The presence of a high level of tumor-infiltrating lymphocytes in residual disease after neoadjuvant chemotherapy is associated with better prognosis in triple-negative breast cancer patients. This parameter may represent a new surrogate of drug efficacy in the neoadjuvant setting and a new risk stratification tool to select patients who may benefit from the inclusion in post-neoadjuvant trials.
Keywords: breast cancer, triple negative, neoadjuvant chemotherapy, tumor lymphocytes
Abstract
Background
There is a need to develop surrogates for treatment efficacy in the neoadjuvant setting to speed-up drug development and stratify patients according to outcome. Preclinical studies showed that chemotherapy induces an antitumor immune response. In order to develop new surrogates for drug efficacy, we assessed the prognostic value of tumor-infiltrating lymphocytes (TIL) on residual disease after neoadjuvant chemotherapy (NACT) in patients with triple-negative breast cancer (TNBC).
Patients and methods
Three hundred four TNBC patients with residual disease after NACT were retrospectively identified in three different hospitals. Hematoxylin and eosin-stained slides from surgical postchemotherapy specimens were evaluated for intratumoral (It-TIL) and stromal (Str-TIL) TIL. Cases were classified as High-TIL if It-TIL and/or Str-TIL >60%.
Results
TIL were assessable for 278 cases. Continuous It-TIL and Str-TIL variables were strong prognostic factors in the multivariate model, both for metastasis-free [hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.77–0.96, P = 0.01 and HR 0.85, 95% CI 0.75–0.98, P = 0.02 for Str-TIL and It-TIL, respectively] and overall survival (HR 0.86, 95% CI 0.77–0.97, P = 0.01 and HR 0.86, 95% CI 0.75–0.99, P = 0.03 for Str-TIL and It-TIL, respectively). The 5-year overall survival rate was 91% (95% CI 68% to 97%) for High-TIL patients (n = 27) and 55% (95% CI 48% to 61%) for Low-TIL patients (HR 0.19, 95% CI 0.06–0.61, log-rank P = 0.0017). The major prognostic impact of TIL was seen for patients with large tumor burden following NACT (residual tumor >2 cm and/or node metastasis). In all but one High-TIL case, It-TIL and Str-TIL values were lower on the prechemotherapy sample.
Conclusions
The presence of TIL in residual disease after NACT is associated with better prognosis in TNBC patients. This parameter may represent a new surrogate of drug efficacy to test investigational agents in the neoadjuvant setting and a new prognostic marker to select patients at high risk of relapse.
introduction
The development of surrogates for drug efficacy is becoming a major challenge in clinical research. Such surrogates could dramatically speed-up drug development by allowing quick selection of successful compounds, avoiding large adjuvant trials and identifying patients at very good outcome. In breast cancer, pathological complete response (pCR) and Ki67 drops have been proposed as surrogates for the efficacy of chemotherapy and endocrine therapy. The Food and Drug Administration recently proposed the use of pCR for drug approval, emphasizing the need for research in this field [1].
Preclinical studies have suggested that cytotoxic agents may partly exert their antitumor activity by inducing immune response against tumor cells [2, 3]. The immunogenic cell death induced by cytotoxic agents allows antigen cross-presentation, activation of dendritic cells and induction of tumor-specific cytotoxic T cells. A few studies on small breast cancer series have suggested that cytotoxic agents, including anthracyclines and taxanes, can induce tumor-specific immune response, and that exposure to such drugs could lead to the attraction of lymphocytes in the tumor bed [4, 5].
Triple-negative breast cancer (TNBC), which lacks the expression of hormone receptors and human epidermal growth factor receptor-2 (HER2) represents the breast cancer subtype with the poorest prognosis. TNBC patients are more likely to relapse within 3 years from diagnosis, with a higher risk of developing visceral disease [6]. Since no targeted drug is available so far, chemotherapy remains the backbone of treatment of this aggressive subtype and can be curative in a proportion of patients. The achievement of a pCR after neoadjuvant chemotherapy (NACT) is associated with good outcome and is now considered as a validated surrogate to develop new drugs [1, 7, 8]. Nevertheless, not all patients with residual disease will eventually relapse, meaning that the population of TNBC patients with residual disease includes a subgroup of patients with good prognosis. Therefore, pCR may not represent an optimal surrogate to evaluate drug efficacy since some patients with residual disease achieve long-term survival. Moreover, most of the drugs under development are not cytotoxics and pCR may not be a good surrogate for each of the new investigational drugs.
With the aim to identify a new potential tool for a better selection of high-risk patients eligible for postneoadjuvant investigational drugs and that could also represent a new surrogate for neoadjuvant drug efficacy for TNBC, we evaluated whether the magnitude of lymphocytic infiltration in the residual disease after NACT could allow identifying a population of TNBC patients at good outcome.
methods
patients
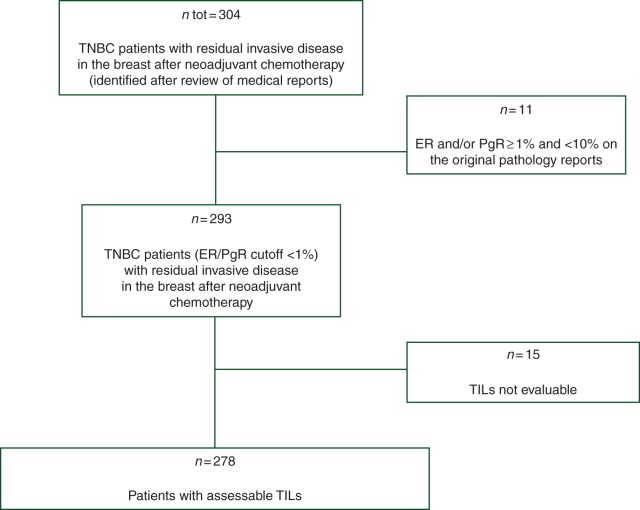
A retrospective series including 304 consecutive TNBC patients with invasive residual disease in the breast after NACT who were treated at three European Institutions (Institut Gustave Roussy, Villejuif, France; Modena University Hospital, Modena, Italy; Istituto Europeo di Oncologia, Milano, Italy) between 1993 and 2010 was included. Patients were first identified by reviewing the medical reports. After original pathology reports of the diagnostic core biopsies were reviewed, 11 patients whose tumors presented a hormone receptors expression by immunohistochemistry between 1% and 10% were excluded, since they did not meet the criteria for the American Society of Clinical Oncology/College of American Pathology definition of hormone receptor-negative status (<1%) (Figure 1) [9]. Clinico-pathological characteristics including histologic type, grade, hormone receptors, HER2, clinical stage at diagnosis, type of surgery and pathologic response were captured from the medical files at each institution and included in one unique dedicated database.
Figure 1.
Flowchart of the study. TNBC, triple-negative breast cancer; ER, estrogen receptor; PR, progesterone receptor; TILs, tumor-infiltrating lymphocytes.
The histological type was defined by using the World Health Organization's classification system. The histological grade was defined according to the Elston and Ellis classification.
The Reporting Recommendations for Tumor marker Prognostic Studies (REMARK) criteria were followed in reporting the results of this study.
pathology
Hematoxilyn and eosin-stained (HES) slides from surgical specimens for each case were retrieved from the respective Institutional Pathology Archives. The evaluation of the percentage of intratumoral (It) and stromal (Str) TILs was carried out according to criteria previously described and published by Denkert et al. [10]. It-TILs were defined as the percentage of tumor cell nests presenting intraepithelial mononuclear cells. Only those mononuclear cells that were within the epithelium or in contact with single-tumor cells were considered. Granulocyte infiltrates were excluded. Str-TILs were defined as the percentage of tumor stroma area that was occupied by mononuclear inflammatory cells. Inflammatory infiltrates in the stroma of noninvasive lesions and normal breast structures were excluded. For each case, all the slides containing residual invasive breast disease were evaluated.
Cases were defined as High-TIL if It-TIL and/or Str-TIL >60%, and as Low-TIL if It-TIL and Str-TIL ≤60%, adopting the same definition as Denkert's et al. since it was the one that was adopted in their seminal paper on prechemotherapy TIL predicting pCR [10]. These cutoff points were defined before any statistical analyses.
For those cases presenting a high lymphocytic infiltration after NACT, HES slides from diagnostic core biopsies before NACT were evaluated for TIL.
statistical analysis
Statistical analysis was carried out using the R project for statistical computing [11].
Two survival end points were evaluated: (i) metastases-free survival (MFS), defined as the time interval between surgery and date of distant relapse or death, and (ii) overall survival (OS), defined as the time interval between surgery and death. Patients who were alive (OS) or without distant relapse (MFS) were censored at the date of last contact. The prognostic value of lymphocytic infiltrate was first tested by considering It-TIL and Str-TIL separately as continuous variables, and then accordingly to the High-TIL versus Low-TIL categorical definition. Hazard ratios (HR) and 95% confidence interval (95% CI) were calculated with the Cox proportional hazard regression model. A multivariate Cox regression analysis including all those parameters that were significantly related with prognosis in the univariate analysis was conducted. The required assumptions of proportionality in the multivariate survival analysis were checked graphically and by Schoenfeld's test. Kaplan–Meier method was used to estimate MFS and OS curves and the log-rank test was used to compare between groups.
The association between TIL status and clinico-pathological variables was calculated using either χ2 tests with continuity correction or Fisher's exact test. Spearman method was used to calculate the correlation between Itr-TIL and Str-TIL. All the statistical tests were two sided, and considered significant when P ≤ 0.05.
results
clinico-pathological characteristics and association with TIL
Postchemotherapy tumor HES slides were evaluable for TIL for 278 of the 293 included patients (Figure 1). The reasons that impaired a correct TIL estimation in 15 cases were decolored staining and tissue artifacts. A significant correlation between It-TILs and Str-TILs was observed (Spearman correlation coefficient 0.72, supplementary Figure S1, available at Annals of Oncology online).
The characteristics of the 278 assessable patients are reported in Table 1. The majority of the patients had a ductal infiltrating carcinoma and a grade 3 tumor. There were no cases of medullary breast cancer diagnosed from core biopsy. Approximately half (48%) of the patients received an anthracycline-based neoadjuvant treatment and 45% an anthracycline/taxane-based neoadjuvant combination. Among those patients treated with anthracycline-based NACT, 36% received further taxane-based adjuvant treatment. Among all the patients who received anthracyclines and/or taxanes as neoadjuvant treatment for whom we had the information on the adjuvant treatment (n = 250), 81 (32%) received further anthracyclines and/or taxanes in the adjuvant setting and 169 (68%) did not. Overall, considering both neoadjuvant and adjuvant treatment, 62% of the patients received an anthracycline/taxane-based treatment of early breast cancer. The median number of NACT cycles was six (range 1–12). Fifty-eight percent of the patients underwent mastectomy, whereas the remaining received breast conservative surgery.
Table 1.
Clinico-pathological characteristics of the patients
| All, n (%) | Low-TIL, n (%) | High-TIL, n (%) | P value | |
|---|---|---|---|---|
| Total population | 278 (100) | 251 (90) | 27 (10) | |
| Age (years) | ||||
| ≥50 | 133 (48) | 121 (91) | 12 (9) | |
| <50 | 145 (52) | 130 (90) | 15 (10) | 0.87 |
| Clinical stage at diagnosis | ||||
| I–II | 138 (52) | 121 (88) | 17 (12) | |
| III | 129 (48) | 119 (92) | 10 (8) | 0.3 |
| Unknown | 1 | 1 | 0 | |
| Histological type | ||||
| Ductal | 253 (93) | 226 (89) | 27 (11) | |
| Lobular | 5 (2) | 5 (100) | 0 | |
| Other | 15 (5) | 15 (100) | 0 | 0.31 |
| Unknown | 5 | 5 | 0 | |
| Grade (at diagnosis) | ||||
| 1–2 | 36 (17) | 32 (89) | 4 (11) | |
| 3 | 176 (83) | 159 (90) | 17 (10) | 0.97 |
| Unknown | 66 | 60 | 6 | |
| Nodal status after neoadjuvant chemotherapy | ||||
| Neg | 128 (46) | 110 (86) | 18 (14) | |
| Pos | 148 (54) | 139 (94) | 9 (6) | 0.04 |
| Unknown | 2 | 2 | 0 | |
| Residual tumor size | ||||
| ≤2 cm | 126 (47) | 104 (82.5) | 22 (17.5) | |
| >2 cm | 142 (53) | 137 (96.5) | 5 (3.5) | 0.0003 |
| Unknown | 10 | 10 | 0 | |
| Neoadjuvant chemotherapy | ||||
| Anthra-based | 133 (48) | 121 (91) | 12 (9) | |
| Anthra + Tax-based | 125 (45) | 115 (92) | 10 (8) | 0.4 |
| Other | 20 (7) | 15 (75) | 5 (25) | |
| Anthra + Tax either before or after surgery | ||||
| Yes | 173 (62) | 161 (93) | 12 (7) | |
| No | 105 (38) | 90 (86) | 15 (14) | 0.07 |
| Number of neoadjuvant chemotherapy cycles | ||||
| <6 | 124 (46) | 111 (45) | 13 (48) | |
| ≥6 | 148 (54) | 134 (55) | 14 (52) | 0.93 |
Anthra, anthracycline; Tax, Taxane.
Twenty-seven patients were classified as High-TIL (It-TIL and/or Str-TIL >60%), the remaining 251 were defined as Low-TIL.
As reported in Table 2, the presence of a high lymphocytic infiltrate in residual disease was significantly associated with the absence of metastatic axillary nodes and a small tumor size (≤2 cm) at pathologic examination after NACT. No correlation with other features, especially with the type of treatment, was observed.
Table 2.
Factors associated with prognosis in univariate and multivariate analyses
| Variables |
Univariate analysis |
Multivariate analysis |
Variables |
Univariate analysis |
Multivariate analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Overall survival | Overall survival | ||||||||||||||
| Str-TIL | Str-TIL (per 10% increase) | 0.79 | 0.71–0.89 | <0.001 | 0.86 | 0.77–0.97 | 0.01 | It-TIL | It-TIL (per 10% increase) | 0.78 | 0.68–0.89 | <0.001 | 0.86 | 0.75–0.99 | 0.03 |
| Age (years) | ≥50 | 1.00 | Age (years) | ≥50 | 1.00 | ||||||||||
| <50 | 0.78 | 0.54–1.13 | 0.19 | <50 | 0.79 | 0.54–1.14 | 0.20 | ||||||||
| cStage | I–II | 1.00 | cStage | I–II | 1.00 | 1.00 | |||||||||
| III | 2.33 | 1.59–3.4 | <0.001 | 1.44 | 0.96–2.15 | 0.08 | III | 2.30 | 1.57–3.36 | <0.001 | 1.43 | 0.95–2.13 | 0.08 | ||
| Histotype | Ductal | 1.00 | Histotype | Ductal | 1.00 | ||||||||||
| Lobular | 0.80 | 0.11–5.75 | 0.83 | Lobular | 0.80 | 0.11–5.76 | 0.83 | ||||||||
| Other | 0.42 | 0.13–1.32 | 0.14 | Other | 0.42 | 0.13–1.32 | 0.14 | ||||||||
| Grade | 1–2 | 1.00 | Grade | 1–2 | 1.00 | ||||||||||
| 3 | 0.95 | 0.55–1.63 | 0.85 | 3 | 0.95 | 0.55–1.64 | 0.86 | ||||||||
| Nodal status after neoadjuvant chemotherapy | Neg | 1.00 | Nodal status after neoadjuvant chemotherapy | Neg | 1.00 | 1.00 | |||||||||
| Pos | 4.53 | 2.9–7.07 | <0.001 | 3.52 | 2.21–5.58 | <0.001 | Pos | 4.61 | 2.94–7.25 | <0.001 | 3.56 | 2.23–5.69 | <0.001 | ||
| Residual tumor size | ≤2 cm | 1.00 | Residual tumor size | ≤2 cm | 1.00 | 1.00 | |||||||||
| >2 cm | 2.64 | 1.77–3.93 | <0.001 | 1.54 | 1–2.36 | 0.05 | >2 cm | 2.65 | 1.77–3.97 | <0.001 | 1.65 | 1.07–2.53 | 0.02 | ||
| Neo | A-based/other | 1.00 | Neo | A-based/other | 1.00 | ||||||||||
| A + T-based | 0.88 | 0.6–1.29 | 0.52 | A + T-based | 0.90 | 0.61–1.32 | 0.59 | ||||||||
| Neo + Adj | A + T | 1.00 | Neo + Adj | A + T | 1.00 | ||||||||||
| Other | 0.87 | 0.59–1.28 | 0.48 | Other | 0.85 | 0.58–1.26 | 0.43 | ||||||||
| Metastasis-free survival | Metastasis-free survival | ||||||||||||||
| Str-TIL | Str-TIL (per 10% increase) | 0.79 | 0.71–0.88 | <0.001 | 0.86 | 0.77–0.96 | 0.01 | It-TIL | It-TIL (per 10% increase) | 0.77 | 0.68–0.88 | <0.001 | 0.85 | 0.75–0.98 | 0.02 |
| Age (years) | ≥50 | 1.00 | Age (years) | ≥50 | 1.00 | ||||||||||
| <50 | 0.75 | 0.53–1.06 | 0.11 | <50 | 0.75 | 0.53–1.07 | 0.12 | ||||||||
| cStage | I–II | 1.00 | cStage | I–II | 1.00 | 1.00 | |||||||||
| III | 2.35 | 1.63–3.39 | <0.001 | 1.45 | 0.98–2.13 | 0.06 | III | 2.32 | 1.61–3.35 | <0.001 | 1.44 | 0.98–2.12 | 0.06 | ||
| Histotype | Ductal | 1.00 | Histotype | Ductal | 1.00 | ||||||||||
| Lobular | 0.75 | 0.1–5.34 | 0.77 | Lobular | 0.74 | 0.1–5.33 | 0.77 | ||||||||
| Other | 0.36 | 0.11–1.13 | 0.08 | Other | 0.36 | 0.11–1.13 | 0.08 | ||||||||
| Grade | 1–2 | 1.00 | Grade | 1–2 | 1.00 | ||||||||||
| 3 | 1.00 | 0.59–1.71 | 1.00 | 3 | 1.01 | 0.59–1.72 | 0.98 | ||||||||
| Nodal status after neoadjuvant chemotherapy | Neg | 1.00 | Nodal status after neoadjuvant chemotherapy | Neg | 1.00 | 1.00 | |||||||||
| Pos | 5.02 | 3.25–7.75 | <0.001 | 4.00 | 2.55–6.28 | <0.001 | Pos | 5.10 | 3.28–7.93 | <0.001 | 4.00 | 2.54–6.32 | <0.001 | ||
| Residual tumor size | ≤2 cm | 1.00 | Residual tumor size | ≤2 cm | 1.00 | 1.00 | |||||||||
| >2 cm | 2.57 | 1.76–3.76 | <0.001 | 1.47 | 0.98–2.22 | 0.06 | >2 cm | 2.57 | 1.75–3.78 | <0.001 | 1.58 | 1.05–2.38 | 0.03 | ||
| Neo | A-based/other | 1.00 | Neo | A-based/other | 1.00 | ||||||||||
| A + T-based | 0.87 | 0.61–1.26 | 0.47 | A + T-based | 0.89 | 0.62–1.28 | 0.53 | ||||||||
| Neo + Adj | A + T | 1.00 | Neo + Adj | A + T | 1.00 | ||||||||||
| Other | 0.87 | 0.6–1.26 | 0.47 | Other | 0.86 | 0.59–1.24 | 0.41 | ||||||||
Str-TIL, stromal tumor-infiltrating lymphocytes; It-TIL, intratumoral tumor-infiltrating lymphocytes; HR, hazard ratio; CI, confidence interval; cStage, clinical stage at diagnosis; Neg, negative; Pos, positive; Neo, neoadjuvant treatment; A, anthracycline; T, taxane; adj, adjuvant treatment.
association of TILs with prognosis
Median follow-up was 6.3 years. Both It-TIL and Str-TIL were significantly correlated as continuous variables to both MFS and OS in univariate analysis (Table 2). For each 10% Str-TIL increment, the risk of metastasis and death was reduced by 21% (HR 0.79, 95% CI 0.71–0.88 and HR 0.79, 95% CI 0.71–0.89 for MFS and OS, respectively; P < 0.001 for both outcomes). For each 10% increase in It-TIL, a 22% reduction in the risk of metastasis (HR 0.78, 95% CI 0.68–0.89) and a 23% reduction in the risk of death (HR 0.77, 95% CI 0.68–0.88) was observed (P < 0.001 in both cases). Clinical stage I–II at diagnosis, absence of metastatic lymph nodes after NACT and residual tumor ≤2 cm were the other parameters associated with better outcome.
We carried out two separate multivariate analyses including the three prognostic factors together with It-TIL and Str-TIL, respectively. As reported in Table 2, both Str-TIL and It-TIL maintained a strong prognostic value in the multivariate model, both for MFS (HR 0.86, 95% CI 0.77–0.96, P = 0.01 and HR 0.85, 95% CI 0.75–0.98, P = 0.02 for Str-TIL and It-TIL, respectively) and OS (HR 0.86, 95% CI 0.77–0.97, P = 0.01 and HR 0.86, 95% CI 0.75–0.99, P = 0.03 for Str-TIL and It-TIL, respectively). The other parameters that were independent predictors of outcome were pathological nodal status and tumor size after NACT.
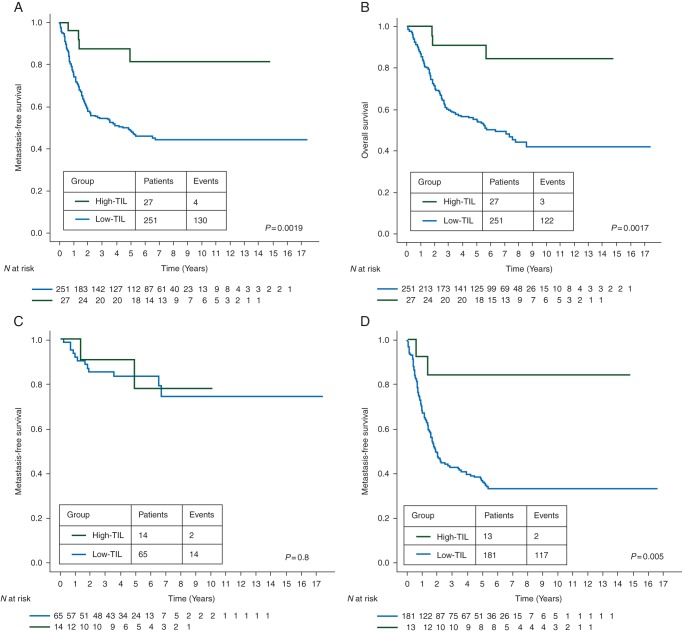
Those patients with a High-TIL residual disease experienced a significantly better MFS when compared with the Low-TIL group. Five-year MFS rates were 81.5% (95% CI 57% to 93%) for the High-TIL group and 46% (95% CI 42% to 55%) for the Low-TIL group (HR 0.24, 95% CI 0.09–0.64, log-rank P = 0.0019). The 5-year OS rate was 91% (95% CI 68% to 97%) for High-TIL patients and 55% (95% CI 48% to 61%) for Low-TIL patients (HR 0.19, 95% CI 0.06–0.61, log-rank P = 0.0017). Kaplan–Meyer survival curves are represented in Figure 2A and B.
Figure 2.
Prognostic value of high lymphocytic infiltration on residual disease after neoadjuvant chemotherapy. Estimated Kaplan–Meyer curves of metastasis-free survival (A) and overall survival (B) for all patients. Estimated Kaplan–Meyer curves of metastasis-free survival for patients with node-negative and ≤2 cm residual disease (C) and for patients with node-positive and/or >2 cm residual disease (D).
TIL prognostic role in high-risk and low-risk populations
As high TIL levels were related with the absence of axillary nodal disease and small tumor size at surgery, we explored the association of TILs with prognosis in two different risk-groups: (i) patients with node-negative and ≤2 cm residual disease, (ii) patients with node-positive and/or >2 cm residual disease. MFS Kaplan–Meyer survival curves in the two groups according to TIL are represented in Figure 2C and D. In patients with node-negative and ≤2 cm residual disease, MFS rates were high independently from the presence of TIL (5-year MFS rates: 78%, 95% CI 35% to 94% and 83%, 95% CI 71% to 91% for the High-TIL and Low-TIL group, respectively; HR 0.82, 95% CI 0.18–3.66, log-rank P = 0.8). TIL was associated with a major prognostic value in patients with node positive and/or >2 cm residual disease. Indeed, 5-year MFS rates were 84% (95% CI 49% to 96%) and 36% (95% CI 29% to 43%) in patients with high and low TIL, respectively (HR 0.17, 95% CI 0.04–0.69, log-rank P = 0.005).
We further evaluated whether TIL had a different prognostic impact in the group of patients treated with additional anthracycline and/or taxane postneoadjuvant treatment (n = 81) compared with the group who received neoadjuvant anthracycline- and/or taxane-based chemotherapy only (n = 169). The test for interaction was not significant (P = 0.48), suggesting that the effect of High-TIL versus Low-TIL is similar in the two populations.
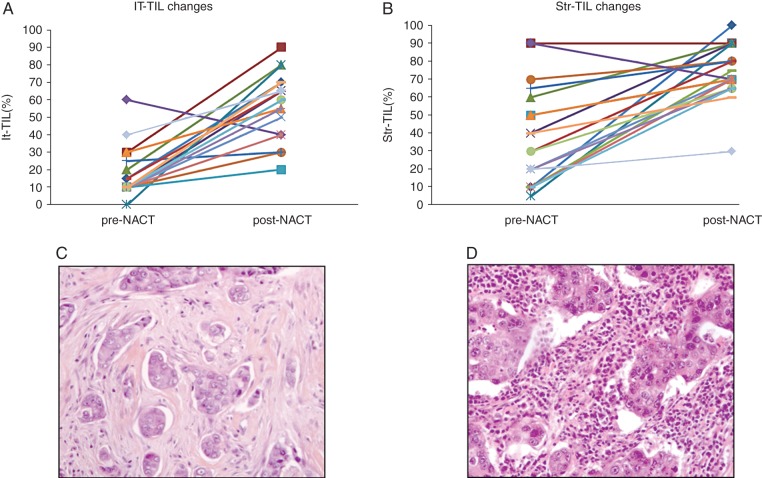
TIL evaluation on diagnostic core biopsies
We evaluated TILs on the core-diagnostic biopsy samples for the 27 patients having a High-TIL residual disease. Pretreatment HES slides were available for 19 cases. In all but one case, It-TIL and Str-TIL were lower on the prechemotherapy compared with the postchemotherapy sample (Figure 3). Four patients had either It-TIL or Str-TIL >60% on the diagnostic core biopsy, all the remaining changed from Low-TIL to High-TIL after chemotherapy.
Figure 3.
Tumor-infiltrating lymphocytes (TILs) changes before and after neoadjuvant chemotherapy. Intratumoral-TIL (It-TIL, A) and stromal-TIL (Str-TIL, B) level changes from diagnostic core biopsy (prechemotherapy) to surgical specimen (postchemotherapy) for patients having a High-TIL residual disease after neoadjuvant chemotherapy. Illustration of a case changing from Low-TIL (core biopsy, C) to High-TIL (residual disease, D).
discussion
Several studies have emphasized the prognostic and predictive impact of TIL in breast cancer. Denkert et al. have described that high baseline TIL levels can predict pCR across all breast cancer subtypes. They suggested that the response to NACT depended on It-TIL and Str-TIL as continuous variables, and they defined a cutoff to be considered for further validation studies (It-TIL and/or Str-TIL >60% versus It-TIL and Str-TIL ≤60%) [10]. More recently, TIL at baseline has been reported as a strong prognostic factor for TNBC patients treated with conventional adjuvant chemotherapy [12]. In this article, we evaluated whether postchemotherapy TIL could identify a subgroup of TNBC patients at good prognosis. The rationale was supported by preclinical studies showing that chemotherapy can induce an antitumor immune response, through immunogenic cell death. We demonstrate that a high level of TIL in residual disease after NACT is a strong prognostic factor in TNBC. A possible limitation of this study is the heterogeneity of the type and timing of administration of neo-/adjuvant treatments. However, treatment variables did not affect prognosis in our series and the effect of TIL was similar for those patients treated with NACT only or with neoadjuvant and adjuvant chemotherapy. Moreover, the presence of High-TIL correlated neither to the number of cycles nor to the type of NACT.
Two data deserve further discussion. First, the presence of High-TIL in residual disease was related to chemotherapy exposure since most of these samples did not present a High-TIL tumor at baseline. Secondly, the outcome in TNBC patients with high TIL levels after chemotherapy was excellent, suggesting that this parameter could be used to select high-risk patients and could be a surrogate for drug efficacy.
The observation that chemotherapy can induce lymphocytes activation and attraction has been reported in small breast cancer series. A study including 21 patients with residual disease after taxane-based NACT described a chemotherapy-induced attraction of lymphocytes to the tumor bed in seven patients [4]. Similarly, Ladoire et al. [5] have suggested that the ratio of CD8/Forkhead box P3 (FoxP3) after chemotherapy could be associated with better outcome. This finding supports the hypothesis that chemotherapy could induce antitumor immune response. Apetoh et al. showed that cancer cell death induced by anthracyclines results in the release of high-mobility group box 1 which activates antigen-presenting cells through Toll-like receptor-4, finally resulting in the induction of antitumor-specific T lymphocytes. In our study, we evaluated prechemotherapy samples only for those cases with a High-TIL residual disease. Our results suggest that dynamic changes in tumor infiltration by lymphocytes are an important topic for research. Nevertheless, we are not able to assess whether a High-TIL case at baseline may become Low-TIL after chemotherapy. This question is extremely important to explain why some TIL+ cancers do not respond to chemotherapy and may represent the aim of further studies in the field.
The finding that postchemotherapy TIL could identify a subset of patients with excellent outcome could have several implications in drug development and patient stratification.
In the present study, patients with both a high residual cancer burden and low postchemotherapy TIL presented a 36% 5-year MFS. Therefore, they could be considered good candidates to enter postneoadjuvant clinical trials. At the opposite, even in the presence of poor prognostic factors, a high lymphocytic infiltration could define a group of patients with very good outcome, who do not deserve the inclusion in adjuvant clinical trials. The identification of surrogates for drug efficacy has become a major challenge in the breast cancer field since it could dramatically speed-up drug development. pCR has been validated as a surrogate for drug efficacy in the neoadjuvant setting [1]. Nevertheless, this surrogate is suboptimal since some patients who did not reach pCR will never relapse and might have benefited from therapy. Also, pCR applies for cytotoxic, but not for all therapies delivered in the preoperative setting. The finding that post-treatment high TIL is associated with 91% OS at 5 years suggests that TIL can further inform measures of the amount of residual disease and might be a surrogate to evaluate drug efficacy. This surrogate could be complementary to chemotherapy, but could also apply to new drugs, including immune checkpoint modulators, or monoclonal antibodies.
Overall, this study suggests that chemotherapy could convert a Low-TIL into a High-TIL tumor, and that this conversion is associated with an excellent 5-year OS rate. This finding supports the concept that chemotherapy could partly exert its antitumor effect through the immune system. Also, it suggests that post-treatment TIL could be a stratification parameter and a surrogate for treatment efficacy in the neoadjuvant setting.
funding
This work was funded by TRANSCAN JCT-2011, French NCI and the Monica Boscolo 2012 research grant.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 2.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 3.André F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- 4.Demaria S, Volm MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 5.Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, et al. Meta-analysis results from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) Cancer Res. 2012;72:S1–11. [Google Scholar]
- 8.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 11.R Development Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.rproject.org/ (July 15, 2013, date last accessed ) [Google Scholar]
- 12.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.