The post-AI recurrence phenotype can be highly variable and reflect multiple mechanisms of resistance. In some cases, loss of ER occurs but there is also increased expression in other instances with evidence of persistent ER functioning, suggesting that further endocrine therapy may be appropriate in these patients. Cases of PTEN loss and HER2 gain also occur. Comprehensive molecular profiling of metastases will be required to direct subsequent targeted therapy with confidence.
Keywords: breast cancer, immunohistochemistry, aromatase inhibitor, relapse
Abstract
Background
The purpose of this study was to identify any differences in key biomarkers associated with estrogen action between biopsies taken at diagnosis and at recurrence or progression during treatment with an aromatase inhibitor (AI).
Patients and Methods
Patients were retrospectively identified from a clinical database as having relapsed or progressed during AI treatment. Immunohistochemistry was carried out against estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2), insulin-like growth factor type-1 receptor (IGF1R), insulin receptor substrate-1 (IRS-1), stathmin, phosphatase and tensin homolog and Ki67.
Results
Fifty-five pairs of samples were identified with ER- and/or PgR-positive diseases. Four (7%) patients were ER-negative at progression. Overall, PgR levels were lower in the recurrence sample, but 35% of cases remained positive. IGF1R levels decreased significantly. There were no substantial changes in HER2, IRS-1 or stathmin levels to indicate a role in resistance. Higher Ki67 levels at resistance indicate more proliferative disease.
Conclusions
The phenotype of AI-recurrent lesions shows high between-tumour heterogeneity. There is evidence of an increase in Ki67, a reduction in IGF1R and a loss of ER expression in some individuals and some activation of growth factor signalling pathways that may explain resistance in individuals and merit treatment targeted to those pathways. Biopsy at recurrence will be necessary to identify the relevant target for individuals.
introduction
Third-generation aromatase inhibitors (AIs) have become the standard of care as first-line endocrine treatment of hormone receptor-positive post-menopausal patients in all settings of the disease [1]. Despite the efficacy of these compounds, response rates for first-line metastatic patients have been described as up to 40%, with all initial responders eventually developing resistance over time and there is no proven survival advantage over tamoxifen in the metastatic setting. Moreover, recurrences occur in a sizeable minority of patients treated with adjuvant AIs [2, 3].
Much effort has been expended in trying to elucidate the mechanisms involved in endocrine resistance [4–10]. The discovery of predictive biomarkers has been primarily based on correlative analysis between the molecular characteristics of the primary tumours and time to recurrence, in patients treated with adjuvant [5, 6] or neoadjuvant therapy [7–10].
Biomarker profiles of breast cancers may change following adjuvant treatment, changes in estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status having been documented in patients treated with tamoxifen and/or chemotherapy [11, 12]. The pattern of changes of key signalling pathways in patients treated with AIs has yet to be characterised, partly due to the limited availability of samples at relapse.
Our aim was to investigate the changes in the expression of biomarkers in patients treated with AIs using immunohistochemical analysis with validated antibodies against ER, progesterone receptor (PgR), HER2, Ki67, insulin-like growth factor type-1 receptor (IGF1R), insulin receptor substrate-1 (IRS-1), phosphatase and tensin homolog (PTEN) and stathmin.
patients and methods
patients
Patients who had relapsed or progressed while on an AI either in the advanced metastatic or locally advanced setting for the treatment of ER and/or PgR-positive breast cancer were identified from a prospectively maintained database at The Royal Marsden Hospital from 1 January 2005 until 31 January 2009. Cases were selected if archival formalin-fixed paraffin embedded tissue was available from both before, either a core-cut biopsy at diagnosis or the surgical excision of primary breast cancer, and from the recurrent lesion after the treatment with the AI.
laboratory analysis
Immunohistochemical analysis is described in supplementary information, available at Annals of Oncology online.
statistical analysis
Analyses were carried out using the GraphPad Prism software, Microsoft Excel and Stata version 11.2 for Windows. The Wilcoxon matched-pairs test was used to assess differences between paired samples, and differences between unpaired groups were assessed by the Mann–Whitney test. Spearman rank was used to determine correlations between variables. Significance was taken as P ≤ 0.05. With 55 paired samples, a difference of 0.5 standard deviation (SD) can be detected with 95% power and 5% two-sided significance level, and conventionally a standardised difference of 0.5 SD is described as a ‘medium’ effect size. With 55 patients, a correlation of 0.4 SD or more is reliably detectable. Only relatively large differences, e.g., 1 SD, are detectable between unpaired groups.
results
patient characteristics and treatment administered
Fifty-five paired samples were identified. Patients and treatment characteristics are summarised in Table 1.
Table 1.
Patients and treatment characteristics (N = 55)
| Age at diagnosis (mean/range) | 56 (27–86) |
| Setting, n (%) | |
| LABC | 9 (16) |
| Following excision of locoregional disease | 17 (31) |
| Metastatic | 29 (53) |
| Metastatic locations, n (%) | |
| Number of locations (mean/range) | 1.6 (1–4) |
| Bone | 13 (45) |
| Visceral | 4 (14) |
| Skin | 10 (35) |
| Other | 19 (66) |
| Type of sample, n (%) | |
| Pre | |
| CB | 20 (36) |
| Surgical specimen | 29 (53) |
| PB | 3 (5.5) |
| Other | 3 (5.5) |
| Post | |
| CB | 9 (16) |
| Surgical specimen | 19 (35) |
| PB | 10 (18) |
| Other | 17 (31) |
| AI administered, n (%) | |
| Letrozole | 30 (54) |
| Anastrozole | 23 (42) |
| Exemestane | 2 (4) |
| Previous TAM, n (%) | |
| Yes | 43 (78) |
| No | 12 (22) |
| Second AI after PD, n (%) | |
| Yes | 37 (67) |
| No | 18 (33) |
| Deceased, n (%) | |
| Yes | 35 (64) |
| No | 16 (29) |
| Lost follow up | 4 (7) |
NA, neoadjuvant; LABC, locally advanced breast cancer; CB, core biopsy; PB, punch biopsy; TTF, time to treatment failure; AI, aromatase inhibitor; TAM, tamoxifen; PD, progressive disease.
The median time from diagnosis to the start of AI treatment was 7 (interquartile range, IQR 2–13) years. A total of 53% (n = 29) of patients received AI for metastatic disease. In the remainder, the AI was administered after removal of locoregional disease following locoregional relapse [removal of local regional disease (ROLD); 31%, n = 17] or as systemic treatment of locoregional relapse (locally advanced breast cancer, LABC; 16%, n = 9). The pre-treatment sample for biomarker analysis was taken at the time of diagnosis or surgical excision before any medical treatment. Before obtaining the recurrence sample, 78% had already received tamoxifen treatment and 9% an AI, either in the adjuvant or in the metastatic setting. Overall, 78% had received previous endocrine treatment. In the 29 metastatic cases, the median time to treatment failure on AI was 11 (IQR 6–27) months, and in the remaining patients, it was 14.5 (IQR 5–26) months, P = 0.65.
biomarker results
The biomarker results are summarised in Table 2 and correlations are presented in Table 3. Although 4 (7%) patients had ER-negative disease at progression on AI (Figure 1), overall there was no significant difference in ER H-score (P = 0.51). PgR score decreased significantly; 40 (73% cases) were PgR-positive pre-treatment and 19 (35%) PgR-positive at progression. In 6 (11%) cases, PgR showed increased expression (≥2-fold), including 2 cases who were PgR negative pre-treatment, becoming positive at progression with an AI. Ki67 levels were higher at progression (P = 0.001) and inversely associated with ER levels at progression (P ≤ 0.05).
Table 2.
Pre- and post-treatment biomarker values
| Geometric mean (95% CI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA/LABC/ROLD (n = 26) |
Metastatic (n = 29) |
Overall |
||||||||||
| Pre | Post | Fold change | P-value | Pre | Post | Fold change | P-value | Pre | Post | Fold change | P-value | |
| ER | 179 | 91 | 0.51 | 0.859 | 153 | 97 | 0.63 | 0.531 | 165 | 94 | 0.57 | 0.505 |
| (163–195) | (45–185) | (0.25–1.02) | (130–181) | (57–165) | (0.37–1.08) | (150–182) | (62–144) | (0.38–0.87) | ||||
| PgR | 26.9 | 5.5 | 0.2 | 0.001 | 34.1 | 6.8 | 0.2 | <0.001 | 30.5 | 6.1 | 0.2 | <0.001 |
| (14.6–49.6) | (2.6–11.6) | (0.09–0.45) | (19.6–59.1) | (3.3–14.1) | (0.09–0.43) | (20.5–45.3) | (3.7–10.2) | (0.12–0.34) | ||||
| Ki67 | 9.1 | 10.5 | 1.15 | 0.361 | 5.1 | 14 | 2.78 | 0.001 | 6.7 | 12.2 | 1.83 | 0.001 |
| (6.6–12.5) | (7.3–15.1) | (0.81–1.64) | (3.1–8.3) | (10.1–19.6) | (1.58–4.89) | (4.9–9.1) | (9.6–15.6) | (1.29–2.61) | ||||
| Stathmin | 2.2 | 1.8 | 0.8 | 0.476 | 1.4 | 2.9 | 2.01 | 0.051 | 1.8 | 2.3 | 1.3 | 0.288 |
| (1.4–3.4) | (1–3.1) | (0.4–1.59) | (0.8–2.5) | (2–4.2) | (1–4.05) | (1.3–2.5) | (1.7–3.2) | (0.79–2.13) | ||||
| IGF1Ra | 2.1 | 1.5 | −0.62 | 0.001 | 2.2 | 1.6 | −0.55 | 0.002 | 2.1 | 1.6 | −0.58 | <0.001 |
| (1.9–2.4) | (1.2–1.9) | (−0.92–−0.31) | (1.9–2.4) | (1.3–1.9) | (−0.85–−0.25) | (2–2.3) | (1.4–1.8) | (−0.79–−0.37) | ||||
| IRS-1 | 7.9 | 7.3 | 0.92 | 0.639 | 6 | 6.4 | 1.08 | 0.841 | 6.9 | 6.8 | 1 | 0.91 |
| (6.5–9.6) | (6.1–8.8) | (0.7–1.21) | (4.6–7.9) | (5.3–7.9) | (0.75–1.54) | (5.8–8.1) | (6–7.8) | (0.8–1.25) | ||||
| Pre/post | n | n | n | |||||||||
| HER2 | neg/neg | 22 | 24 | 46 | ||||||||
| neg/pos | 2 | 1 | 3 | |||||||||
| pos/neg | 0 | 1 | 1 | |||||||||
| pos/pos | 2 | ns | 2 | ns | 4 | ns | ||||||
| PTEN | neg/neg | 2 | 1 | 3 | ||||||||
| neg/pos | 1 | 1 | 2 | |||||||||
| pos/neg | 0 | 2 | 2 | |||||||||
| pos/pos | 14 | ns | 18 | ns | 32 | ns | ||||||
aNot geometric.
Table 3.
Spearman's rank correlation coefficients for the biomarkers pre- and post-treatment
| ER | PgR | HER2 | Ki67 | Stathmin | IGF1-1R | IRS-1 | |
|---|---|---|---|---|---|---|---|
| Pre-treatment | |||||||
| PgR | −0.17 | 1 | |||||
| HER2 | 0 | −0.27 | 1 | ||||
| Ki67 | 0.18 | −0.17 | −0.07 | 1 | |||
| Stathmin | 0.31* | −0.01 | 0.04 | 0.38* | 1 | ||
| IGF1-1R | 0.28 | −0.06 | −0.08 | −0.17 | 0.02 | 1 | |
| IRS-1 | 0.48* | −0.11 | −0.03 | 0.48* | 0.17 | 0.2 | 1 |
| PTEN | −0.28 | 0.08 | −0.02 | −0.12 | −0.44* | 0.05 | −0.13 |
| Post-treatment | |||||||
| PgR | 0.21 | 1 | |||||
| HER2 | 0.15 | −0.09 | 1 | ||||
| Ki67 | −0.29* | 0.02 | −0.1 | 1 | |||
| Stathmin | −0.01 | −0.06 | −0.33* | 0.41* | 1 | ||
| IGF1-1R | 0.52* | 0.22 | −0.09 | −0.27 | 0 | 1 | |
| IRS-1 | 0.52* | −0.02 | 0.17 | 0.08 | 0.18 | 0.22 | 1 |
| PTEN | 0.16 | −0.04 | 0.1 | 0.13 | 0.28 | −0.14 | 0.45* |
*P ≤ 0.05.
Figure 1.
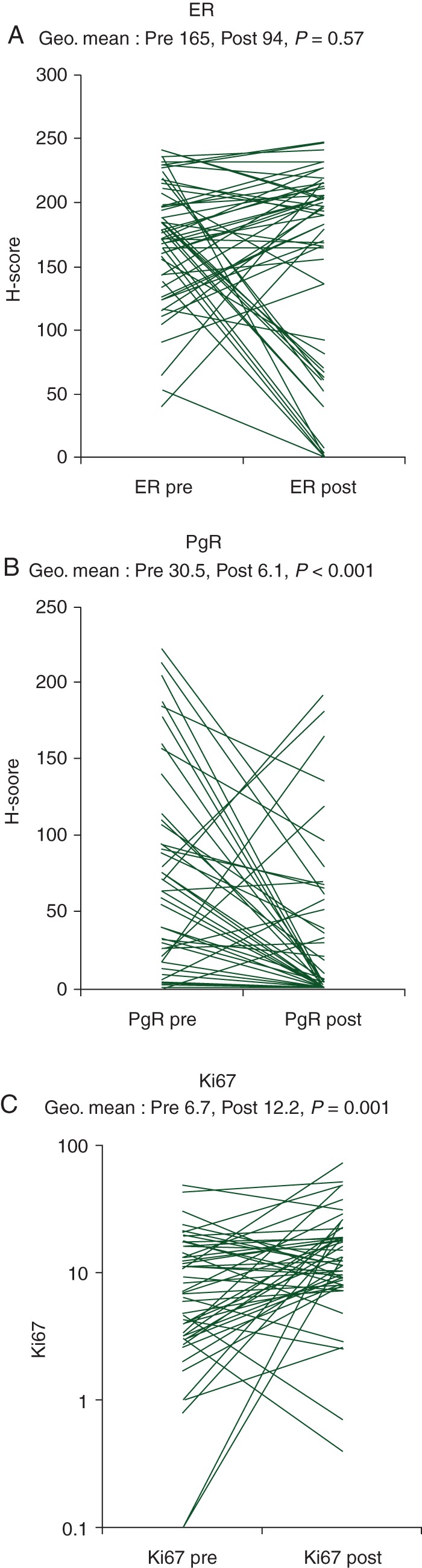
(A) Estrogen receptor levels determined by H-score pre- and post-treatment, (B) PgR levels determined by H-score pre- and post-treatment and (C) % Ki67 levels pre- and post-treatment.
Fifty-four patients had paired HER2 values. In 37 cases, values were either immunohistochemical (IHC) 0 or 1+ in both samples, and in 8 cases, values were 2+ or 3+ at presentation and remained so at progression. In 3 cases, 3+ status was acquired by the time of progression and in 1 case, there was a change in the primary tumour to HER2 negative at progression. FISH was carried out in those cases that were IHC 2+, being negative in all cases except in one pre-AI, that was concordant with post-AI. For those cases with increases from 0/1+ to 2+, FISH tests showed no amplification of HER2.
IGF1R significantly decreased after treatment with a mean score of 2.1 before and 1.6 at progression (P < 0.001). There was a trend in pre-AI samples for IGF1R to correlate with ER levels (Rs: 0.28; P = 0.06), while at progression this correlation became stronger (Rs: 0.52; P < 0.001). Moreover, changes in ER were significantly positively correlated with that in IGF1R (Rs: 0.38; P = 0.005). The correlation of ER with levels of IRS-1 did not differ between pre-treatment (Rs: 0.48; P < 0.001) and recurrent lesions (Rs: 0.52; P < 0.001).
No significant changes were observed for stathmin levels (P = 0.29), but a positive correlation between stathmin and Ki67 pre- and post-treatment was observed (Rs: 0.38, P = 0.002 and Rs: 0.41, P < 0.001, respectively). PTEN was analysable in 39 paired samples, and no significant changes were observed after progression.
conclusions
The response rates for AIs as the first-line treatment of advanced ER-positive breast carcinoma in post-menopausal patients vary from 21% to 46% [13–16], and most of the patients who experience an initial response will develop resistance over time. The recurrent phenotype is likely to be the predominant determinant of responsiveness, but there is little published evidence on predictive biomarkers. An earlier study from our group revealed that higher PgR and Ki67 levels were significantly associated with increased and decreased time to treatment failure, respectively [17].
Levels of ER did not show a significant change overall in the current study, but there was a 43% fall and in four cases, a conversion from ER-positive to ER-negative phenotype was observed. Loss of or low ER is likely to explain the resistance to AI. In the remainder, alternative explanations must be considered as discussed below. A number of ESR1 mutations have been described; the impact of these on resistance could usefully be evaluated in this series. Of note, the increased expression of ER in some patients is consistent with the data on long-term estrogen deprived cells such that hypersensitivity to estrogen may lead to resistance to AIs [5].
As an estrogen-dependent protein, PgR [18] may help in assessing whether signalling through ER persists and lower levels of PgR expression were seen at progression on AI compared with presentation samples. In the IMPACT and P024 (letrozole versus tamoxifen) neoadjuvant trials [7, 19], decreases in PgR levels were observed in most of the AI-treated patients. Estrogenic signalling may therefore not be activated in some patients, but the marked increases in PgR levels in a small number of cases are consistent with hypersensitivity to estrogens.
The higher Ki67 levels at progression are consistent not only with disease progression, but also with the development of a more aggressive phenotype compared with the disease at presentation.
Preclinical and clinical studies have suggested that the IGF-1 pathway may be involved in resistance. Receptor binding of IGF-1 results in the recruitment of proteins such as the IRS-1 family members, triggering the PI3K/AKT and MAPK [20, 21] pathways. IGF1R has been found to be overexpressed (up to 14-fold) in ER-positive breast cancer cells compared with levels in normal epithelial cells [22], and high cytoplasmic IRS-1 has been correlated with shorter disease-free survival in patients with ER-positive breast tumours [23, 24]. In our study, both IGF1R and IRS1 levels were significantly correlated with the levels of ER expression pre- and post-AI especially in ER-positive cases. Changes in IGF1R and IRS-1 were also correlated with those in ER expression. However, no correlation was observed between IGF1R and IRS-1, which might be related to the fact that IRS-1 is downstream of other growth factor receptors. There was no support from our observations for IGF1R being activated as a resistance mechanism to AIs even among those patients who remained ER positive.
Loss of PTEN expression was documented in only two patients, but in these patients, it is plausible that PTEN loss of function may have constituted a resistance mechanism. Stathmin expression has been reported to act as an integrative marker for the signalling of the PI3-kinase/PTEN pathway [25]. No significant changes were seen; however, the correlation seen between stathmin and Ki67 may reflect a role for stathmin in proliferation due to its activity on microtubules, a function that may disturb its acting as a good marker for the activity of PI3-kinase. A recent study of the mammalian target of rapamycin (mTOR) inhibitor, everolimus, strongly supported the involvement of the PI3K/AKT/mTOR pathway in the mechanism of resistance [26]. A study of the role of PI3-kinase mutational status in acquired resistance might be worthwhile.
HER2 status was largely stable in the current study, but in three cases IHC3+ status, which is regarded as unequivocally positive, was acquired. Discordance of steroid receptor and HER2 status between primary tumours and metastatic lesions is increasingly recognised. For example, in a prospective study of biopsies of suspected metastasis in 121 women diagnosed with breast cancer, discordance in ER, PgR and HER2 between the primary and the metastasis was 16%, 40% and 10%, respectively. Biopsy led to a reported change of management in 14% of women (95% confidence interval 8.4%–21.5%) [12].
Activation of growth factor pathways is reflected by the increased expression of phosphorylated substrates. We did not perform IHC analyses of phosphorylated markers in this study because of the earlier observation by our group that extreme loss of phospho-staining can occur during routine fixation of resection specimens [27].
Scepticism has been expressed at the degree to which variation between paired observations in primary disease and metastases reflects biological tumour differences as opposed to sampling differences from internally heterogeneous tumours and/or methodological variability [28]. The latter is unlikely since all tissues were examined in the same laboratory with paired samples in the same batch. Similarly, the loss of PgR expression is unlikely to be due to heterogeneity given its statistical significance. It is important to note, given that this study, like others, compared a lesion at presentation with a recurrent lesion in a different site, phenotypic differences between the tumours could have existed before starting the AI. Many patients had received medical treatment before the AI, mostly tamoxifen. Given that the molecular phenotype of the recurrent lesion was measured while still on the AI that phenotype is relevant to AI resistance and to subsequent treatment choices, but the changes from baseline may not have been induced by the AI itself in all cases. The molecular changes may have occurred as a result of phenotypic drift, which may have been greater with longer time between baseline and resistance biopsies, as well as being influenced by the other intervening treatments. The possibility of the phenotype varying between recurrent lesions in the same patients was not addressed here.
The degree to which the observations made in this series are generalisable is unknown and may, for example, be affected by the availability of the tumour samples for research, which tends to select for larger lesions and may also be affected by the fact the Royal Marsden is a tertiary referral centre.
In summary, this study of post-AI recurrence phenotype provides support for highly variable phenotype and multiple mechanisms of resistance at recurrence on AI. Loss of ER in some cases, similar to that seen with some patients recurring on tamoxifen, occurs but also enhanced expression in others with evidence of persistent ER functioning despite the AI treatment. The latter cases are better candidates for further endocrine therapy. Occasional cases of PTEN loss and HER2 gain emphasise the variable nature of putative mechanisms of resistance. Sampling of metastases with comprehensive molecular profiling will be required to direct subsequent targeted therapy with confidence.
funding
This study was funded in part by Breakthrough Breast Cancer and National Institute of Health Research Biomedical Research Centre at the Royal Marsden Hospital. MA was supported by the Cridland Fund. RA was supported by CRUK (grant number C1491/A9895).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431–2442. doi: 10.1056/NEJMra023246. doi:10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 2.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. doi:10.1016/S0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 3.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. doi: 10.1056/NEJMoa052258. doi:10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 4.Martin LA, Ghazoui Z, Weigel MT, et al. An in vitro model showing adaptation to long-term oestrogen deprivation highlights the clinical potential for targeting kinase pathways in combination with aromatase inhibition. Steroids. 2011;76(8):772–776. doi: 10.1016/j.steroids.2011.02.035. doi:10.1016/j.steroids.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. J Clin Oncol. 2008;26(7):1059–1065. doi: 10.1200/JCO.2007.12.9437. doi:10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 6.Viale G, Giobbie–Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569–5575. doi: 10.1200/JCO.2008.17.0829. doi:10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol. 2005;23(11):2477–2492. doi: 10.1200/JCO.2005.07.559. doi:10.1200/JCO.2005.07.559. [DOI] [PubMed] [Google Scholar]
- 8.Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11(2 Pt 2):951s–958s. [PubMed] [Google Scholar]
- 9.Ellis MJ, Lin L, Crowder R, et al. Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2010;119(2):379–390. doi: 10.1007/s10549-009-0575-y. doi:10.1007/s10549-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis MJ, Miller WR, Tao Y, et al. Aromatase expression and outcomes in the P024 neoadjuvant endocrine therapy trial. Breast Cancer Res Treat. 2009;116(2):371–378. doi: 10.1007/s10549-008-0161-8. doi:10.1007/s10549-008-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593–599. doi: 10.1200/JCO.2010.33.8889. doi:10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–592. doi: 10.1200/JCO.2010.33.5232. doi:10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101–2109. doi: 10.1200/JCO.2003.04.194. doi:10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 14.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18(22):3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 15.Bonneterre J, Thurlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18(22):3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 16.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(30):4883–4890. doi: 10.1200/JCO.2007.14.4659. doi:10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson H, Hills M, Zabaglo L, et al. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol. 2011;22(8):1770–1776. doi: 10.1093/annonc/mdq700. doi:10.1093/annonc/mdq700. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978;103(5):1742–1751. doi: 10.1210/endo-103-5-1742. doi:10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- 19.Ellis MJ, Coop A, Singh B, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;63(19):6523–6531. [PubMed] [Google Scholar]
- 20.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68(3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. doi:10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 21.Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–S43. doi: 10.1677/erc.1.01280. doi:10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- 22.Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5(1):95–105. doi: 10.1023/a:1009523501499. doi:10.1023/A:1009523501499. [DOI] [PubMed] [Google Scholar]
- 23.Rocha RL, Hilsenbeck SG, Jackson JG, et al. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3(1):103–109. [PubMed] [Google Scholar]
- 24.Lee AV, Jackson JG, Gooch JL, et al. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13(5):787–796. doi: 10.1210/mend.13.5.0274. doi:10.1210/me.13.5.787. [DOI] [PubMed] [Google Scholar]
- 25.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104(18):7564–7569. doi: 10.1073/pnas.0702507104. doi:10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. doi:10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinhel IF, Macneill FA, Hills MJ, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12(5):R76. doi: 10.1186/bcr2719. doi:10.1186/bcr2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15(11):1164–1168. doi: 10.1634/theoncologist.2010-0059. doi:10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


