Abstract
Objective. To identify modifiable cardio-metabolic and lifestyle risk factors among indigenous populations from Australia (Aboriginal Australians/Torres Strait Islanders), New Zealand (Māori), and the United States (American Indians and Alaska Natives) that contribute to cardiovascular disease (CVD). Methods. National health surveys were identified where available. Electronic databases identified sources for filling missing data. The most relevant data were identified, organized, and synthesized. Results. Compared to their non-indigenous counterparts, indigenous populations exhibit lower life expectancies and a greater prevalence of CVD. All indigenous populations have higher rates of obesity and diabetes, hypertension is greater for Māori and Aboriginal Australians, and high cholesterol is greater only among American Indians/Alaska Natives. In turn, all indigenous groups exhibit higher rates of smoking and dangerous alcohol behaviour as well as consuming less fruits and vegetables. Aboriginal Australians and American Indians/Alaska Natives also exhibit greater rates of sedentary behaviour. Conclusion. Indigenous groups from Australia, New Zealand, and the United States have a lower life expectancy then their respective non-indigenous counterparts. A higher prevalence of CVD is a major driving force behind this discrepancy. A cluster of modifiable cardio-metabolic risk factors precede CVD, which, in turn, is linked to modifiable lifestyle risk factors.
1. Introduction
Cardiovascular disease (CVD) is considered the primary influencing factor in the life expectancy discrepancy between indigenous and nonindigenous groups in many countries [1]. Preceding CVD, many groups exhibit a cluster of cardiometabolic risk factors, which, in turn, is linked with a number of modifiable lifestyle risk factors (Figure 1). Multiple studies have revealed that modifiable risk factors are responsible for a large number of premature deaths due to CVD [2, 3]. Recently, it was reported that the single largest risk factor for cardiovascular mortality in the US was high blood pressure, directly responsible for 45% of all CVD deaths, closely followed by obesity, physical inactivity, high cholesterol, and smoking [2]. Fortunately, many of these metabolic and lifestyle risk factors are modifiable and relatively simple to monitor.
Figure 1.
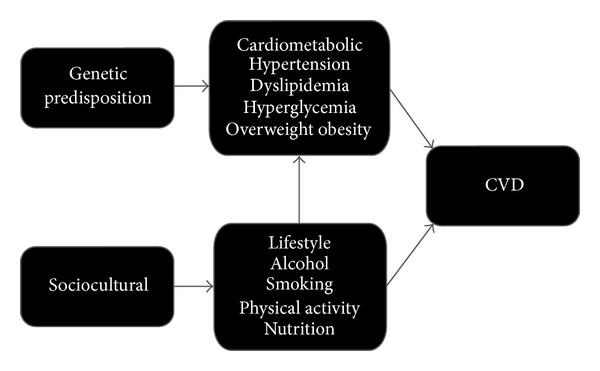
Causation pathway for cardiovascular disease (CVD) [4].
The current review will focus on known modifiable cardio-metabolic (overweight obesity, diabetes, high cholesterol, and high blood pressure; see Table 1) and common lifestyle (physical inactivity, poor nutrition, dangerous alcohol behaviour, and cigarette smoking; see Table 2) risk factors among indigenous populations from Australia, New Zealand, and the United States. Comparisons will be made with nonindigenous groups, and discussion will focus on the association of lifestyle factors and cardiovascular-metabolic conditions. Recommendations will be provided for measuring and tracking each of these risk factors.
Table 1.
Prevalence of cardio-metabolic risk factors among adults.
| Group | Population | Life expect. yrs |
CVD | Body weight |
High cholest. % |
HT | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| million | % | Prev. % |
Mortality % |
Over % |
Obese % |
Diabetes % |
% | ||||
| AU | 20.8 | 100 | 81 | 17 | 9 | 29 | 22 | 4 | 7 | 10 | |
| White AU | 20.3 | 98 | 81 | 17 | 9 | 29 | 22 | 4 | 7 | 10 | [13, 114, 115] |
| Indigenous AU | 0.52 | 2.5 | 62 | 22 | 27 | 35 | 27 | 12 | 6 | 15 | |
| NZ | 4.03 | 100 | 80 | 5 | 31 | 36 | 27 | 5 | 8 | 14 | |
| White NZ | 2.61 | 68 | 81 | 4 | 32 | 32 | 24 | 4 | 8 | 13 | [6, 14, 16, 26, 116] |
| Indigenous NZ | 0.57 | 15 | 73 | 7 | 32 | 32 | 42 | 8 | 9 | 17 | |
| U.S. | 309 | 100 | 78 | 21 | 34 | 33 | 33 | 8 | 16 | 34 | |
| White U.S. | 309 | 100 | 78 | 21 | 34 | 33 | 31 | 6 | 17 | 33 | [7, 17, 19, 54, 107] |
| Indigenous U.S. | 5.22 | 1.7 | 75 | 23 | 25 | 28 | 42 | 15 | 31 | 30 | |
CVD: cardiovascular disease; HT: Hypertension.
Notes: a body mass index (BMI) ≥ 25.0 kg/m2 is considered overweight, ≥30.0 kg/m2 is considered obese.
AU: CVD, cholesterol, diabetes (includes high sugar levels), and body weight data are self-reported and age-adjusted for adults ≥ 18 yrs [13].
NZ: diabetes = physician diagnosed; high cholesterol: individuals medicated for high total cholesterol [26]; HT = currently taking prescribed blood pressure medication [26]; HT, cholesterol, diabetes, and body weight data are for adults aged ≥ 15 [26]; CVD data are age-adjusted for adults ≥18 [14, 16].
Table 2.
Prevalence of modifiable lifestyle risk factors.
| Group | Activity behaviour | Nutrition | Alcohol behaviour | Smokers % |
References | |||
|---|---|---|---|---|---|---|---|---|
| Sedentary % |
Prescribed % |
Veg. % ≥2 day |
Fruit % ≥2 day |
Any % |
Risky % |
|||
| AU | 33 | 33 | 78 | 52 | 83 | 14 | 21 | |
| White AU | 33 | 33 | 78 | 52 | 83 | 14 | 21 | [13] |
| Indigenous AU | 51 | 21 | 43 | 26 | 49 | 15 | 46 | |
| New Zealand | 15 | 51 | 64 | 60 | 85 | 13 | 20 | |
| White NZ | 14 | 51 | 67 | 63 | 90 | 12 | 19 | [26, 81] |
| Indigenous NZ | 14 | 51 | 62 | 56 | 85 | 24 | 38 | |
| U.S. | 39 | 11 | 23 | 55 | 7 | 21 | ||
| White U.S. | 37 | 12 | N/A | 60 | 8 | 22 | [10, 17, 82, 107] | |
| Indigenous U.S. | 40 | 10 | 17 | 48 | 12 | 24 | ||
AU: sedentary activity behavior = <50 mins/week, moderate: >800 mins/week, for adults ≥15 yrs [13]; risky alcohol behavior = ≥ 5 standard drinks/day for males (or ≥15/week) and ≥4 for females (or ≥8/week) for adults aged ≥ 18 yrs [13]; smoker: any type of tobacco consumption [13]; smoking, activity and nutrition data are age-adjusted for adults ≥18 yrs [13].
NZ: prescribed activity behavior = recommended ≥30 mins/day most days or at least 150 mins/week, sedentary activity behavior = <30 mins/week [26]; risky alcohol behavior = weekly binge (≥6 standard drinks on one occasion for males and ≥4 for females) drinking, age-adjusted for adults aged 16–64 [81]; smoker = cigarette smoking [26]; vegetable = ≥3 servings/day [26]; smoking, activity and nutrition date are age-adjusted for adults aged 16–64 [26].
US: prescribed activity behavior = ≥30 mins/day most days or at least 150 mins/week (self-reported, ≥18 yrs), age-adjusted for adults aged ≥18 yrs [107]; nutrition = ≥5 servings/day of vegetables/fruit, age-adjusted for adults aged ≥18 yrs [10]; risky alcohol behaviour = ≥ 5 standard drinks/day on ≥5 days in past 30 days [82]; smoking = cigarette smoking, age-adjusted for adults aged ≥20 [107].
2. Methods
2.1. Data Sources
Electronic databases included PubMed, Medline, and Google Scholar. All titles were exported to Endnote and checked for duplicates.
2.2. Study Inclusion and Exclusion Criteria
National health surveys were identified where available. Electronic databases identified sources for filling missing data. Criteria for inclusion of articles included (a) published in a peer-reviewed English-language journal or government report; (b) contained data from nonindigenous cohorts for use as a comparison group; (c) cited in health science, nursing, medical, or exercise science literature. The largest sample studies containing data for CVD prevalence and mortality published between 2002 and July 2012 were selected to compare data of CVD and lifestyle risk factors and conditions (Tables 1 and 2).
2.3. Data Extraction and Data Synthesis
Search terms included Aboriginal Australians, Māori, American Indians/Alaska Natives, indigenous, cardiovascular disease, heart disease, overweight, obesity, diabetes, cholesterol, blood pressure, hypertension, alcohol, physical activity, exercise, nutrition, cigarette smoking, and tobacco.
3. Demographics
3.1. Australia
For the purpose of this paper, the term indigenous Australian refers to those of Aboriginal origin and Torres Strait Islanders. The indigenous population is estimated to be 2.5% of the total Australian population, approximately 90% of which self identify as Aboriginal, 6% as Torres Strait Islander, and 4% both mixed [5]. The indigenous population is relatively young, with a median age of 20.5 years compared to 36.6 years for the nonindigenous population [5]. Around 26% of indigenous people live in remote areas, compared with only 2% nonindigenous peoples [5], and many continue to maintain a strong connection to their traditional culture, language, and lands.
3.2. New Zealand
For the purpose of this paper, the term indigenous New Zealander encompasses those of Māori descent. Māori people comprise 15% of the total population [6], with the predominance (84.4%) residing in urban areas [6]. The Māori population has a median age of 22.7 years compared to 35.9 years for the nonindigenous population [6]. Māori culture continues to be an important thread of New Zealand society with 23.7% of Māori being able to hold a conversation in te Reo Māori [6].
3.3. United States
For the purpose of this paper, the term indigenous for a person from the United States encompasses American Indians and Alaska Natives. There are approximately 5.2 million reported indigenous Americans in the United States, representing 1.7% of the population, including those of more than one race [7]. The indigenous population is younger than the nonindigenous population (30.3 years compared to (cf.) 36.6 years, resp., [8]), and is varied with 566 federally recognized tribes [9]. These tribal groups often have different histories, unique languages, and varied cultural traditions, reside in numerous geographic regions, and show various degrees of societal assimilation [10]. In 2010, the majority (78%) of indigenous Americans lived outside of native reservation areas [11].
3.4. Cardiovascular Disease
CVD covers all diseases and conditions of the heart and blood vessels. Coronary heart disease (CHD), stroke, heart failure, and peripheral vascular disease contribute approximately 30% to the CVD burden in developed countries [12]. In 2001, CVD was the primary cause of death worldwide, with indigenous peoples leading the way [1].
3.4.1. Australia
CVD is the principal cause of death among all ethnic groups in Australia [13]. The 2004-05 National Aboriginal and Torres Strait Islander Health Survey (NATSIHS, the largest health survey of Indigenous Australians, [13]) found age-adjusted rates for CVD that were 30% higher among the indigenous population (22%) compared to the nonindigenous (17%) population, with CVD mortality three times higher (27% cf. 9%, resp.). Indigenous Australians show a marked increase in the prevalence of CVD from around 35 years of age onwards, some 10 years earlier than in the nonindigenous population.
3.4.2. New Zealand
Across all ethnic groups, CVD mortality peaked between 1966 and 70, and since then, death rates have fallen by over 60% in all age and sex groups [14, 15]. However, the decline has been slower among indigenous New Zealanders. Between 1981 and 2004, CVD mortality rates decreased by 43% among indigenous New Zealanders compared to 65% among nonindigenous New Zealanders [14, 15]. Indigenous New Zealanders continue to have a higher prevalence of CVD compared to their nonindigenous counterparts (7% cf. 4%, resp., [16]). Chan et al. [16] found that CVD prevalence begins to rise after age 35 years among all age groups, but the rise in prevalence rate is greater among indigenous New Zealanders.
3.4.3. United States
Among all ethnicities, CVD accounted for 34% of all deaths in 2010 [17], 33% of which occurred before the age of 75 years—well before the average life expectancy of 78 years. According to the National Center for Health Statistics (NCHS) [18], if all forms of major CVD were eliminated, life expectancy would rise by almost 7 years. Despite a lower life expectancy and a slightly higher prevalence of CVD among indigenous versus nonindigenous Americans (23% cf. 21%), CVD mortality is lower (25% cf. 34%) [17]. These discrepant findings may be partially explained by higher rates of mortality among indigenous Americans for tuberculosis (600% higher), alcoholism (510% higher), motor vehicle crashes (229% higher), diabetes (189% higher), unintentional injuries (152% higher), homicide (61% higher), and suicide (62% higher) [19].
4. Modifiable Cardiometabolic Metabolic Risk Factors
4.1. Obesity
Excess body fat increases the risk of developing a range of health problems, including high blood pressure, diabetes mellitus, and CVD [20–22]. According to results from the Framingham Heart Study [20], age-adjusted relative risk for CVD is increased for overweight and obese men (21% and 46%, resp.) and women (20% and 64%, resp.) when compared with normal weight individuals. Population studies, including those retrieved for the current paper, typically estimate the proportion of people that are obese by calculating an individual's body mass index (BMI). BMI is based on the assumption that the ratio between body mass and height provides an indication of body fatness; however, this often discriminates against individuals (and/or populations) that have a higher proportion of muscle mass. Alternatively, waist circumference, waist-to-height ratio, and waist-to-hip ratio (WHR) take into consideration body-fat distribution, especially central (abdominal) obesity [23]. A recent study compared the predictive power of BMI, waist circumference, waist-to-height ratio, and WHR for diabetes mellitus, hypertension, and dyslipidemia in Australian Aboriginal and Torres Strait Islander adults [24]. WHR was found to have the greatest predictive power. A WHR of ≥0.90 and ≥0.80, for males and, females respectively, is considered optimal. However, studies over the past two decades indicate that the rate of risk for a given WHR differs between ethnic groups; therefore these reference values should not be used to ascertain absolute risk [25].
4.1.1. Australia
After adjusting for age differences between the two populations, the 2004/05 NATSIHS [13] reported that indigenous Australians are 1.2 times more likely to be overweight/obese than nonindigenous Australians (62% cf. 51%). In each age group, the disparity between indigenous and nonindigenous groups was greater for females than for males and was more pronounced within remote geographical areas.
4.1.2. New Zealand
The 2006/07 New Zealand National Health Survey (NZNHS) [26] reported that 36% of adults were overweight and a further 27% were obese. The obesity burden is particularly prevalent among indigenous New Zealanders, with 42% of this population being obese compared to 24% of their white counterparts [26]. However, there was no significant increase reported for either ethnic group between 2002/3 and 2006/07.
4.1.3. United States
An estimated 144,100,000 people or 66% of the total US adult population is overweight or obese [17]. The rates of overweight/obesity are comparable between indigenous and white Americans. However, while indigenous Americans are less likely to be overweight (28%) than white Americans (33%) they are more likely to be obese (42% cf. 31%, resp.). These findings have been corroborated by other studies [10].
4.2. Diabetes
Diabetes mellitus is a group of metabolic diseases in which hyperglycaemia results from defective insulin secretion, insulin action, or both [27, 28]. There are several forms of diabetes mellitus, each with a different cause and clinical history. The two most prominent forms are type 1 and type 2 diabetes, which differ according to their underlying pathophysiology, with type 1 often attributed to an autoimmune response and type 2 often related to several lifestyle factor. type 2 diabetes accounts for 90–95% of diabetes cases and is a major risk factor for CVD [17, 29–34]. A meta-analysis [34], encompassing 6,573 subjects found that type 2 diabetes resulted in greater CVD mortality risk (RR 3.42, 95% CI: 2.23 to 5.23) than hypertension (RR 1.57, 95% CI: 1.10 to 2.24) or hypercholesteremia (RR 1.49, 95% CI: 1.05 to 2.10). In turn, diabetes is modified by lifestyle factors, including physical inactivity and poor nutrition, in addition to any genetic predisposition and the natural ageing process [35–39]. Diabetes mellitus risk can be monitored by measuring glucose tolerance or fasting blood glucose, where a fasting blood glucose of <100 mg/dL is considered optimal [40].
4.2.1. Australia
The 2007/08 Australian National Health Survey [41] reported that an estimated 4% of the total population had diagnosed diabetes. According to the 2004/05 NATSIHS [13], the age-standardized prevalence of diabetes/elevated blood glucose (i.e., prediabetes) among indigenous Australians was 3.4 times the rate of that observed in nonindigenous people (12% cf. 4%). The prevalence of diabetes among indigenous Australians increases rapidly after 35 years of age, rising from 10% at age 35–44 years to 32% at age 55 years and over. By contrast, the prevalence increases from 2% to 12% for nonindigenous people. Prevalence rates among indigenous Australians are similar between genders, but those in remote areas are almost twice as likely to have diabetes [13].
4.2.2. New Zealand
The 2006/07 NZNHS [26] reported 5% of the total population was diagnosed with diabetes. Indigenous New Zealanders were twice as likely to be diagnosed with diabetes (8% cf. 4%, resp.). There has been no significant change in diabetes prevalence between 1996/97 and 2006/07 for both indigenous and nonindigenous populations or for males and females [26].
4.2.3. United States
In 2006, an estimated 17,200,000 Americans had diagnosed diabetes, representing 8% of the adult population [17]. A further estimated 6,100,000 had undiagnosed diabetes and 29% had prediabetes with abnormal fasting glucose levels. Diabetes was once rare among indigenous Americans, but the prevalence is rising dramatically with rates almost twice as high when compared with their non-Hispanic white counterparts (15% cf. 8%, resp.) [17].
4.3. Cholesterol
The two most common blood lipids are cholesterol and triglycerides. These two blood fats are carried on particles called lipoproteins, the most important of which are low density lipoprotein (LDL) and high density lipoprotein (HDL). Both carry cholesterol, but high levels of LDL cholesterol have been shown to be atherogenic [42–44]. Similarly, low levels of HDL cholesterol are associated with increased CHD morbidity and mortality [45–47]. High HDL cholesterol levels conversely convey reduced risk [45–48]. In population studies, serum total cholesterol is often used as a surrogate for LDL cholesterol levels; however, LDL concentrations confers more predictive value. The best way to determine the true prevalence of high cholesterol in the community is through blood samples [26]. An LDL cholesterol level <100 mg/dL is considered optimal [49].
4.3.1. Australia
The 2004/05 NATSIHS [13] found the prevalence of high age-adjusted serum total cholesterol levels to be similar for indigenous (6%) and nonindigenous (7%) groups. The same report found the prevalence of high total cholesterol levels to drastically increase with age, starting at 4% for both indigenous and nonindigenous groups when aged 35–44 years, rising to 18% and 20%, respectively, aged 55 years or over.
4.3.2. New Zealand
The 2006/07 NZNHS [26] reported that 8% of the total adult (≥15 years) population were currently taking medication for high cholesterol. Men (8%) were significantly more likely than women (6%) to be taking medication for high cholesterol, when standardized for age. Age-standardized cholesterol levels are similar between indigenous and nonindigenous groups. However, the lack of differences in cholesterol between populations may be misleading as the NZNHS data only details the number of individuals on medication for high cholesterol, therefore excluding those who remain undiagnosed.
4.3.3. United States
In 2006, an estimated 102,200,000 people or 47% the total US adult population (≥20) had total cholesterol levels above ≥200 mg/dL, with an estimated 16% registering a cholesterol level ≥240 mg/dL [17]. The prevalence of high total cholesterol (≥240 mg/dL) is substantially greater for indigenous Americans compared to nonindigenous Americans (31% cf. 17%) [17].
4.4. Hypertension
Hypertension is a major risk factor for CVD. For every 20 mmHg systolic or 10 mmHg diastolic increase in resting blood pressure there is a twofold increase in risk of death from ischemic heart disease or stroke [50]. Hypertension is associated with shorter overall life expectancy and earlier onset of CVD [51]. According to the WHO, hypertension is likely the leading risk factor for death worldwide [52]. In part, this is because hypertension is common and because management of hypertension is suboptimal [53]. An ideal blood pressure is one with a systolic pressure <120 mmHg and a diastolic pressure <80 mmHg [40].
4.4.1. Australia
In 2004/05, hypertension was the most commonly reported CVD condition among indigenous Australians, with prevalence rates 50% greater than for nonindigenous Australians when adjusted for age (10% cf. 15%, resp.) [13]. Hypertension is of particular concern to remote indigenous groups with an overall prevalence of 6% among urban dwellers versus 10% for remote dwellers. For both ethnic groups prevalence increases dramatically with age, from 12 to 43% for indigenous and 4–33% for nonindigenous groups between the ages 35–44 years and 55+ years, respectively.
4.4.2. New Zealand
In 2006/07 one in seven adults (14%) reported that they were currently taking medication for high blood pressure [26]. After adjusting for age, indigenous New Zealanders were 26% more likely to have elevated blood pressure than the general population.
4.4.3. United States
Data from the 2006 National Health and Nutrition Examination Survey (NHANES) [54] indicate that 34% of US adults over 20 years have hypertension. Rates of hypertension are slightly lower among indigenous (30%) than nonindigenous Americans (33%) [54].
5. Modifiable Lifestyle Risk Factors
5.1. Nutrition
Poor dietary habits affect multiple cardiovascular risk factors including blood pressure, cholesterol levels, glucose levels, and obesity [55–61]. A diet high in nutrient-rich (vitamins, minerals, antioxidants and fibre) fruits and vegetables can reduce the risk for many leading causes of death [17, 55–57, 62]. In meta-analyses of prospective cohort studies, each daily serving of fruits or vegetables was associated with a 4% lower risk of CHD (RR 0.96, 95% CI: 0.93 to 0.99) and a 5% lower risk of stroke (RR: 0.95, 95% CI 0.92 to 0.97) [56, 57]. Five or more daily servings of fruits and vegetables are recommended for optimal nutrition [17, 62]. Direct observation is considered the “gold standard” for monitoring dietary intake [63–65]. However, this approach can be time consuming and impractical for use in large-scale studies. Alternatively, a food frequency questionnaire (FFQ), including the freely available National Cancer Institute Diet History Questionnaire (http://riskfactor.cancer.gov/dhq2/) [66, 67], allows for assessment of the usual patterns of food intake over an extended period of time [68, 69] and is considerably less burdensome in both time and cost than other measurement tools [70, 71].
5.1.1. Australia
In 2004/05, 78% of the total Australian population consumed two or more servings of vegetables per day compared with just 43% of the indigenous population [13]. Similarly, 53% of the total Australian population consumed two or more servings of fruit compared with just 26% of the indigenous population. Rates of consumption were also reportedly much lower for indigenous groups living in remote locations, wherein 20% reported no daily fruit intake and 15% no daily vegetable intake.
5.1.2. New Zealand
In 2006/07, two out of every three adults (64%) consumed the recommended three or more servings of vegetables each day, and 60% consumed the recommended two or more servings of fruits each day [26]. Consumption of the recommended servings of vegetables and fruits was higher for the nonindigenous (67% and 63%, resp.) than the indigenous population (62% and 56%, resp.).
5.1.3. United States
Daily consumption of fruits and vegetables is poor across the general US population, with one recent study [10] estimating that only 23% of the population consume five or more servings of fruits and vegetables per day, with an even lower rate of consumption among the indigenous population (18%). These data are consistent with other nutritional studies in indigenous communities [72–76].
5.2. Alcohol
Accumulating scientific evidence indicates that light to moderate alcohol consumption may significantly reduce the risk of CVD and all-cause mortality [77–79]. However, excessive alcohol intake is toxic to both the heart and overall health [77–79]. In particular, binge drinking, even among otherwise light drinkers, increases cardiovascular events and mortality [77–79]. Alcohol should not be universally prescribed for health enhancement owing to the lack of randomised outcome data and the potential for developing irresponsible drinking habits. The American Heart Association warns those who have never consumed alcohol against initiating such behaviours due to the inability to predict the potential for alcohol abuse [80]. Alcohol intake can be monitored using a food frequency survey (FFQ) (see above).
5.2.1. Australia
The 2004/05 NATSIHS [13] reported a lower prevalence of alcohol consumption among indigenous (49%) than nonindigenous (83%) Australians. However, after standardizing for age, indigenous Australians are just as likely as nonindigenous Australians (15% cf. 14%, resp.) to consume higher than recommended daily intakes of alcohol (≥5 standard drinks/day for males and ≥4 standard drinks/day for females). Increased alcohol consumption was reported to be greatest among remote indigenous Australians (19%). For both groups, the prevalence of high alcohol consumption had risen by 3% since the previous (2001) NATSIHS.
5.2.2. New Zealand
In 2007/08, 85% of the New Zealand population reported that they had consumed alcohol in the past year, with a slightly higher rate among the nonindigenous (90%) than indigenous (85%) population [81]. However, the indigenous population were twice as likely (24% cf. 12%, resp.) to exhibit excessive alcohol consumption behaviours (males: ≥6 standard drinks on one occasion; females: ≥4 standard drinks on one occasion).
5.2.3. United States
In 2006/07, a greater portion of the non-Hispanic white population had consumed alcohol in the past month compared to the indigenous population (55% cf. 60%, resp.) [82]. However, a greater proportion of the indigenous population consumed higher than the recommended levels of alcohol consumption (≥5 standard drinks/day for ≥5 days in the past 30 days) than the nonindigenous population (12% cf. 8%, resp.). It is important to note, however, that tribal diversity has been reported for alcohol drinking tendency [83, 84]. Beals et al. [84] compared two culturally and geographically distinct tribes and found that current drinking rates were higher for a Northern Plains tribe than for a Southwest tribe. Gender differences have also been demonstrated, with May and Gossage [85] reporting higher levels of binge drinking among Northern tribe males than females (3 days cf. 1.3 days of drinking ≥5 standard drinks in the past 30 days, resp.).
5.3. Physical Activity
It has been estimated that physical inactivity is responsible for 12% of the global burden of myocardial infarction [86]. Regular physical activity reduces CVD risk in its own right and also improves CVD risk factors such as obesity, hypertension, dyslipidemia, and type 2 diabetes [87–92]. The American College of Sports Medicine (ACSM) recommends at least 30 minutes of moderate-intensity physical activity (e.g., walking briskly, dancing, swimming, and bicycling) at least 5 days a week [93]. A number of tools have been developed to measure physical activity, ranging from objective measures, such as accelerometry, to subjective questionnaires [94]. While questionnaires are prone to technical error, they are inexpensive and practical for use in population studies and can provide information about physical activity type and context [94]. The International Physical Activity Questionnaire (IPAQ) (http://www.ipaq.ki.se/ipaq.htm) is a freely available, cross-national monitoring tool which has been validated for use in adults [95–99] and children [100–103].
5.3.1. Australia
In 2004-05 an estimated one in three people (33%) in Australia were sedentary, with an even higher rate (51%) among the indigenous population [13]. Physical inactivity contributes an estimated 7% of Australia's disease burden and 10% of all deaths [104] and accounts for 12% of the health gap between indigenous and nonindigenous Australians [105]. Despite the publicized importance of physical activity levels have remained stagnant in recent National Health Surveys [5, 13].
5.3.2. New Zealand
In 2006/07, half of all adults reported that they met physical activity guidelines (≥30 mins/day on most days), with 15% of all adults identified as sedentary [26]. No significant differences were reported between indigenous and nonindigenous populations. For both ethnic groups, men were more likely to be physically active than women (55% cf. 48%, resp.), and for both groups physical activity levels remained constant between 2002/03 and 2006/07 national surveys. However, while similar physical activity levels have been reported for indigenous and nonindigenous groups, large-scale studies using validated instruments are limited. The national surveys collect physical activity data using a New Zealand version of the IPAQ. Only one study has tested the validity of the NZPAQ and reported poorer accuracy when used on Māori and Pasifika compared to European/other [106].
5.3.3. United States
The 2007 US National Health Interview Survey [107] estimated that 39% of all adults were sedentary, with a higher prevalence among women (41%) versus men (37%). Compared to the nonindigenous population, the indigenous population had a higher rate of sedentary behaviour (40% cf. 37%, resp.) and a lower rate of meeting prescribed physical activity (30 min/day on most days) (10% cf. 12%, resp.). These data agree with other studies showing lower rates of physical activity among indigenous Americans compared to the general population [10, 108–113].
5.4. Tobacco Use
Cigarette smoking, which is estimated to kill five million people worldwide each year [117], has been established as a risk factor for CVD since the 1940s [118]. The relationship between smoking and CVD is resultant upon the interaction of multiple mechanisms which contribute to atherosclerosis, vascular injury, vascular dysfunction, and thrombosis, although these precise mechanisms are largely unknown [119]. Cigarette smoking increases the incidence of CVD in a dose-dependent manner [120–122], with even occasional smoking increasing the risk of CVD [123]. Conversely, long-term prospective studies have demonstrated considerable mortality risk reduction with smoking cessation [124–126].
5.4.1. Australia
In 2004/05, an estimated one in five people (21%) in Australia were smoking cigarettes daily [13]. Among the indigenous population, smoking rates are higher for those living in remote (52%) versus nonremote (49%) areas and particularly for males living in remote areas (58% for males cf. 47% for females). For both ethnic groups, daily smoking prevalence declined between 2001 and 2004/05, decreasing from 49% to 46% and from 22% to 21% among indigenous and nonindigenous populations, respectively.
5.4.2. New Zealand
In 2006/07, 20% of the New Zealand population were current cigarette smokers, with a prevalence rate that is twice as high among the indigenous (38%, age adjusted) compared to the nonindigenous population [26]. After adjusting for age, indigenous women were more than twice as likely to be smokers than women in the total population, while indigenous men were 1.5 times more likely to smoke than men in the total population. The prevalence of smoking decreased from 23% in 2002/03 to 19% in 2006/07 among the total population and from 47% to 38% among the indigenous population.
5.4.3. United States
Between 2000 and 2004, cigarette smoking resulted in an estimated 443,000 premature deaths in the US each year [127]. In adults aged 35 years or over, 33% of these deaths were related to CVD. From 1965 to 2007, smoking in the US declined by 50% among people aged 18 years or over [128]. However, despite this progress, in 2008 an estimated 21% of the total US population were current cigarette smokers [107]. The prevalence of cigarette smoking was higher among indigenous Americans at 23%, increasing to 31% when mixed race indigenous Americans were included. There also appear to be notable differences between tribes [10, 129, 130].
6. Discussion
There are more than 370 million indigenous people in 70 countries worldwide. Indigenous peoples are not monolithic; there is significant variation between and within peoples in terms of worldview, political forces, education, socioeconomic status, living conditions, and familial factors. However, many indigenous groups do share a striking commonality, a discrepancy in life expectancy when compared to their nonindigenous counterparts. Three such examples can be seen in the life expectancy of indigenous groups in Australia, New Zealand, and America. A higher prevalence of CVD may be considered the driving force behind this discrepancy [1], which is being fuelled by lifestyle and subsequent cardiometabolic risk factors.
The indigenous populations from each of these nations exhibit a cluster of cardiometabolic conditions. Compared to their respective nonindigenous counterparts, all three indigenous groups have higher rates of obesity and diabetes, hypertension is greater for the indigenous populations of New Zealand and Australia, and high cholesterol is greater among indigenous groups in the United States. While each of these conditions has independently been shown to accelerate CVD [131–134], the effects are also thought to be additive [34]. Poor lifestyle choices may precede and contribute to these cardiometabolic outcomes. Compared to their nonindigenous counterparts, all three indigenous groups exhibit higher rates of smoking and dangerous alcohol behaviour, as well as lower consumption of fruits and vegetables. The indigenous groups of Australia and the United States also exhibit greater rates of sedentary behaviour, while there remains a need to collect valid physical activity data in New Zealand [106].
Holistic strategies, which recognize the complex interactions between lifestyle factors, may assist in promoting positive changes. For example, a recent systematic review and meta-analysis [135] reported that physical activity interventions have had only a small effect on children's overall activity levels. This implies that lifestyle strategies to promote physical activity should be sensitive to total daily physical activity as well as other lifestyle factors, including nutrition and sleep behaviour, each of which may be influenced by increased physical activity levels and may affect cardiometabolic outcomes [136, 137].
In order to maximize potential positive outcomes, strategies which aim to promote positive changes in lifestyle should not only be physiologically appropriate; they should also be sensitive to sociocultural norms. For example, within Australia an indigenous person's connections to family, ancestors, the wider community, and the land are very important to the choices they make about all aspects of their lives [138]. Exercising alone for personal benefit may prevent a person from spending time with family and loved ones, and this may be seen as superficial. Similarly, the Māori people of New Zealand show a decided preference for physical activities which involve whanaungatanga/kotahitanga (a team environment), a forum to experience feelings of whanau (extended family) [139]. In this regard an argument can be made that appropriate physical activity prescription can be used as a vehicle to experience, discover, and reconnect to indigenous cultural heritage [140]. However, it must also be recognized that sociocultural norms may substantially differ by group, including within a given nation. For example, in the United States there are 566 federally recognized tribes [9], with different histories, unique languages, varied cultural traditions, and various degrees of societal assimilation [10].
Even within a given nation, strategies to promote lifestyle changes must be specific to a group, not to the population as a whole, especially when a geographical area includes different language and culturally distinct groups [141]. Diversity competence involves knowledge, skills, and abilities that enable a researcher to deal with a specific population. The National Standards for Culturally and Linguistically Appropriate Services in Health and Health Care (the National CLAS Standards) [142] in the United States intend to advance health equity, improve quality, and help eliminate health care disparities by providing a blueprint for individuals and health and health care organizations to implement culturally and linguistically appropriate services. Adoption of these standards is highly recommended for health care providers interested in respecting such diversity competences.
7. Limitations
To ensure reliable comparisons between ethnic groups, the largest data sources available were utilized for a given cohort (nation), where available. Wherever large data sets were not available, reliable sets of relevant data were used to consolidate thought and formulate a comprehensive picture. All data used in the current publication spanned from 2002 to 2012. This would have introduced selection and methodological and historical bias, limiting our ability to make accurate comparisons across nations. Furthermore, the preponderance of the literature on the health of indigenous populations is focused on describing or understanding problems [143], rather than on testing the effectiveness of potential solutions. Further studies are required to determine causality between lifestyle and cardiometabolic risk factors and to determine whether causality is moderated by ethnicity.
8. Implications
This review has described the relationship between common lifestyle choices, cardiometabolic conditions (i.e., lifestyle-related disease), and CVD. It is evident that disparities in CVD prevalence, mortality, and associated risk factors exist between indigenous and nonindigenous populations. Causality, however, has yet to be conclusively determined and is essential if we are to develop effective solutions for decreasing disease burden in a number of groups. While the described model will assist future research focusing on indigenous health outcomes, it must also be recognised that such research must be sensitive to differences in culture between indigenous groups within a country, in addition to being sensitive to national cultural norms.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Huffman MD, Galloway JM. Cardiovascular health in indigenous communities: successful programs. Heart Lung and Circulation. 2010;19(5-6):351–360. doi: 10.1016/j.hlc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle and metabolic risk factors. PLoS Medicine. 2009;6(4) doi: 10.1371/journal.pmed.1000058.e1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Stoner L, Shultz SP, Lambrick DM, Krebs J, Weatherall M, Palmer BR. The combating obesity in Māori and Pasifika adolescent school-children study: COMPASS methodology and study protocol. International Journal of Preventive Medicine. 2013;4(5):67–81. [PMC free article] [PubMed] [Google Scholar]
- 5.AIHW. Australian Institute of Health and Welfare. Canberra, Australia: 2006. Australia's health 2006. [Google Scholar]
- 6.SNZ. Census 2006. 2007, http://www.stats.govt.nz/Census/2006CensusHomePage.aspx.
- 7.USCB. Census data 2010. 2011, http://2010.census.gov/2010census/data/index.php.
- 8.USCB. U.S. Hispanic Population Surpasses 45 Million Now 15 Percent of Total. 2008, http://www.census.gov/newsroom/releases/archives/population/cb08-67.html.
- 9.Affairs UDoII. Tribal directory. 2013.
- 10.Holm JE, Vogeltanz-Holm N, Poltavski D, McDonald L. Assessing health status, behavioral risks, and health disparities in American Indians living on the northern plains of the U.S. Public Health Reports. 2010;125(1):68–78. doi: 10.1177/003335491012500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010. Washington, DC, USA: US Census Bureau; 2010. [Google Scholar]
- 12.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112(23):3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 13.ABS. National Aboriginal and Torres Strait Islander Health Survey, 2004-05. Canberra, Australia: Australian Bureau of Statistics; 2006. [Google Scholar]
- 14.MOH. Mortality and Demographic Data 2004. Wellington, New Zealand: Ministry of Health; 2007. [Google Scholar]
- 15.Tobias M, Sexton K, Mann S, Sharpe N. How low can it go? projecting ischaemic heart disease mortality in New Zealand to 2015. New Zealand Medical Journal. 2006;119(1232) [PubMed] [Google Scholar]
- 16.Chan WC, Wright C, Riddell T, et al. Ethnic and socioeconomic disparities in the prevalence of cardiovascular disease in New Zealand. New Zealand Medical Journal. 2008;121(1285):11–20. [PubMed] [Google Scholar]
- 17.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G. Heart disease and stroke statistics-2010 update: a report from the American heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RN. United States Life Tables Eliminating Certain Causes of Death. Vol. 1. National Center for Health Statistics: Hyattsville, Md, USA; 1999. U.S. decennial life tables for 1989–91. [Google Scholar]
- 19.IHS. Facts on Indian Health Disparities. Rockville, Md, USA: Indian Health Service; 2006. [Google Scholar]
- 20.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the framingham experience. Archives of Internal Medicine. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 21.Chrostowska M, Szyndler A, Paczwa P, Narkiewicz K. Impact of abdominal obesity on the frequency of hypertension and cardiovascular disease in Poland—results from the IDEA study (international day for the evaluation of abdominal obesity) Blood Pressure. 2011;20(3):145–152. doi: 10.3109/08037051.2010.538996. [DOI] [PubMed] [Google Scholar]
- 22.Balkau B, Deanfield JE, Despres JP, et al. International day for the evaluation of abdominal obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116(17):1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality-fat or fiction? Nature Reviews Cardiology. 2011;8(4):233–237. doi: 10.1038/nrcardio.2010.209. [DOI] [PubMed] [Google Scholar]
- 24.Li M, McDermott RA. Using anthropometric indices to predict cardio-metabolic risk factors in Australian indigenous populations. Diabetes Research and Clinical Practice. 2010;87(3):401–406. doi: 10.1016/j.diabres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. European Journal of Clinical Nutrition. 2010;64(1):42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- 26.MOH. A Potrait of Health: Key Results of the 2006-07 New Zealand Health Survey. Wellington, New Zealand: Ministry of Health; 2008. [Google Scholar]
- 27.WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 28.ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Supplement 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. Journal of the American College of Cardiology. 2010;55(13):1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 31.Mellbin LG, Anselmino M, Ryden L. Diabetes, prediabetes and cardiovascular risk. European Journal of Cardiovascular Prevention and Rehabilitation. 2010;17(Supplement 1):S9–S14. doi: 10.1097/01.hjr.0000368192.24732.2f. [DOI] [PubMed] [Google Scholar]
- 32.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus and the framingham heart study. Trends in Cardiovascular Medicine. 2010;20(3):90–95. doi: 10.1016/j.tcm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the framingham heart study. Circulation. 2009;120(3):212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagami T, Qiao Q, Tuomilehto J, et al. Screen-detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: the DECODA study. European Journal of Cardiovascular Prevention and Rehabilitation. 2006;13(4):555–561. doi: 10.1097/01.hjr.0000183916.28354.69. [DOI] [PubMed] [Google Scholar]
- 35.Rajpathak SN, Aggarwal V, Hu FB. Multifactorial intervention to reduce cardiovascular events in type 2 diabetes. Current Diabetes Reports. 2010;10(1):16–23. doi: 10.1007/s11892-009-0084-8. [DOI] [PubMed] [Google Scholar]
- 36.Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34(5):1228–1237. doi: 10.2337/dc10-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salazar MR, Carbajal HA, Espeche WG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diabetes and Vascular Disease Research. 2011;8(2):109–116. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 38.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. Journal of Clinical Hypertension. 2011;13(4):238–243. doi: 10.1111/j.1751-7176.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowden DW, Cox AJ, Freedman BI, et al. Review of the diabetes heart study (DHS) family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. The Review of Diabetic Studies. 2010;7(3):188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Journal of the American Medical Association. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 41.ABS. National Health Survey: Summary of Results, 2007-2008. Canberra, Australia: Australian Bureau of Statistics; 2009. [Google Scholar]
- 42.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: a population-based study. Atherosclerosis. 2006;189(2):369–374. doi: 10.1016/j.atherosclerosis.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clinic Proceedings. 2009;84(4):345–352. doi: 10.1016/S0025-6196(11)60544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teramoto T, Nakaya N, Yokoyama S, Ohashi Y, Mizuno K, Nakamura H. Association between lowering low-density lipoprotein cholesterol with pravastatin and primary prevention of cardiovascular disease in mild to moderate hypercholesterolemic Japanese. Journal of Atherosclerosis and Thrombosis. 2010;17(8):879–887. doi: 10.5551/jat.4176. [DOI] [PubMed] [Google Scholar]
- 45.De Freitas EV, Brandao AA, Pozzan R, et al. Importance of high-density lipoprotein-cholesterol (HDL-C) levels to the incidence of cardiovascular disease (CVD) in the elderly. Archives of Gerontology and Geriatrics. 2011;52(2):217–222. doi: 10.1016/j.archger.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Windler E, Schoffauer M, Zyriax BC. The significance of low HDL-cholesterol levels in an ageing society at increased risk for cardiovascular disease. Diabetes and Vascular Disease Research. 2007;4(2):136–142. doi: 10.3132/dvdr.2007.032. [DOI] [PubMed] [Google Scholar]
- 47.Briel M, Ferreira-Gonzalez I, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. The British Medical Journal. 2009;338(7693) doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson MH. Targeting high-density lipoprotein cholesterol in the management of cardiovascular disease. The American Heart Hospital Journal. 2007;5(4):210–216. doi: 10.1111/j.1541-9215.2007.07423.x. [DOI] [PubMed] [Google Scholar]
- 49.Stone NJ, Bilek S, Rosenbaum S. Recent national cholesterol education program adult treatment panel III update: adjustments and options. The American Journal of Cardiology. 2005;96(4, Supplement):53–59. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 51.Franco OH, Peeters A, Bonneux L, De Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46(2):280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 52.WHO. World Health Organization. Geneva, Switzerland: 2002. The world health report 2002. [Google Scholar]
- 53.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. part 1: estimates of blood pressure levels. Journal of Hypertension. 2006;24(3):413–422. doi: 10.1097/01.hjh.0000199801.72563.6f. [DOI] [PubMed] [Google Scholar]
- 54.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2012 update: a report from the American heart association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. Journal of Human Hypertension. 2007;21(9):717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 56.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. Journal of Nutrition. 2006;136(10):2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 57.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology. 2005;65(8):1193–1197. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- 58.Nishida C, Uauy R. WHO scientific update on health consequences of trans fatty acids: introduction. European Journal of Clinical Nutrition. 2009;63(2):S1–S4. doi: 10.1038/ejcn.2009.13. [DOI] [PubMed] [Google Scholar]
- 59.Mitrou PN, Kipnis V, Thiebaut AC, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP diet and health study. Archives of Internal Medicine. 2007;167(22):2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 60.Keast DR, O'Neil CE, Jones JM. Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults: national health and nutrition examination survey, 1999–2004. Nutrition Research. 2011;31(6):460–467. doi: 10.1016/j.nutres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Agostoni C, Braegger C, Decsi T, et al. Role of dietary factors and food habits in the development of childhood obesity: a commentary by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology and Nutrition. 2011;52(6):662–669. doi: 10.1097/MPG.0b013e3182169253. [DOI] [PubMed] [Google Scholar]
- 62.CDC. State-specific trends in fruit and vegetable consumption among adults—United States, 2000–2009. Morbidity and Mortality Weekly Report. 2010;59(35):1125–11130. [PubMed] [Google Scholar]
- 63.Frank GC. Taking a bite out of eating behavior: food records and food recalls of children. The Journal of School Health. 1991;61(5):198–200. doi: 10.1111/j.1746-1561.1991.tb06010.x. [DOI] [PubMed] [Google Scholar]
- 64.Mertz W. Food intake measurements: is there a “gold standard”? Journal of the American Dietetic Association. 1992;92(12):1463–1465. [PubMed] [Google Scholar]
- 65.Block G. A review of validations of dietary assessment methods. The American Journal of Epidemiology. 1982;115(4):492–505. doi: 10.1093/oxfordjournals.aje.a113331. [DOI] [PubMed] [Google Scholar]
- 66.Flood A, Subar AF, Hull SG, Zimmerman TP, Jenkins DJ, Schatzkin A. Methodology for adding glycemic load values to the national cancer institute diet history questionnaire database. Journal of the American Dietetic Association. 2006;106(3):393–402. doi: 10.1016/j.jada.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The national cancer institute diet history questionnaire: validation of pyramid food servings. American Journal of Epidemiology. 2006;163(3):279–288. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- 68.Subar AF. Developing dietary assessment tools. Journal of the American Dietetic Association. 2004;104(5):769–770. doi: 10.1016/j.jada.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Carithers TC, Talegawkar SA, Rowser ML, et al. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson heart study. Journal of the American Dietetic Association. 2009;109(7):1184–1193. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kushi LH. Gaps in epidemiologic research methods: design considerations for studies that use food-frequency questionnaires. The American Journal of Clinical Nutrition. 1994;59(Supplement 1):180S–184S. doi: 10.1093/ajcn/59.1.180S. [DOI] [PubMed] [Google Scholar]
- 71.Willett W. Nutritional Epidemiology. 2nd edition. New York, NY, USA: Oxford University Press; 1998. [Google Scholar]
- 72.Harnack L, Sherwood N, Story M. Diet and physical activity patterns of urban American Indian women. American Journal of Health Promotion. 1999;13(4):233–236. doi: 10.4278/0890-1171-13.4.233. [DOI] [PubMed] [Google Scholar]
- 73.Harnack L, Story M, Rock BH. Diet and physical activity patterns of Lakota Indian adults. Journal of the American Dietetic Association. 1999;99(7):829–835. doi: 10.1016/S0002-8223(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 74.DeGonzague B, Receveur O, Wedll D, Kuhnlein HV. Dietary intake and body mass index of adults in 2 Ojibwe communities. Journal of the American Dietetic Association. 1999;99(6):710–716. doi: 10.1016/S0002-8223(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 75.Jones SE, Anderson K, Lowry R, Conner H. Risks to health among American Indian/Alaska native high school students in the United States. Preventing Chronic Disease. 2011;8(4, article A76) [PMC free article] [PubMed] [Google Scholar]
- 76.LaRowe TL, Adams AK, Jobe JB, Cronin KA, Vannatter SM, Prince RJ. Dietary intakes and physical activity among preschool-aged children living in rural American Indian communities before a family-based healthy lifestyle intervention. Journal of the American Dietetic Association. 2010;110(7):1049–1057. doi: 10.1016/j.jada.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. The British Medical Journal. 2011;342, article d671 doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95(10):1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 79.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health. the razor-sharp double-edged sword. Journal of the American College of Cardiology. 2007;50(11):1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 80.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: research challenges and opportunities. Journal of the American College of Cardiology. 2005;45(12):1916–1924. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 81.MOH. Alcohol Use in New Zealand: Key Results of the 2007/08 New Zealand Alcohol and Drug Use Survey. Wellington, New Zealand: Ministry of Health; 2009. [Google Scholar]
- 82.SAMHSA. 2007 National survey on drug use and health, detailed tables, dependence, abuse and treatment, table 2.46B. 2008, http://oas.samhsa.gov/NSDUH/2k7NSDUH/tabs/Sect2peTabs43to84.htm#Tab2.
- 83.Szlemko WJ, Wood JW, Thurman PJ. Native Americans and alcohol: past, present, and future. Journal of General Psychology. 2006;133(4):435–451. doi: 10.3200/GENP.133.4.435-451. [DOI] [PubMed] [Google Scholar]
- 84.Beals J, Spicer P, Mitchell CM, et al. Racial disparities in alcohol use: comparison of 2 American Indian reservation populations with national data. American Journal of Public Health. 2003;93(10):1683–1685. doi: 10.2105/ajph.93.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.May PA, Gossage P. New data on the epidemiology of adult drinking and substance use among American Indians of the northern states: male and female data on prevalence, patterns and consequences. American Indian and Alaska Native Mental Health Research. 2001;10(2):1–26. doi: 10.5820/aian.1002.2001.1. [DOI] [PubMed] [Google Scholar]
- 86.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 87.Sattelmair J, Pertman J, Ding EL, Kohl HW, III, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mansikkaniemi K, Juonala M, Taimela S, Hirvensalo M, Telama R, Huupponen R. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. the cardiovascular risk in young finns study. Annals of Medicine. 2011;44(7):733–744. doi: 10.3109/07853890.2011.590146. [DOI] [PubMed] [Google Scholar]
- 89.Moholdt T, Wisloff U, Nilsen TI, Slordahl SA. Physical activity and mortality in men and women with coronary heart disease: a prospective population-based cohort study in Norway (the HUNT study) The European Journal of Cardiovascular Prevention and Rehabilitation. 2008;15(6):639–645. doi: 10.1097/HJR.0b013e3283101671. [DOI] [PubMed] [Google Scholar]
- 90.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Progress in Cardiovascular Diseases. 2011;53(6):412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Medicine. 2004;34(6):371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 92.Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. Journal of Clinical Hypertension. 2011;13(4):244–251. doi: 10.1111/j.1751-7176.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garber CE, Blissmer B, Deschenes MR, et al. American college of sports medicine position stand. quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and Science in Sports and Exercise. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 94.Dollman J, Okely AD, Hardy L, Timperio A, Salmon J, Hills AP. A hitchhiker’s guide to assessing young people’s physical activity: deciding what method to use. Journal of Science and Medicine in Sport. 2009;12(5):518–525. doi: 10.1016/j.jsams.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 95.Alomari MA, Keewan EF, Qhatan R, et al. Blood pressure and circulatory relationships with physical activity level in young normotensive individuals: IPAQ validity and reliability considerations. Clinical and Experimental Hypertension. 2011;33(5):345–353. doi: 10.3109/10641963.2010.531848. [DOI] [PubMed] [Google Scholar]
- 96.Bauman A, Ainsworth BE, Sallis JF, et al. The descriptive epidemiology of sitting a 20-country comparison using the international physical activity questionnaire (IPAQ) The American Journal of Preventive Medicine. 2011;41(2):228–235. doi: 10.1016/j.amepre.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Schembre SM, Riebe DA. Non-exercise estimation of VO(2)max using the international physical activity questionnaire. Measurement in Physical Education and Exercise Science. 2011;15(3):168–181. doi: 10.1080/1091367X.2011.568369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomioka K, Iwamoto J, Saeki K, Okamoto N. Reliability and validity of the international physical activity questionnaire (IPAQ) in elderly adults: the Fujiwara-kyo study. Journal of Epidemiology. 2011;21(6):459–465. doi: 10.2188/jea.JE20110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 100.Ottevaere C, Huybrechts I, De Bourdeaudhuij I, et al. Comparison of the IPAQ-A and actigraph in relation to VO2max among European adolescents: the HELENA study. Journal of Science and Medicine in Sport. 2011;14(4):317–324. doi: 10.1016/j.jsams.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Rangul V, Holmen TL, Kurtze N, Cuypers K, Midthjell K. Reliability and validity of two frequently used self-administered physical activity questionnaires in adolescents. BMC Medical Research Methodology. 2008;8, article 47 doi: 10.1186/1471-2288-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hagstromer M, Bergman P, De Bourdeaudhuij I, et al. Concurrent validity of a modified version of the international physical activity questionnaire (IPAQ-A) in European adolescents: the HELENA Study. International Journal of Obesity. 2008;32(5):S42–S48. doi: 10.1038/ijo.2008.182. [DOI] [PubMed] [Google Scholar]
- 103.Ottevaere C, Huybrechts I, De Meester F, De Bourdeaudhuij I, Cuenca-Garcia M, De Henauw S. The use of accelerometry in adolescents and its implementation with non-wear time activity diaries in free-living conditions. Journal of Sports Sciences. 2011;29(1):103–113. doi: 10.1080/02640414.2010.521169. [DOI] [PubMed] [Google Scholar]
- 104.AIHW. The Burden of Disease and Injury in Australia 2003. Canberra, New Zealand: Australian Institute of Health and Welfare (AIHW); 2007. [Google Scholar]
- 105.Vos T, Barker B, Begg S, Stanley L, Lopez AD. Burden of disease and injury in Aboriginal and Torres Strait Islander peoples: the indigenous health gap. International Journal of Epidemiology. 2009;38(2):470–477. doi: 10.1093/ije/dyn240. [DOI] [PubMed] [Google Scholar]
- 106.Moy KL, Scragg RK, McLean G, Carr H. The New Zealand physical activity questionnaires: validation by heart-rate monitoring in a multiethnic population. Journal of Physical Activity and Health. 2008;5(1):S45–S61. doi: 10.1123/jpah.5.s1.s45. [DOI] [PubMed] [Google Scholar]
- 107.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: national health interview survey, 2008. Vital and Health Statistics. 2009;10(242):1–157. [PubMed] [Google Scholar]
- 108.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethnicity and Disease. 2007;17(1):143–152. [PubMed] [Google Scholar]
- 109.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska natives-findings from the behavioral risk factor surveillance system, 2000–2006. Cancer. 2008;113(Supplement 5):1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 110.Denny CH, Holtzman D, Cobb N. Surveillance for health behaviors of American Indians and Alaska natives. findings from the behavioral risk factor surveillance system, 1997–2000. Morbidity and Mortality Weekly Report. 2003;52(7):1–13. [PubMed] [Google Scholar]
- 111.Hodge FS, Cantrell BG, Kim S. Health status and sociodemographic characteristics of the morbidly obese American Indians. Ethnicity and Disease. 2011;21(1):52–57. [PMC free article] [PubMed] [Google Scholar]
- 112.Duncan GE, Goldberg J, Buchwald D, Wen Y, Henderson JA. Epidemiology of physical activity in American Indians in the education and research towards health cohort. American Journal of Preventive Medicine. 2009;37(6):488–494. doi: 10.1016/j.amepre.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Storti KL, Arena VC, Barmada MM, et al. Physical activity levels in American-Indian adults. the strong heart family study. The American Journal of Preventive Medicine. 2009;37(6):481–487. doi: 10.1016/j.amepre.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.ABS. Population Distribution, Aboriginal and Torres Strait Islander Australians, 2006. Canberra, Australia: Australian Bureau of Statistics; 2007. [Google Scholar]
- 115.AIHW. Life expectancy. 2011, http://www.aihw.gov.au/life-expectancy/
- 116.SNZ. New Zealand life tables: 2005–07. 2008, http://www.stats.govt.nz/browse_for_stats/health/life_expectancy/nzlifetables_hotp05-07.aspx.
- 117.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3(11, article e442) doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. The British Medical Journal. 1997;315(7114):973–980. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 120.Conen D, Everett BM, Kurth T, et al. Smoking, smoking status, and risk for symptomatic peripheral artery disease in women. Annals of Internal Medicine. 2011;154(11):719–726. doi: 10.1059/0003-4819-154-11-201106070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee YH, Shin MH, Kweon SS, et al. Cumulative smoking exposure, duration of smoking cessation and peripheral arterial disease in middle-aged and older Korean men. BMC Public Health. 2011;11, article 94 doi: 10.1186/1471-2458-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tomiyama H, Hashimoto H, Tanaka H, et al. Continuous smoking and progression of arterial stiffening: a prospective study. Journal of the American College of Cardiology. 2010;55(18):1979–1987. doi: 10.1016/j.jacc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 123.Stoner L, Sabatier MJ, Black CD, McCully KK. Occasional cigarette smoking chronically affects arterial function. Ultrasound in Medicine and Biology. 2008;34(12):1885–1892. doi: 10.1016/j.ultrasmedbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 124.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. The British Medical Journal. 2004;328(7455):1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Archives of Internal Medicine. 2000;160(7):939–944. doi: 10.1001/archinte.160.7.939. [DOI] [PubMed] [Google Scholar]
- 126.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. Journal of the American Medical Association. 2003;290(1):86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 127.CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 128.NCHS. Health, United States, 2008 With Chartbook. Hyattsville, Md, USA: National Center for Health Statistics; 2009. [Google Scholar]
- 129.Eichner JE, Wang W, Zhang Y, Lee ET, Welty TK. Tobacco use and cardiovascular disease among American Indians: the strong heart study. International Journal of Environmental Research and Public Health. 2010;7(10):3816–3830. doi: 10.3390/ijerph7103816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith JJ, Ferucci ED, Dillard DA, Lanier AP. Tobacco use among Alaska native people in the EARTH study. Nicotine and Tobacco Research. 2010;12(8):839–844. doi: 10.1093/ntr/ntq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mattsson N, Ronnemaa T, Juonala M, et al. Arterial structure and function in young adults with the metabolic syndrome: the cardiovascular risk in young finns study. The European Heart Journal. 2008;29(6):784–791. doi: 10.1093/eurheartj/ehm576. [DOI] [PubMed] [Google Scholar]
- 132.Kerr SM, Livingstone MB, McCrorie TA, Wallace JM. Endothelial dysfunction associated with obesity and the effect of weight loss interventions. Proceedings of the Nutrition Society. 2011;70(4):418–425. doi: 10.1017/S0029665111001674. [DOI] [PubMed] [Google Scholar]
- 133.Palmieri V, Russo C, Pezzullo S, Di Minno MN, Celentano A. Relation of flow-mediated dilation to global arterial load: impact of hypertension and additional cardiovascular risk factors. International Journal of Cardiology. 2011;152(2):225–230. doi: 10.1016/j.ijcard.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 134.Bleda S, De Haro J, Varela C, Esparza L, Rodriguez J, Acin F. Improving total-cholesterol/HDL-cholesterol ratio results in an endothelial dysfunction recovery in peripheral artery disease patients. Cholesterol. 2012;2012:6 pages. doi: 10.1155/2012/895326.895326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Metcalf B, Henley W, Wilkin T. Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54) The British Medical Journal. 2012;345, article e5888 doi: 10.1136/bmj.e5888. [DOI] [PubMed] [Google Scholar]
- 136.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. Journal of Pediatrics. 2011;158(4):617–623. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.BHC. Aboriginal health—barriers to physical activity. 2012, http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Aboriginal_health_physical_activity.
- 139.Te Rito P. Leadership in Māori, European cultures and in the world of sport. MAI Review. 2006;1(8):1–19. [Google Scholar]
- 140.Bergin P. Maori sport and cultural identity in Australia. The Australian Journal of Anthropology. 2002;13(3):257–269. [Google Scholar]
- 141.Jamieson LM, Paradies YC, Eades S, Chong A, Maple-Brown L, Morris P. Ten principles relevant to health research among Indigenous Australian populations. The Medical Journal Australia. 2012;197(1):16–18. doi: 10.5694/mja11.11642. [DOI] [PubMed] [Google Scholar]
- 142.OMH. The national CLAS standards. 2013, http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15.
- 143.Paul CL, Sanson-Fisher R, Stewart J, Anderson AE. Being sorry is not enough. the sorry state of the evidence base for improving the health of indigenous populations. The American Journal of Preventive Medicine. 2010;38(5):566–568. doi: 10.1016/j.amepre.2010.02.001. [DOI] [PubMed] [Google Scholar]


