthesis [3], water status [4], carbon assimilation processes [5] and membrane stability [6]. At the cellular level, heat stress results to protein denaturation and aggregation, increased fluidity of membrane lipids, inactivation of enzymes in chloroplast lamella and mitochondria, inhibition of protein synthesis and secondary oxidative stress through the production of reactive oxygen species (ROS) [7].
Consequently, plants manifest different mechanisms for adaptation and protection in elevated temperatures. The initial heat stress signal, probably perceived as the increase of plasmalemma lipid bilayer fluidity [8], triggers downstream signaling processes for transcriptional regulation [9]. Up-regulation of mitogen activated protein kinase (MAPK) transduction pathway through the induction of Ca2+ influx [10], ROS signaling and hormonal activation, as well as heat shock protein (HSP)/chaperone signal transduction pathways, seems to be the key players in plant transcriptional regulation under heat stress [11]. As a result, major thermotolerance mechanisms, such as the induction of antioxidant machinery, accumulation of heat shock proteins, osmolytes and secondary metabolite adjustments, are activated, driving to cellular homeostasis and repairing of damaged proteins and membranes [12].
Extensive plant breeding efforts and more recent transgenic approaches have largely validated that heat stress tolerance is a multigenic trait [13]. In addition, benefits from transgenic approaches have been limited and have not led to agronomically improved crops for heat tolerance under field conditions [14]. Thus, considerable attention has been devoted in alleviating the detrimental effects of high temperatures in plants through the exogenous application of various priming agents. Exogenously applied calcium [7], ascorbic acid [15], abscisic acid [16], salicylic acid [17], H2O2 and NO [18] managed to enhance thermotolerance of treated plants. Furthermore, seed pretreatment with H2O2 improved heat tolerance of wheat seedlings through the alleviation of oxidative damage and the up-regulation of stress proteins [19].
Sulfur-containing defense compounds (SDCs) are crucial for the survival of plants under biotic and abiotic stress [20]. Recent evidence revealed the role of H2S in orchestrating plant responses to environmental stimuli [21,22]. More precisely, exogenous application of H2S donor sodium hydrosulfide (NaHS) managed to alleviate heavy metal toxicity in germinating wheat [23], cucumber [24] and barley seedlings [25]. Exogenous application of NaHS was found to promote osmotic stress tolerance in sweet potato [26], soybean seedlings [27] and strawberry plants [28]. Furthermore, H2S promoted root organogenesis [29] and was also found to be involved in guard cell signaling [30].
Recent reports showed that NaHS pretreatment significantly increased heat tolerance in tobacco suspension cultured cells [31] and maize seedlings [32,33], respectively. However, whether H2S priming could transcriptionally induce a systemic activation of plant defense mechanisms for providing tolerance to subsequent exposure of a heat-sensitive soft fruit crop such as strawberry remains largely unexplored. In the present study we hypothesized that transient pre-exposure of strawberry plant roots to H2S may induce systemic thermotolerance to subsequent exposure of plants to heat shock treatment (42°C, 8 h). Therefore, the effects of root pretreatment with H2S donor NaHS on several key components of stress tolerance mechanisms in the leaves of strawberry plants were investigated following a combined physiological, biochemical and molecular approach. As far as is known, this is the first study dealing with the employment of H2S for the protection of a fruit crop from temperature extremes, as well as the first report to implicate the transcriptional regulation of HSPs and aquaporins in the response.
Results
Phenotypic observations
Exposure of strawberry plants to 42°C for 8 h resulted in mild wilting and leaf curling (Figure 1C), while NaHS root pretreatment prior to stress exposure exhibited obvious mitigating effect, as evidenced by the conservation of plant leaf turgor and structure (Figure 1D). Non-stressed NaHS-treated plants (Figure 1B) displayed similar phenotype with control plants (Figure 1A), verifying the non-toxic effects of NaHS at the concentration applied.
Figure 1.
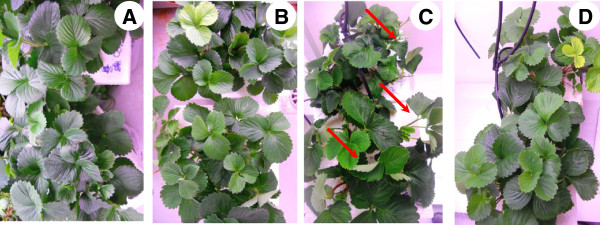
Phenotypic effects of H2S donor NaHS (100 μM) on strawberry plants exposed to heat shock treatment (42°C) for 8 h. {(A) Control: pretreated with H2O and subjected to 23°C for 8 h, (B) NaHS: pretreated with NaHS and subjected to 23°C for 8 h, (C) Heat: pretreated with H2O and subjected to 42°C for 8 h and (D) NaHS → Heat: pretreated with NaHS and subjected to 42°C for 8 h}. Red arrows indicate wilted, curled leaves.
Hydrogen sulfide content
Sodium hydrosulfide root pretreatment resulted in significantly higher absolute H2S content in strawberry leaves compared with control samples, thus verifying its status as an H2S donor (data not shown). Heat stress caused a marked modulation in H2S leaf content, manifested by a significant increase after 1, 4 and 8 h of exposure to 42°C compared with control samples. A significant increase in H2S content was also recorded in NaHS-pretreated plants after 1 h exposure to heat stress, gradually lowering to control levels thereafter (Figure 2).
Figure 2.
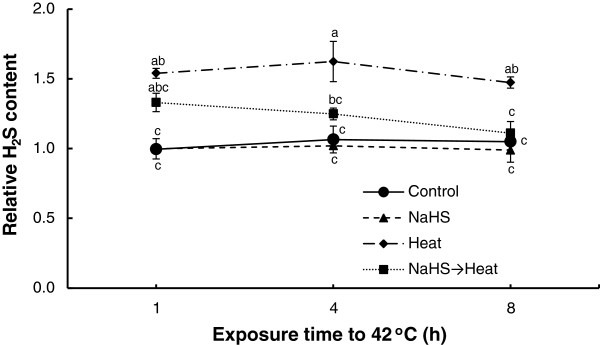
Effect of H2S donor NaHS (100 μM) on H2S content of strawberry leaves exposed to heat shock treatment (42°C) for 8 h, relatively to content at time point 0 h. Treatment acronyms are described in Figure 1 caption. Data are means ± SE of three replications. Bars with different letters are significantly different at p < 0.05.
Chlorophyll fluorescence
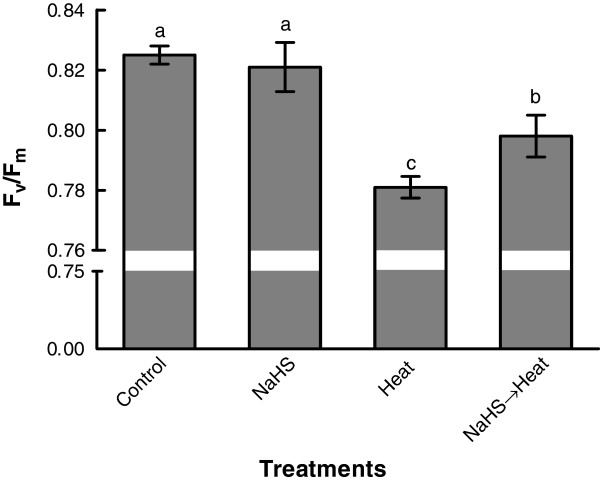
Apparent negative effects of heat stress on Fv/Fm ratio of strawberry plants were registered; a significant reduction on Fv/Fm ratio was measured in plants subjected to heat stress for 8 h. Nevertheless, root pretreatment with NaHS prior to heat exposure enabled strawberry plants subjected for 8 h to heat shock treatment to maintain higher quantum efficiency of photosystem II compared with plants directly exposed to heat stress (Figure 3).
Figure 3.
Effect of H2S donor NaHS (100 μM) on chlorophyll fluorescence (Fv/Fm) of strawberry leaves exposed to 42°C for 8 h. Treatment acronyms are described in Figure 1 caption. Data are means ± SE of three replications. Bars with different letters are significantly different at p < 0.05.
Cellular damage effects
Heat stress enhanced membrane damage, resulting in increased MDA content (Figure 4). MDA content was doubled within 1 h of exposure to 42°C, following an increasing pattern thereafter. Inversely, NaHS root pretreatment prior to stress exposure managed to mitigate the levels of lipid peroxidation, validated by the lower MDA content compared with non-pretreated stressed plants.
Figure 4.
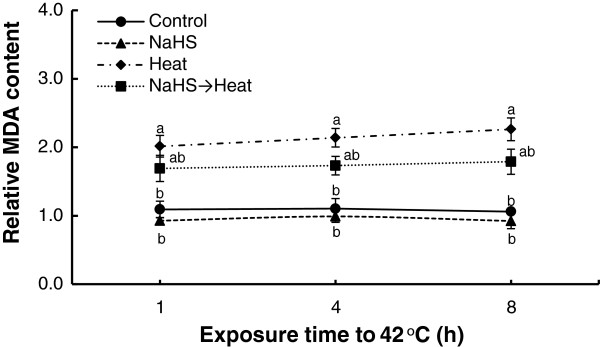
Effect of H2S donor NaHS (100 μM) on malondialdehyde (MDA) content of strawberry plant leaf tissue exposed to heat shock treatment (42°C) for 8 h, relatively to content at time point 0 h. Treatment acronyms are described in Figure 1 caption. Data are means ± SE of three replications. Bars with different letters are significantly different at p < 0.05.
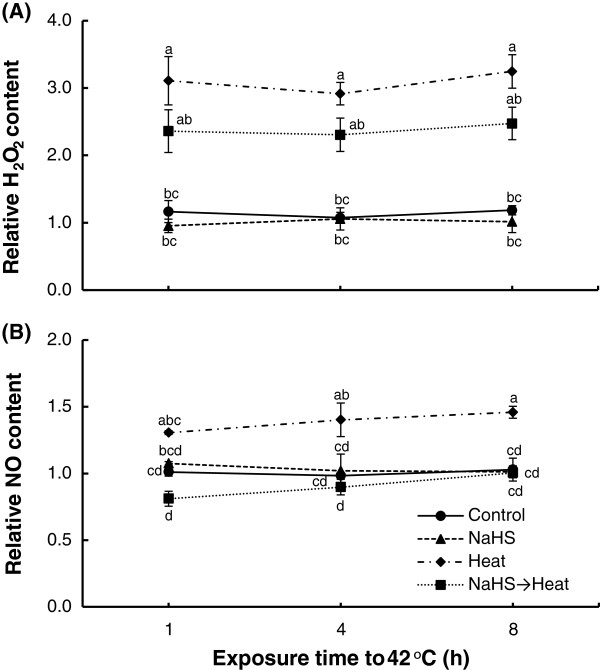
Hydrogen peroxide and nitric oxide content
Progressive heat shock caused a marked increase in both H2O2 and NO content, reaching maximum levels after 8 h (Figure 5). However, lower rates of H2O2 accumulation were recorded in NaHS-pretreated plants subsequently exposed to progressive heat shock treatment, albeit significantly higher than control, unstressed plants (Figure 5A). In turn, no significant modulation in NO content was recorded in NaHS-treated plants exposed to heat treatment, compared with control samples (Figure 5B).
Figure 5.
Effect of H2S donor NaHS (100 μM) on hydrogen peroxide (H2O2) (A) and nitric oxide (NO) (B) content of strawberry plant leaf tissue exposed to heat shock treatment (42°C) for 8 h, relatively to content at time point 0 h. Treatment acronyms are described in Figure 1 caption. Data are means ± SE of three replications. Bars with different letters are significantly different at p < 0.05.
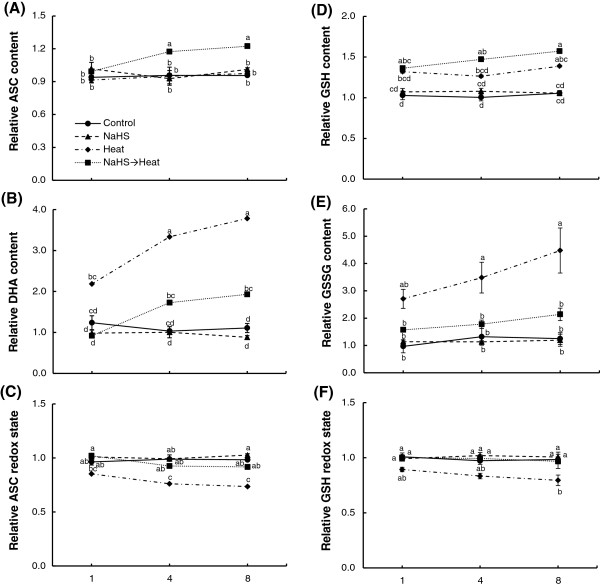
ASC and GSH content/redox state
Exposure to elevated temperature for 8 h resulted in a significant increase in both total ascorbate and total glutathione content, while NaHS root pretreatment prior to heat exposure resulted to a greater increase in both antioxidants’ pools. The increase is likely attributed to the increase of both reduced and oxidized forms of ascorbate and glutathione. More precisely, heat stress resulted in non-significant increase in ASC content, while a significant increase in GSH leaf content was recorded. NaHS pretreatment prior to heat exposure resulted in a greater increase of both reduced forms (Figure 6A and D). On the other hand, a substantial increase in both DHA and GSSG content was registered after heat exposure for 8 h. Interestingly, root pretreatment with NaHS managed to ameliorate further oxidation of both antioxidant molecules as evidenced by the lower levels of DHA and GSSG content (Figure 6B and E). Overall, NaHS root pretreatment managed to significantly alleviate both ascorbate and glutathione redox state disturbances compared with non-pretreated stressed plants (Figure 6C and F). In addition, total ascorbate and glutathione pools of NaHS-treated unstressed plants were maintained in similar levels to those observed in control samples.
Figure 6.
Effect of H2S donor NaHS (100 μM) on ascorbate and glutathione pool and redox state of strawberry plant leaf tissue exposed to heat shock treatment (42°C) for 8 h, relatively to content at time point 0 h. {reduced ascorbate (ASC) (A), dehydroascorbate (DHA) (B), ascorbate redox state (C), reduced glutathione (GSH) (D), oxidized glutathione (GSSG) (E) and glutathione redox state (F)}. Treatment acronyms are described in Figure 1 caption. Data are means ± SE of three replications.
Gene expression levels
The relative expression ratio of a diverse set of specific genes involved in antioxidant machinery, cellular redox regulation, signal transduction and protein structure stability, assayed by quantitative real-time RT-PCR, is presented in Figure 7. These included key enzymatic antioxidant (cytosolic ascorbate peroxidase, cAPX; catalase, CAT; manganese superoxide dismutase, MnSOD; glutathione reductase, GR), ascorbate and glutathione biosynthesis (L-galactose dehydrogenase, GDH; glutamylcysteine synthetase, GCS; glutathione synthetase, GS), NO biosynthesis (nitrate reductase, NR), transcription factor (dehydration-responsive element binding factor, DREB), heat shock proteins (HSP70, HSP80, HSP90) and aquaporin (PIP) genes. Overall, heat stress alone or in combination with NaHS root pretreatment significantly induced mRNA expression levels of most examined genes, being highly dependent on the duration of heat exposure.
Figure 7.
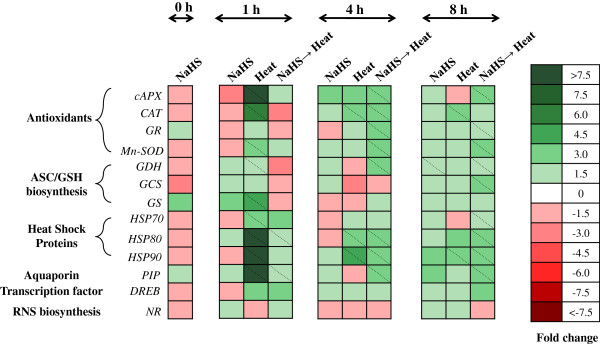
Heat map showing temporal expression pattern of selected genes associated with enzymatic antioxidants, RNS biosynthesis, redox homeostasis, heat shock proteins and aquaporins in leaves of strawberry plants under non-stress and heat stress conditions. Following root pre-treatment with 100 μΜ NaHS, the experimental plants were grown either under normal (23°C) or extreme temperature (42°C) conditions for 8 h, as described in Figure 1. Relative mRNA abundance was evaluated by real-time RT-PCR using three biological repeats. Up-regulation is indicated in green; down-regulation is indicated in red. Diagonal dotted lines represent statistically significant values at p < 0.05. A scale of color intensity is presented as a legend. Actual relative expression data, obtained from 3 independent replicates, are shown in Additional file 1: Table S1.
The main trends observed were the overall low levels of regulation of all genes examined in plants treated solely with NaHS, compared with control samples, except for GDH 8 h after the end of root incubation (Figure 7). In turn, 1 h of heat stress imposition revealed a significant up-regulation of most genes examined (with the exceptions of GR, NR, GCS and DREB), greatly ameliorated after progressive heat stress exposure. Furthermore, heat treatment caused the rapid accumulation of high levels of cAPX, PIP, HSP80 and HSP90 transcripts as early as 1 h after heat stress imposition (see Additional file 1: Table S1). Nevertheless, the expression pattern of most genes examined in non-treated plant suffering heat stress for 8 h was generally similar to respective controls.
Interestingly, the protective effect of NaHS root pre-treatment on heat stress tolerance in terms of transcriptional induction of defense-related genes was recorded 4 h after stress imposition, reaching maximal induction levels for most genes examined (except for CAT, NR, GS and DREB) after 8 h of heat stress (Figure 7). Conversely, no significant activation of defense-related molecular machinery was recorded 1 h after stress imposition in NaHS-pretreated and subsequently stressed plants.
Discussion
Heat stress affects a broad spectrum of cellular components and metabolism, often causing irreversible damage to plant growth and development. Despite the constant advances being made towards understanding plant responses to temperatures extremes and improving thermotolerance, examples of plants with heat tolerance through conventional breeding and transgenic approaches are limited and condensed into laboratory conditions [14]. Therefore, compounds that might result in the mitigation of high temperature detrimental effects could potentially be of great importance. We have recently postulated the priming effect of H2S in the alleviation of salinity and non-ionic osmotic stress [28]. In the current study, an array of physiological, biochemical and molecular approaches provided novel evidence that root pretreatment with H2S donor NaHS enhanced thermotolerance of strawberry plants subsequently exposed to temperature extremes, supporting the notion that H2S is a key signaling molecule in plants. This seems to be of pivotal importance for strawberry cultivation, since strawberry growth and productivity are found to be greatly affected by temperature extremes [34]. Furthermore, this study revealed that the role of NaHS in alleviating heat shock stress could be attributed to H2S, as the levels of endogenous H2S increased following NaHS pretreatment and subsequent stress imposition, in accordance with similar findings by Zhang et al. [27]. Importantly, Zhang et al. [26,29] reported that only H2S, and no other Na+- or sulfur-containing compounds released from NaHS, have a protective role during abiotic stresses, while similar findings were provided by Li et al. [32] in maize plants under heat stress following an H2S inhibitor approach.
Photosystem II (PSII) is the most thermally sensitive component of photosynthesis and its activity is greatly reduced or even partially stopped under high temperatures [35]. Our results are in agreement with previous findings highlighting that maximal photochemical efficiency (Fv/Fm) in leaves of several plant species was reduced under heat stress conditions [7,36]. However, NaHS application results in the alleviation of heat stress injuries to PSII activity, as evidenced by the higher Fv/Fm ratio preserved in pre-treated stressed strawberry plants compared with untreated stressed plants (Figure 3).
Furthermore, in order to verify the role of NaHS root pretreatment in alleviating heat shock derived oxidative damage, lipid peroxidation and H2O2 leaf content were assayed. Results showed that NaHS mitigated heat stress-induced MDA and H2O2 content increase in strawberry leaves, thus suggesting that oxidative damage to membranes and other cell components was reversed following H2S treatment (Figures 4 and 5). Our findings further support previous results showing that NaHS pretreatment resulted in lower content of both MDA and H2O2 in stressed plants. More precisely, NaHS resulted to the conservation of MDA and H2O2 content increase against osmotic stress [26,27], or under high aluminum concentration [23]. In addition, we recently provided evidence that NaHS root pretreatment resulted in lower levels of MDA and H2O2 in strawberry plants suffering ionic and non-ionic osmotic stress [28]. Rapid (within 1 h) accumulation of H2O2 in heat-stressed plants alone, may suggest a role for H2O2 in triggering the early expression of heat-shock proteins in stressed plants, as previously reported [37,38]. Our findings are further supported from the transcript levels of major enzymatic antioxidants (cAPX, CAT, MnSOD, GR), which were found to be induced in NaHS-pretreated plants in comparison with untreated ones, after 4 and 8 h of exposure at 42°C. Transcript levels are in line with previous observations in NaHS-pretreated strawberry plants exposed to NaCl and PEG-6000 treatment [28], as well as with enhanced antioxidant enzymatic activities in NaHS-treated plants under cadmium [39], heat [32] and drought [27] stress, as well as in NaHS-treated salt-stressed germinating seeds [40], thus rendering the apparent induction of antioxidant enzymes of prime importance for the enhanced tolerance observed.
Besides ROS, NO and other NO-derived products, cumulatively called reactive nitrogen species (RNS), may also be overproduced under abiotic stress conditions, causing secondary nitrosative stress in plants [41,42]. NO has also been shown to act in parallel with other signaling molecules for regulating many biological processes, including responses to abiotic stresses [43-45]. Results indicated that NaHS pretreatment managed to sustain NO content in levels similar to control, as supported by the non-significant modulation of NR relative expression compared with control samples. In contrast, strawberry plants experiencing heat shock treatment exhibited a marked increase in leaf NO content, despite similar NR expression levels with control samples. Such a negative correlation could be attributed to feedback inhibition of NR [46], possibly due to NO toxicity [47]. However, the increase in NO content in heat-stressed plants may be the key factor driving to the rapid accumulation of HSP transcripts observed. Xuan et al. [48] reported that NO acts upstream of AtCaM3 in thermotolerance for the stimulation of DNA-binding activity of heat shock transcription factors and the accumulation of HSPs. Interestingly, recent findings by Li et al. [32] suggested that H2S may be a downstream signal molecule in NO-induced heat tolerance of maize seedlings, further supporting the interplay between reactive species towards induced tolerance.
Beside their participation in oxidative processes that may lead to cell damage, ROS participate in redox state-based sensing mechanisms that are activated or amplified in response to environmental stimuli [49,50]. In parallel with ROS detoxification, ascorbate and glutathione are molecules with a regulatory role, since they participate in the redox signaling of the plant cell under abiotic stress conditions [51]. In recent years, several studies have strengthened the notion that high ASC/DHA and/or GSH/GSSG ratios, sustained by increased ASC and GSH or diminution of DHA and GSSG cellular production, may be the key element for enhanced tolerance during abiotic stress exposure [52]. In the current study, we showed that transient root exposure to H2S donor NaHS prior to stress exposure managed to sustain higher ratios of ASC/DHA and GSH/GSSG, compared with non-pretreated heat stressed plants, as evidenced by higher ASC and GSH redox states. Heat stress resulted in direct and progressive increase of reduced and oxidized glutathione and oxidized ascorbate. However, NaHS pretreatment prior to heat exposure resulted in an additional increase of reduced ascorbate and glutathione and the diminution of their oxidized forms. The induced expression of GDH and GCS provides support for these observations, since these enzymes contribute to ASC and GSH biosynthesis and redox homeostasis, respectively. Our results are in agreement with those of Shan et al. [53,54], who reported that the observed induced tolerance in NaHS-pretreated wheat seedlings under water and copper stress was attributed to the increased activity of enzymatic antioxidants and ASC (L-galactono-1,4-lactone dehydrogenase; GalLDH) and GSH biosynthesis (GCS) enzyme activities, as well as to the increased contents of ASC, GSH, total ascorbate and total glutathione, in comparison with untreated stressed seedlings.
Changes in genotypic expression leading to increase synthesis of heat shock proteins (HSPs) is known to be an early and important adaptive strategy in cells that are subjected to all types of stresses [55]. The HSPs, ranging in molecular mass from about 10 to 200 kDa, have been found to accumulate in great amounts during heat stress in various cellular structures, such as cell wall, chloroplasts, ribosomes and mitochondria [2,56]. Their role in maintaining cellular homeostasis under heat stress, mainly by assisting the correct folding of stress-accumulated misfolded proteins, preventing their aggregation and promoting proteolytic degradation of misfolded or denatured proteins, as well as in participating in signal transduction, has been recently reviewed [57]. In the current study, HSPs appeared to be substantially accumulated in cells immediately after heat stress exposure, since HSP70, HSP80 and HSP90 expression levels were found to be significantly induced, compared with control samples, as early as after 1 h of exposure to 42°C. The increased expression levels of the examined HSPs may be attributed to the rapid accumulation of H2O2 and NO, which function as signaling molecules for the production of HSPs, as also previously reported [37,38,48]. Induction of HSPs in stressed plants was lowered after prolonged exposure to heat treatment. Our results are in agreement with previously reported findings, based on transcriptomic and proteomic analyses, highlighting the importance of the early accumulation of HSPs for the acquisition of thermotolerance in plants experiencing temperature extremes [57,58]. The role of HSP70 in Arabidopsis thaliana heat shock responses and thermotolerance has recently been elucidated [59]. On the contrary, NaHS treatment prior to heat stress induced the up-regulation of HSPs only after 4 h of exposure to heat shock conditions, providing evidence for their possible contribution to the observed mitigation of heat stress devastating effects, made apparent after 4 h of exposure to 42°C. The lower H2O2 and NO contents during the early stages of heat exposure in NaHS-pretreated plants can be attributed to late accumulation of HSPs (after 4 h of stress imposition), since both active molecules function in parallel with HSPs biosynthesis. In turn, acclimation of Aloe vera plants to less severe temperature extremes resulted in elevated expression of HSPs[60].
The molecular and functional characterization of aquaporins (PIPs), a class of membrane proteins that facilitate water diffusion across cell membranes, has revealed the significance of their regulation in response to adverse environmental stimuli [61,62]. In plants, aquaporins are localized in abundance in the plasmalemma and the vacuolar membrane [63]. Recent studies shed light to the possible role of aquaporins in abiotic stress tolerance. Ayadi et al. [62] confirmed the role of PIP1 and PIP2 in osmotic and salinity stress tolerance in durum wheat. Furthermore, Iglesias-Acosta et al. [64] reported a decline in PIP1 and PIP2 transcript abundance in the roots of broccoli plants under increasing temperature, while Chen and Arora [65] highlighted the role of aquaporins (SoPIP2;1 and SoδTIP) during the recovery of spinach leaves from reversible freeze-thaw injury. In the current study, gene expression analysis revealed that PIP had the same expression pattern as HSPs, suggesting that heat exposure caused the early induction (after 1 h) of PIP expression, which was eliminated after prolonged heat stress. On the other hand, NaHS pretreatment-induced PIP up-regulation was apparent after 4 h exposure at 42°C (see Additional file 1: Table S1).
Conclusion
A coordinated, transient induction of antioxidants, HSPs and aquaporin gene expression was registered when plants were exposed to heat shock treatment, which was de-escalated as stress imposition progressed. The early (1 h) and transient up-regulation of defense-related genes in plants exposed directly to heat stress conditions seems to provide inadequate signal for transcriptional regulation of defense pathways, leading to weak heat stress responses. On the contrary, NaHS root priming demonstrated a ‘delayed’ (after 4 h) but prolonged (maximized after 8 h) transcriptional activation of defense responses, resulting in acquired thermotolerance via the sufficient production of protective molecules such as HSPs and antioxidants. The energy-consuming coordinated orchestration of several independent pathways is most likely feasible through increased photosynthetic capacity in NaHS-treated plants [66]. Overall, data reported herein provide novel information for the improvement of crop tolerance to heat stress and lends additional support to the suggested role of H2S in plant responses to environmental stimuli. The current state of knowledge in defining the contribution of H2S in plant tolerance mechanisms to abiotic stress warrants further investigation, including the potential application of synthetic inhibitors of H2S biosynthesis (e.g [67]).
Methods
Plant growth and stress treatments
Forty-eight strawberry (cv. ‘Camarosa’) plants were grown in peat in greenhouse for six months and subsequently transferred and grown hydroponically in continuously aerated half-strength Hoagland nutrient solution in a growth room with 16 h photoperiod (250 μmol m-2 s-1), 23°C/20°C day/night temperature and 65% relative humidity. After one week, roots of one half of the plants were incubated in deionized water containing an H2S donor, sodium hydrosulfide (NaHS; 100 μM for 48 h; changed every 12 h). At the end of the incubation period, plants were transferred to half-strength Hoagland nutrient solution. As a result, plants either pretreated or not with NaHS and grown hydroponically in continuously aerated half-strength Hoagland nutrient solution were simultaneously exposed (0 h, stress imposition) or not to elevated temperature treatment (42°C) for 8 h. Overall, strawberry plants were subjected to 4 treatments, as presented in detail in Figure 1 and described schematically in Additional file 2: Figure S1. Each treatment was independently run in triplicate, and each replicate consisted of 4 individual plants. Fully expanded leaves were sampled immediately after the imposition of heat stress treatment (0 h) and after 1, 4 and 8 h of exposure to 42°C. Leaves were flash-frozen in liquid nitrogen and stored at -80°C, unless otherwise stated.
Physiological and biochemical measurements
The ratio of variable fluorescence to maximum fluorescence (Fv/Fm), representing the maximum photochemical efficiency of photosystem II (PSII), was determined using a portable chlorophyll fluorometer (OptiSci OS-30p Chlorophyll Fluorometer, Opti-Sciences Inc, USA). Leaves were incubated in dark for 1 h prior to measurements. The comparative rates of lipid peroxidation in strawberry leaves were assayed in terms of MDA content according to Heath and Packer [68].
Reactive species quantification
Nitric oxide content was indirectly assayed by measuring nitrite (NO2–), a stable and non-volatile breakdown product of NO reduction, via the Griess reaction, as described by Zhou et al. [69]. Leaf H2O2 content was assayed as described by Loreto and Velikova [70], while H2S content was determined following the methodology described by Nashef et al. [71]. Descriptions of all reactive species quantification protocols followed can be found in [28].
ASC and GSH content/redox state
Reduced ascorbate (ASC) and dehydroascorbate (oxidized ascorbate; DHA) were measured according to Foyer et al. [72]. Redox state of ascorbate was expressed as the ratio of ASC to total ascorbate (ASC/ASC + DHA). The levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were assayed as described by Griffith [73], while the glutathione redox state was expressed as the ratio of GSH to total glutathione (GSH/GSH + GSSG).
RNA isolation, cDNA synthesis and gene expression analysis
Total RNA from strawberry leaves was isolated following the protocol described by [74]. The integrity of total RNA was checked spectrophotometrically (A260/A280) using a NanoDrop Spectrophotometer ND-1000 (Labtech International Ltd, Rigmer, UK), followed by gel electrophoresis. For first strand cDNA synthesis, 1 μg of total RNA was reverse-transcribed using the Primescript 1st Strand Synthesis kit, according to manufacturer’s instructions (Takara Bio Inc., Japan). Quantitative real-time RT-PCR was performed in a final volume of 10 μl, containing 4 μl of ten-fold diluted first strand cDNA, 0.5 μl of each of the gene specific primers (10 pM) and 5 μl of KAPA SYBR® FAST qPCR supermix (Takara Bio Inc). The initial denaturation stage was at 95°C for 3 min, followed by 40 cycles of amplification (95°C for 30 s, Ta°C for 45 s, and 72°C for 45 s) and a final elongation stage at 72°C for 5 min. Gene amplification cycle was followed by a melting curve run, carrying out 61 cycles with 0.5°C increment between 65°C - 95°C. PCR reactions of each treatment were performed in triplicate with an iQ5 real-time PCR detection system (Bio-Rad Laboratories, Inc., California, USA). Fold change in RNA expression was estimated using threshold cycles. The housekeeping reference gene used was 18S (Ta = 46°C) [75]. The statistical analysis of qRT-PCR results (pairwise fixed reallocation randomization test) was performed using the REST software, according to Pfaffl et al. [76]. The list of gene-specific primers used is presented in Additional file 3: Table S2.
Statistical analysis
Statistical analysis was carried out using the software package SPSS v17.0 (SPSS Inc., Chicago, USA) and the comparison of averages of each treatment was based on the analysis of variance (One-Way ANOVA) according to Duncan’s multiple range test at significance level 5% (P ≤ 0.05).
Abbreviations
cAPX: Cytosolic ascorbate peroxidase; ASC: Reduced ascorbate; CAT: Catalase; DREB: Dehydration-responsive element binding factor; GCS: Glutamylcysteine synthetase; GDH: L-galactose dehydrogenase; GR: Glutathione reductase; GS: Glutathione synthetase; GSH: Reduced glutathione; H2S: Hydrogen sulfide; HSPs: Heat shock proteins; MnSOD: Manganese superoxide dismutase; NaHS: Sodium hydrosulfide; NO: Nitric oxide; NR: Nitrate reductase; PIP: Aquaporin; RNS: Reactive nitrogen species; ROS: Reactive oxygen species.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AC, GAM and VF designed the study, while VF also designed the oligonucleotide primers used herein. AC performed the experiments and took care of the plants. AC and PF carried out the laboratory work and data analysis. AC, GAM and VF wrote the manuscript and prepared the figures. All authors read and approved the manuscript.
Supplementary Material
Effects of H2S donor NaHS on the relative mRNA expression (fold change) of enzymatic antioxidants, heat shock proteins, aquaporins and enzymes involved in RNS biosynthesis, redox homeostasis and transcription regulation, in leaves of strawberry plants under non-stress and heat shock conditions compared with controls, as determined by qRT-PCR.
Schematic representation of the experimental design.
Oligonucleotides used as primers for real-time RT-PCR.
Contributor Information
Anastasis Christou, Email: anastasis.christou@ari.gov.cy.
Panagiota Filippou, Email: panagiota.filippou@cut.ac.cy.
George A Manganaris, Email: george.manganaris@cut.ac.cy.
Vasileios Fotopoulos, Email: vassilis.fotopoulos@cut.ac.cy.
Acknowledgements
The authors would like to thank Ms. Chrystalla Antoniou and Ms. Anna Mlynarczyk for excellent technical assistance, as well as Ms. Diane Abdulahad for language editing. This work was partly supported by Cyprus University of Technology Internal Grant EX032 to VF. The authors would also like to acknowledge support by the CUT Open Access Author Fund.
References
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61:199–223. [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev S, Kreslavski V, Klimov V, Los D, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- Wahid A, Close T. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plantarum. 2007;51:104–109. [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta. 2007;1767:414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Huang W-D, Liu Y-P, Pan Q-H. Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. J Integr Plant Biol. 2005;47:959–970. [Google Scholar]
- Tan W, Meng QW, Brestic M, Olsovska K, Yang X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol. 2011;168:2063–2071. doi: 10.1016/j.jplph.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Horvath I, Glatz A, Varvasovszki V, Balogh G, Kovacs E. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci U S A. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Saidi Y, Finka A, Goloubinoff P. Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol. 2011;190:556–565. doi: 10.1111/j.1469-8137.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- von Koskull-Doring P, Scharf K-D, Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007;12:452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ahn Y-J, Claussen K, Lynn Zimmerman J. Genotypic differences in the heat-shock response and thermotolerance in four potato cultivars. Plant Sci. 2004;166:901–911. [Google Scholar]
- Howarth CJ. In: Abiotic Stresses: Plant Resistance Through Breeding and Molecular Approaches. Ashraf M, Harris PJC, editor. New York: Howarth Press Inc; 2005. Genetic improvements of tolerance to high temperature; pp. 277–300. [Google Scholar]
- Sung D-Y, Kaplan F, Lee KJ, Guy CL. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003;8:179–187. doi: 10.1016/S1360-1385(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kaur R, Kaur N, Bhandhari K, Kaushal N, Gupta K, Bains TS, Nayyar H. Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol Plant. 2011;33:2091–2101. [Google Scholar]
- Larkindale J, Huang B. Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul. 2005;47:17–28. [Google Scholar]
- Wang L-J, Fan L, Loescher W, Duan W, Liu G-J, Cheng J-S, Luo H-B, Li S-H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010;10:34. doi: 10.1186/1471-2229-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002;163:515–523. [Google Scholar]
- Wahid A, Perveen M, Gelani S, Basra SMA. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol. 2007;164:283–294. doi: 10.1016/j.jplph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Rausch T, Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci. 2005;10:503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Christou A, Manganaris GA. In: In Molecular Approaches in Plant Abiotic Stress. Gaur RK, Sharma P, editor. UK: CRC Press; 2013. Hydrogen sulfide as a potent regulator of plant responses to abiotic stress factors; pp. 353–373. [Google Scholar]
- Li Z-G. Hydrogen sulfide: a multifunctional gaseous molecule in plants. Rus J Plant Phys. 2013;60:733–740. [Google Scholar]
- Zhang H, Tan Z-Q, Hu L-Y, Wang S-H, Luo J-P, Jones RL. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol. 2010;52:556–567. doi: 10.1111/j.1744-7909.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- Wang B-L, Shi L, Li Y-X, Zhang WH. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta. 2010;231:1301–1309. doi: 10.1007/s00425-010-1134-9. [DOI] [PubMed] [Google Scholar]
- Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang G. Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem. 2013;32:2234–2239. doi: 10.1002/etc.2309. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ye Y-K, Wang S-H, Luo J-P, Tang J, Ma D-F. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul. 2009;58:243–250. [Google Scholar]
- Zhang H, Jiao H, Jiang C-X, Wang S-H, Wei Z-J, Luo J-P, Jones R. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol Plant. 2010;32:849–857. [Google Scholar]
- Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot. 2013;64:1953–1966. doi: 10.1093/jxb/ert055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tang J, Liu X-P, Wang Y, Yu W, Peng W-Y, Fang F, Ma D-F, Wei Z-J, Hu L-Y. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol. 2009;51:1086–1094. doi: 10.1111/j.1744-7909.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010;188:977–984. doi: 10.1111/j.1469-8137.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- Li Z-G, Gong M, Xie H, Yang L, Li J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012;185–186:185–189. doi: 10.1016/j.plantsci.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Li Z-G, Yang S-Z, Long W-B, Yang G-X, Shen Z-Z. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013;36:1564–1572. doi: 10.1111/pce.12092. [DOI] [PubMed] [Google Scholar]
- Li Z-G, Ding X-J, Du P-F. Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Phys. 2013;170:741–747. doi: 10.1016/j.jplph.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Kadir S, Sidhu G, Al-Khatib K. Strawberry (Fragaria x ananassa Duch.) growth and productivity as affected by temperature. HortSci. 2006;41:1423–1430. [Google Scholar]
- Rowland JG, Pang X, Suzuki I, Murata N, Simon WJ, Slabas AR. Identification of components associated with thermal acclimation of photosystem II in Synechocystis sp. PCC6803. PLoS One. 2010;5:e10511. doi: 10.1371/journal.pone.0010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Chow WS, Sun L, Li C, Peng C. Acclimation of photosystem II to high temperature in two Wedelia species from different geographical origins: implications for biological invasions upon global warming. J Exp Bot. 2010;61:4087–4096. doi: 10.1093/jxb/erq220. [DOI] [PubMed] [Google Scholar]
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 2010;152:1471–1483. doi: 10.1104/pp.109.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov R, Panchuk I, Mullineaux P, Schöffl F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol. 2006;61:733–746. doi: 10.1007/s11103-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Li L, Wang Y, Shen W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. BioMetals. 2012;25:617–631. doi: 10.1007/s10534-012-9551-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Cui W, Xu S, Shen W, Wang R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil. 2012;351:107–119. [Google Scholar]
- Filippou P, Antoniou C, Fotopoulos V. Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal Behav. 2011;6:270–277. doi: 10.4161/psb.6.2.14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ. Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants. 2013;5:052. doi: 10.1093/aobpla/pls052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasimowicz M, Floryszak-Wieczorek J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007;172:876–887. [Google Scholar]
- Molassiotis A, Fotopoulos V. Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signal Behav. 2011;6:210–214. doi: 10.4161/psb.6.2.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippou P, Bouchagier P, Skotti E, Fotopoulos V. Proline and reactive oxygen/nitrogen species metabolism is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ Exp Bot. 2014;97:1–10. [Google Scholar]
- Antoniou C, Filippou P, Mylona P, Fasoula D, Ioannides I, Polidoros A, Fotopoulos V. Developmental stage and concentration-specific sodium nitroprusside application results in nitrate reductase regulation and the modification of nitrate metabolism in leaves of Medicago truncatula plants. Plant Signal Behav. 2013;8:e25479. doi: 10.4161/psb.25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD. Nitric oxide signaling in plants. Vitam Horm. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- Xuan Y, Zhou S, Wang L, Cheng Y, Zhao L. Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 2010;153:1895–1906. doi: 10.1104/pp.110.160424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos V, Ziogas V, Tanou G, Molassiotis A. In: Ascorbate-Glutathione Pathway and Stress Tolerance in Plants. Anjum NA, Chan M-T, Umar S, editor. Netherlands: Springer; 2010. Involvement of AsA/DHA and GSH/GSSG Ratios in Gene and Protein Expression and in the Activation of Defence Mechanisms Under Abiotic Stress Conditions; pp. 265–302. [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Potters G, Horemans N, Jansen MAK. The cellular redox state in plant stress biology - a charging concept. Plant Physiol Biochem. 2010;48:292–300. doi: 10.1016/j.plaphy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Szalai G, Kellős T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul. 2009;28:66–80. [Google Scholar]
- Shan C-J, Zhang S-L, Li D-F, Zhao Y-Z, Tian X-L, Zhao X-L, Wu Y-X, Wei X-Y, Liu R-Q. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol Plant. 2011;33:2533–2540. [Google Scholar]
- Shan C, Dai H, Sun Y. Hydrogen sulfide protects wheat seedlings against copper stress by regulating the ascorbate and glutathione metabolism in leaves. Aust J Crop Sci. 2012;6:248–254. [Google Scholar]
- Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Yang KA, Lim CJ, Hong JK, Park CY, Cheong YH, Chung WS, Lee KO, Lee SY, Cho MJ, Lim CO. Identification of cell wall genes modified by a permissive high temperature in Chinese cabbage. Plant Sci. 2006;171:175–182. [Google Scholar]
- Rampino P, Mita G, Pataleo S, De Pascali M, Di Fonzo N, Perrotta C. Acquisition of thermotolerance and HSP gene expression in durum wheat (Triticum durum Desf.) cultivars. Environ Exp Bot. 2009;66:257–264. [Google Scholar]
- Xu C, Huang B. Root proteomic responses to heat stress in two Agrostis grass species contrasting in heat tolerance. J Exp Bot. 2008;59:4183–4194. doi: 10.1093/jxb/ern258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-X, Wang C, Yang C-Y, Wang J-Y, Chen L, Bao X-M, Zhao Y-X, Zhang H, Liu J. The role of Arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010;62:539–548. doi: 10.1111/j.1365-313X.2010.04173.x. [DOI] [PubMed] [Google Scholar]
- Huerta C, Freire M, Cardemil L. Expression of hsp70, hsp100 and ubiquitin in Aloe barbadensis Miller under direct heat stress and under temperature acclimation conditions. Plant Cell Rep. 2013;32:293–307. doi: 10.1007/s00299-012-1363-4. [DOI] [PubMed] [Google Scholar]
- Luu D-T, Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 2005;28:85–96. [Google Scholar]
- Ayadi M, Cavez D, Miled N, Chaumont F, Masmoudi K. Identification and characterization of two plasma membrane aquaporins in durum wheat (Triticum turgidum L. subsp. durum) and their role in abiotic stress tolerance. Plant Physiol Biochem. 2011;49:1029–1039. doi: 10.1016/j.plaphy.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P. The role of aquaporins in cellular and whole plant water balance. Biomembranes. 2000;1465:324–342. doi: 10.1016/s0005-2736(00)00147-4. [DOI] [PubMed] [Google Scholar]
- Iglesias-Acosta M, Martínez-Ballesta MC, Teruel JA, Carvajal M. The response of broccoli plants to high temperature and possible role of root aquaporins. Environ Exp Bot. 2010;68:83–90. [Google Scholar]
- Chen K, Arora R. Understanding the cellular mechanism of recovery from freeze–thaw injury in spinach: possible role of aquaporins, heat shock proteins, dehydrin and antioxidant system. Physiol Plant. (in press), DOI: 10.1111/ppl.12090. [DOI] [PubMed]
- Chen J, Wu F-H, Wang W-H, Zheng C-J, Lin G-H, Dong X-J, He J-X, Pei Z-M, Zheng H-L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot. 2011;62:4481–4493. doi: 10.1093/jxb/err145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Wang L, Liu J, Hou L, Liu X. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J Integr Plant Biol. 2013;55:277–289. doi: 10.1111/jipb.12004. [DOI] [PubMed] [Google Scholar]
- Heath R, Packer L. Photooxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Zhou B, Guo Z, Xing J, Huang B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot. 2005;56:3223–3228. doi: 10.1093/jxb/eri319. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Nashef AS, Osuga DT, Feeney RE. Determination of hydrogen sulfide with 5,5β’-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem. 1977;79:394–405. doi: 10.1016/0003-2697(77)90413-4. [DOI] [PubMed] [Google Scholar]
- Foyer C, Rowell J, Walker D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Griffith O. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Christou A, Georgiadou E, Filippou P, Manganaris GA, Fotopoulos V. Establishment of a rapid, inexpensive protocol for the extraction of high quality RNA from small amounts of strawberry plant tissues and other recalcitrant fruit crops. Gene. 2014;537:169–173. doi: 10.1016/j.gene.2013.11.066. [DOI] [PubMed] [Google Scholar]
- Bustamante CA, Rosli HG, Añón MC, Civello PM, Martinez GA. β-xylosidase in strawberry fruit: isolation of a full-length gene and analysis of its expression and enzymatic activity in cultivars with contrasting firmness. Plant Sci. 2006;171:497–504. doi: 10.1016/j.plantsci.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of H2S donor NaHS on the relative mRNA expression (fold change) of enzymatic antioxidants, heat shock proteins, aquaporins and enzymes involved in RNS biosynthesis, redox homeostasis and transcription regulation, in leaves of strawberry plants under non-stress and heat shock conditions compared with controls, as determined by qRT-PCR.
Schematic representation of the experimental design.
Oligonucleotides used as primers for real-time RT-PCR.