Abstract
Purpose
The aims of this study were to: (1) estimate the volumetric and metabolic growth rate of non-small cell lung cancer (NSCLC), (2) evaluate disease progression prior to treatment, and (3) explore the effects of tumor growth rate and time to treatment (TTT) on survival outcome.
Methods
Patients with inoperable stages I–III NSCLC with serial pre-treatment PET/CT scans were eligible for this study. PET-derived metabolic tumor volumes (PET-MTV) and CT-derived gross tumor volumes (CT-GTV) were contoured using PET/CT information. Normalized standardized uptake values (NSUV) in tumors including the NSUVmean and NSUVmax were measured. Tumor growth rates expressed as doubling time (DT) were estimated using an exponential model. Pre-treatment disease progression defined as the development of any new site of disease on PET/CT and change in TNM stage (AJCC 7th ed.) were recorded. Growth rate and tumor progression were analyzed with respect to overall (OS) and progression free survival (PFS).
Results
Thirty-four patients with a median inter-scan interval (ISI) of 43 days and TTT of 48 days were analyzed. Tumor volumes showed remarkable inter-scan growth while NSUV did not increase significantly. The DT for PET-MTV, CT-GTV, NSUVmean and NSUVmax were 124, 139, 597, and 333 days, respectively. Pre-treatment disease progression occurred in 20.6% patients with longer ISI being a significant risk factor (OR = 1.027, p = 0.02). The optimal threshold ISI to predict progression was 58 days (4.8% vs. 46.2%, p = 0.007). Neither tumor growth rates nor TTT were significantly correlated to OS or PFS.
Conclusions
NSCLC displays rapid tumor volume growth whereas NSUVmean and NSUVmax are relatively stable over the same time period. Longer delays before initiation of treatment are associated with higher risk of pre-treatment disease progression.
Keywords: Positron emission tomography/computed, tomography, Non-small cell lung cancer, Doubling time, Disease progression, Waiting time
1. Introduction
Non-small cell lung cancer (NSCLC) is a biologically aggressive tumor, with rapid growth and metastatic spread leading to dismal survival outcomes. By the time tumor is detected on imaging modalities, it is likely to have been present as microscopic disease for a longer duration. Treatment delay in cancer patients is not an uncommon occurrence in daily practice often with multiple contributing factors such as scheduling delay during the diagnosis and staging process, patient delay related to anxiety or hesitation, and even issues relating to insurance policies [1]. Lung cancer genotyping is being increasingly performed prior to starting treatment and can contribute to delays as well. Excessive waiting time may lead to interval tumor growth and metastatic spread which can consequently alter treatment intent and strategy as well as clinical outcome. Therefore a more detailed understanding of the natural time-course of growth and disease progression in untreated NSCLC would assist with clinical decision making, determination of appropriate treatment strategies and surveillance protocols, and defining acceptable waiting time without compromise to patient outcomes [2,3].
Several studies of lung cancer presenting initially as a small pulmonary nodule detected by X-ray or CT based screening programs have shown great heterogeneity in tumor volume doubling time (VDT) [4–8]. However, there is little published data on the natural growth of lung cancer detected by routine medical care, when tumor size is generally larger and regional nodal metastases may already be present. Changes in tumor volumes and metabolic activity for untreated NSCLC on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) have not been well-described.
The aims of this study, through serial PET/CT scans prior to administration of any active treatment, were to: (1) estimate the volumetric and metabolic growth rate of NSCLC, (2) evaluate disease progression prior to treatment, and (3) explore the effects of tumor growth rate and time to treatment (TTT) on survival outcome including overall survival (OS) and progression free survival (PFS).
2. Patients and methods
2.1. Study population
This is a secondary analysis of a subgroup of patients prospectively enrolled in a series of functional imaging-related studies at the University of Michigan. These investigational studies were approved by the local institutional review board (IRB). Patients with either unresectable or inoperable stages I–III NSCLC treated with radiation therapy (RT), with or without concurrent chemotherapy, were eligible. Per protocol, pre-RT PET/CT scans were performed within two weeks of the CT simulation. Repeated PET/CT scans were implemented for patients whose initial diagnostic PET/CT scans were more than two weeks before simulation, leading to a situation where many patients had two pre-RT PET/CT studies available for comparison before any treatment had been initiated.
2.2. PET/CT image acquisition
All scans were performed on integrated PET/CT scanners at two institutions: University of Michigan Hospital (UMH) and Veterans Administration Health Center/Veterans Affairs Medical Center, Ann Arbor (VA-AA) between 2003 and 2010. The PET protocols used in both institutions were standardized throughout the time period at both UMH and VA-AA. Details on our PET/CT scanning protocols have been described in a previous publication [9]. At the UMH between 2003 and 2006 the PET/CT imaging was performed on a Siemens Biograph Classic (Siemens Medical Solutions, Hoffman Estates, IL, USA), and between 2006 and 2010 on a Siemens Biograph T6. At the VA Ann Arbor Medical Center the PET/CT imaging was performed on a Siemens Biograph T6.
2.3. Image analysis and tumor delineation
FDG-PET/CT images were analyzed on an in-house functional imaging analysis workstation (FIAT). PET and CT images were co-registered using a rigid body method. A 1 cc volume of interest (VOI) was set in the ascending aorta (AA), extending downward from the transitional slice between AA and aortic arch. The mean standardized uptake value (SUV) of this VOI was recorded to represent the average FDG metabolic activity of the mediastinal blood pool. The SUV in the tumor was normalized to that of the corresponding AA (NSUV) to minimize the potential for inter-scan variation in FDG distribution. A NSUV threshold of 1.5 on PET was used for auto-segmentation of gross tumor volume considering both primary and lymph node sites to define PET-derived metabolic tumor volume (PET-MTV). In conjunction with registered CT, manual edits on auto-generated PET-MTV were performed to exclude the normal structures. Mean and maximum NSUV (NSUVmean and NSUVmax) as well as the geometric volume of PET-MTV were recorded for further analysis. Non-contrast CT-derived gross tumor volume (CT-GTV) was auto-contoured with a self-defined threshold on FIAT to visually encompass entire tumor. Manual editing was performed to exclude normal structures as necessary [10].
2.4. Computation of tumor growth rate and definition of disease progression
Tumor volumetric and metabolic growth rates were estimated based on an exponential model assuming a constant doubling time (DT) calculated as follows [8]:
where t = days between two pre-RT scans, Q1 = volume or activity on the first PET/CT, Q2 = volume or activity on the second PET/CT and ln = natural logarithm.
Growth fraction and cell loss were not taken into consideration. Previous studies have observed that tumor volume and metabolic activity in NSCLC may decrease as part of the natural history [8,11]. Under such circumstances, the DT determined by the above equation would lead to a negative value, implying an infinite DT. In order to avoid the confusion caused by this situation, we converted DT to the reciprocal of DT (RDT = 365/DT), indicating the number of doubling events within one year. Therefore a smaller RDT represents slower tumor growth, regardless of whether the value is positive or not.
TTT was recorded for all patients, referring to the interval between the first PET/CT scan and the beginning of treatment. We defined pre-treatment disease progression as visual identification of new sites of disease involvement on PET/CT and recorded whether this led to T, N, or M upstaging (AJCC 7th ed.). This definition is consistent with that published in a descriptive study discussing the natural progression of NSCLC [1]. Notably, not every site of disease progression was confirmed with histology. However the sites of new disease identified by PET/CT were reviewed at tumor board, discussed by a multidisciplinary team, and invariably management decisions included the metabolic information and changed the RT planning volumes.
2.5. Statistical analysis
The two-related-sample Wilcoxon test was used to compare individual characteristics between two PET/CT scans. The difference in baseline characteristics between various categories was evaluated using the Mann–Whitney U test. Logistic regression and receiver operating characteristic (ROC) analysis were used to determine correlations between corresponding characteristics and clinical progression. Kaplan–Meier survival analysis and log-rank test were performed to compare survival between different groups. OS was defined as the time elapsing from treatment beginning to the latest follow-up or death. PFS was defined as the duration from start of treatment to the date of first progression or death. p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Patients characteristics
A total of 118 patients were enrolled in a series of functional imaging-related studies between 2003 and 2010 and eventually 34 patients were eligible for this analysis. Thirty patients received both scans at the same institution and 4 patients had crossover PET/CT studies at both UMH and VA-AA. The demographics and tumor characteristics of the patients in this secondary analysis were similar to those who were not included. General patient demographics and tumor characteristics are shown in Table 1.
Table 1.
Patient demographics and NSCLC tumor characteristics.
| Characteristics | Number of patients (%) | ||
|---|---|---|---|
| Age | 68 (50, 84)a | ||
| Gender | Male | 23 (67.6%) | |
| Female | 11 (32.4%) | ||
| Histology | Adenocarcinoma | 6 (17.6%) | |
| Squamous cell carcinoma | 12 (35.3%) | ||
| NSCLC-NOS | 16 (47.1%) | ||
| Concurrent chemotherapy | Yes | 21 (61.8%) | |
| No | 13 (38.2%) | ||
| Physical RT dose (Gy) | 66 (45.0, 85.5)a | ||
| Inter-scan interval (day) | 43 (11, 281)a | ||
| Time to treatment (day) | 48 (18, 293)a | ||
| First scan | Second scan | ||
| Overall stage | I | 6 (17.6%) | 5 (14.7%) |
| II | 5 (14.7%) | 5 (14.7%) | |
| III | 23 (67.6%) | 22 (64.7%) | |
| IV | 0 | 2 (5.9%) | |
| T stage | Tx | 2 (5.9%) | 2 (5.9%) |
| T1 | 9 (26.5%) | 9 (26.5%) | |
| T2 | 5 (14.7%) | 5 (14.7%) | |
| T3 | 10 (29.4%) | 9 (26.5%) | |
| T4 | 8 (23.5%) | 9 (26.5%) | |
| N stage | N0 | 10 (29.4%) | 9 (26.5%) |
| N1 | 3 (8.8%) | 3 (8.8%) | |
| N2 | 14 (41.2%) | 14 (41.2%) | |
| N3 | 7 (20.6%) | 8(23.5%) |
NSCLC-NOS, non-small cell lung cancer – not otherwise speci.ed; RT, radiation therapy
Presented as median (range).
3.2. Volumetric and metabolic changes on PET/CT
Table 2 displays the actual tumor volume and metabolic activity measurements from each scan, and the absolute and relative changes between scans. The PET-MTV and CT-GTV both increased remarkably during the inter-scan period (p < 0.001). NSUVmean showed a trend towards marginal increase (p = 0.06) while NSUVmax did not significantly change (p = 0.12). There was stable or decreased PET-MTV between the pre-treatment PET/CT scans in 4 (11.8%) patients, stable or decreased NSUVmean in 11 (32.4%) patients and stable or decreased NSUVmax in 10 (29.4%) patients. No patients had a decrease in CT-GTV between scans.
Table 2.
Change in tumor volume and metabolic activity on PET/CT.
| CT-GTV |
PET-MTV |
NSUVmean |
NSUVmax |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 1st scan (cc) | 99.7 | 54.1, 145.4 | 80.2 | 38.9, 121.6 | 3.0 | 2.7, 3.4 | 7.9 | 6.3, 9.5 |
| 2nd scan (cc) | 129.7 | 75.8, 183.6 | 99.5 | 52.5, 146.6 | 3.2 | 2.8, 3.6 | 8.6 | 6.9, 10.4 |
| Actual change (cc) | 29.9 | 13.2, 46.6 | 19.3 | 8.9, 29.7 | 0.2 | −0.03, 0.4 | 0.7 | −0.5, 1.9 |
| Relative change (%) | 69.1 | 31.6, 106.6 | 54.7 | 28.3, 81.1 | 5.7 | −0.23, 11.6 | 13.8 | 0.9, 26.7 |
| pa | <0.001 | <0.001 | 0.064 | 0.122 | ||||
CT-GTV, gross tumor volume on CT; PET-MTV, metabolic tumor volume on PET; NSUVmean, average tumor metabolic activity normalized to aorta; NSUVmax, maximum tumor metabolic activity normalized to aorta; 95% CI, 95% of con.dence interval.
Results from two-related-sample Wilcoxon test.
3.3. Doubling time of tumor
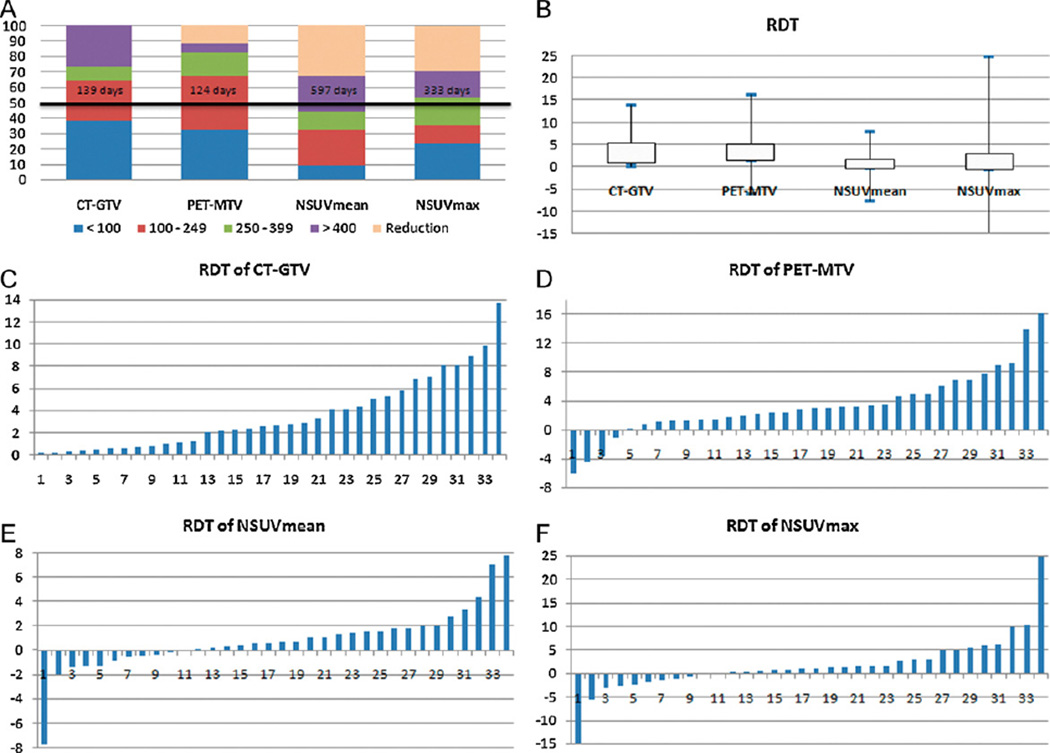
The median DT for PET-MTV, CT-GTV, NSUVmean and NSUVmax was 124, 139, 597 and 333 days, respectively. Approximately half of the patients had metabolic DT longer than 400 days, whereas more than two thirds of patients were estimated to have volumetric DT less than 250 days. PET-MTV and CT-GTV both demonstrated significantly faster growth than metabolic activity (NSUVmean and NSUVmax) (p < 0.01). Fig. 1 summarizes the RDTs for each patient.
Fig. 1.
Volumetric and metabolic doubling time (DT) determined on PET/CT. (A) DT distribution for each parameter based on all patients. Y-axis indicates the percentage distribution for each DT category and the solid line shows the median DT for each parameter. (B) Comparison in terms of RDT among parameters. (C, D, E, F) Waterfall plots of RDTs for CT-GTV, PET-MTV, NSUVmean and NSUVmax, respectively. CT-GTV, gross tumor volume on CT; PET-MTV, metabolic tumor volume on PET; NSUVmean, mean tumor metabolic activity normalized to aorta; NSUVmax, maximum tumor metabolic activity normalized to aorta; RDT, reciprocal of doubling time = 365/DT.
Twelve patients had non-contiguous primary and metastatic lymph node sites. There was no significant difference in volumetric growth whereas lymph node seemed to have faster growth in NSUVmax than primary tumor, corresponding to the mean RDT of 4.0 and 0.5 (p = 0.034).
3.4. Pre-treatment disease progression
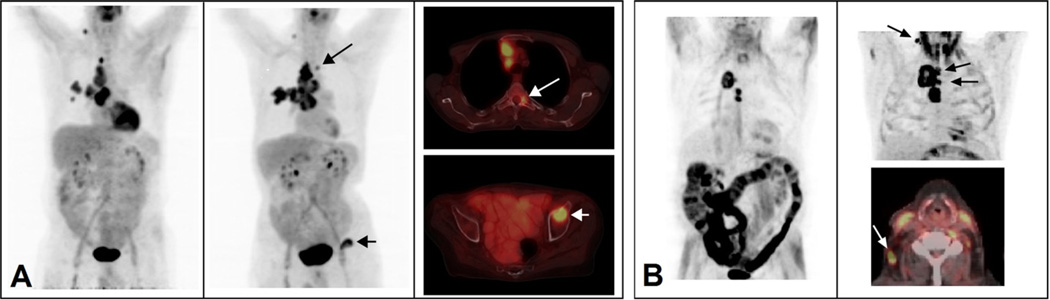
The median ISI for stages I, II, III and total patients, was 32, 43, 54, and 43 days, respectively (p = 0.804). The median interval from the second PET/CT scan to RT beginning was 7 days. Seven patients (20.6%) developed pre-treatment disease progression based on our criteria and 6 patients (17.6%) were upstaged on TNM during the ISI. Patient-specific details of disease progression are summarized in Table 3. Two examples of pre-treatment disease progression on PET/CT are shown in Fig. 2. Using logistic regression, initial stage did not correlate with the occurrence of interval disease progression. Viewed as a continuous variable, longer ISI was a risk factor for disease progression (OR = 1.027, 95% CI: 1.004–1.050, p = 0.02). Based on ROC analysis, the area under the curve (AUC) of ISI was 0.847 (p = 0.005) for the prediction of disease progression with the optimal criterion of 58 days. The occurrence of disease progression was 4.8% for patients with ISI ≤ 58 days and 46.2% for those with ISI > 58 days (p = 0.007). No significant difference was found in DT between patients that had inter-scan progression and those who did not.
Table 3.
Pre-treatment disease progression on PET/CT.
| Patients no. | Primary location | TNM 1st PET/CT |
TNM 2nd PET/CT |
Sites of progression |
|---|---|---|---|---|
| 1 | LUL | T1aN0M0 | T1bN0M0 | T upstaging |
| 3 | RUL | T3N3M0 | T3N3M1 | LN involvement at a new station bone metastasis |
| 13 | RUL | T4N2M0 | T4N3M0 | N upstaging |
| 21 | RLL | T4N2M0 | T4N2M1 | New retrocrural and periaortic LN involvement |
| 23 | RUL | T3N2M0 | T4N2M0 | T upstaging, LN involvement at a new station |
| 29 | RUL, RML | T4N2M0 | T4N2M0 | LN involvement at a new station |
| 30 | RUL | T1N0M0 | T1N2M0 | N upstaging |
LUL, left upper lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe; LN, lymph node.
Fig. 2.
Imaging examples of clinical disease progression prior to treatment. (A) A patient with a hypermetabolic right upper lobe mass, adjacent satellite lung nodule and FDG-avid right hilar, mediastinal and right supraclavicular lymph nodes. (Left) Maximum intensity projection images (MIPS) at the 1st pre-treatment PET scan demonstrates T3N3M0 disease. (Middle) MIPS at the 2nd pre-treatment PET scan demonstrates new abnormal FDG uptake in the thoracic spine (long arrow) and left acetabulum (short arrow). (Right top) 2nd pre-treatment axial fused PET/CT demonstrates a left T3 pedicle bone metastasis (long arrow). (Right bottom) 2nd pre-treatment axial fused PET/CT demonstrates a left acetabulum bone metastasis (short arrow) compatible with T3N3M1 disease. (B) A second patient with a hypermetabolic right upper lobe mass and FDG-avid lower mediastinal lymph nodes. (Left) MIPS at the 1st pre-treatment PET scan demonstrates T4N2M0 disease. (Right top) MIPS at the 2nd pre-treatment PET scan demonstrates new upper paratracheal lymph nodes and new ipsilateral neck lymph nodes with abnormal metabolic activity (arrows) indicating T4N3M0 disease. (Right bottom) 2nd pre-treatment axial fused PET/CT at the level of the right neck lymph nodes (arrow).
3.5. Post-treatment outcome
By January 2012, 32 out of 34 patients regularly followed up for at least 6 months were included in the patient-outcome analysis. The minimum follow-up time for surviving patients was 7.0 months. Referring to the published literature [12], we used DT of 180 days as the threshold to divide patients into fast- and slow-growth groups, and found that neither PET-MTV (p = 0.247) nor CT-GTV (p = 0.870) based grouping could achieve statistically significant difference in OS. Similarly, no significant difference was found between groups in terms of PFS as well (p = 0.516 for PET-MTV and p = 0.361 for CT-GTV). Using the optimal inter-scan duration of 58 days and the median interval of 7 days from the second scan to RT start, we identified a discriminative point to divide patients into short-TTT (≤65 days) and long-TTT (>65 days) groups. However, the effect of TTT on either OS (p = 0.713) or PFS (p = 0.210) did not reach statistical significance.
4. Discussion
In this study of NSCLC diagnosed during routine clinical care, tumor volumetric measurements increased remarkably in a short interval. In contrast, tumor metabolic activity (NSUVmean and NSUVmax) remained relatively stable during the same period of time. VDT of early stage lung cancer has been widely studied based on X-ray and CT evaluations of pulmonary nodules and has a strikingly broad range [3–8,13,14]. One study found that the mean VDT of pulmonary nodule was approximately 150 days on chest radiographs and 480 days on CT in screening studies, compared to a VDT of 135 days for those detected during routine medical care [14]. Using direct volume measurement on the non-contrast CT portion from the PET/CT imaging, we found a median VDT of 139 days. The distribution of CT-VDT in our study was consistent with a recent report based on routine CT detection, in which each VDT category (<100, 100–249 and ≥250 days) accounted for approximate one third of the overall study cohort [15].
Metabolic tumor volume (PET-MTV) on PET/CT is being increasingly studied as a characteristic of tumor biological behavior. At present PET-MTV does not have a consistent definition [16–19]. To our knowledge, there have been only two studies exploring the changes in PET-MTV in untreated lung cancer [11,20]. One reported a 32% mean relative increase in PET-MTV with a median interval of 24 days between scans and an expected DT of 66 days [11]. Another study of 11 NSCLC patients found a 51% average increase in PET-MTV with a median interval of 33 days between PET scans, with 4 patients demonstrating a DT less than 45 days [20]. In our study of PET-MTV, we observed a 55% mean increase with a median interval of 43 days and an estimated median DT of 124 days in 34 patients. Interestingly, the DT of PET-MTV did not significantly differ from the median CT-GTV derived DT based on the near-simultaneously acquired CT imaging. Interval PET-MTV shrinkage was observed in our study, including two patients with very small regression (<10 cc) in the primary tumor, and two with regression in hilar nodes. These findings are similar to the first study above mentioned who found 24% of their patients underwent a PET-MTV remission at the second PET/CT scan. Decreases in tumor volume without treatment intervention has also been reported on numerous CT based studies [6–8] and may be attributable to multiple factors such as measuring variation, respiratory motion, misregistration, proximity to the mediastinum, re-expansion of adjacent consolidated lung tissue, and tumor necrosis due to insufficient blood and nutrition supply.
The natural history of metabolic activity in lung cancer remains poorly understood and currently we are aware of only one published study investigating the NSCLC SUV change prior to treatment [11]. In this study, a significant increase in SUV measurements was observed within a median interval of 24 days, including 19% enhancement in maximum SUV and 15.6% in average SUV. Notably, 24% of patients underwent interim reduction in maximum SUV. In our study, the observed change in metabolic activity was less significant. Within the median interval of 43 days, NSUVmean achieved a marginally increase while NSUVmax was relatively stable. Nearly one third of our patients displayed interval reduction in metabolic activity. It should be noted that different methods of SUV measurements were used; absolute SUV in the above-mentioned published study and internally normalized SUV in our study. In addition, it is important to note that maximum SUV measurements are more susceptible to noise, which may exacerbate observed percentage changes between scans [21].
We found metabolic evidence for pre-treatment disease progression in 21% patients and TNM upstaging in 18% after a relatively short median inter-scan interval of 43 days. Longer intervals before start of treatment appear to have higher risk of pre-treatment progression, lending credence to the notion that excessive delay before treatment may lead to worsened patient-outcomes. Using criteria for pre-treatment disease progression similar to that in our study, one study found disease progression prior to treatment occurred in 13%, 31% and 46% of patients at 4 weeks, 8 weeks and 16 weeks after initial clinical detection with upstaging in 13% of patients at 8 weeks, and 21% at 16 weeks [1]. Another study reported an even greater chance of tumor progression within a median interval of 28 days, with upstaging in TNM scores in 39% of patients, and changes in treatment intent from curative to palliative in 29% of patients [11]. Given these findings of significant pre-treatment disease progression, repeated staging appears justified after certain delays in treatment initiation. One study recommended complete restaging after 4–8 weeks of treatment initiation delay [1]. In our study, longer inter-scan interval (ISI) was found significantly correlated with higher risk of interval disease progression prior to treatment. Based on the estimation of progression probability, we suggest that restaging of PET-CT should be performed for NSCLC patients with longer than 2 months (58 days) waiting after the initial PET/CT examination.
Studies investigating the effects of delays to treatment on patient-outcomes have found that local control rates decreased with increasing TTT, raising the possibility that these increased rates of local recurrence might in turn translate into worse survival [22–25]. In our study, no significant difference was detected between TTT and OS or PFS. Furthermore, we did not find that DT significantly affected OS or PFS, which was counter-intuitive. These negative findings may be explained by our small sample size, heterogeneity of tumor parameters, and the lack of multivariate modeling of additional prognostic factors. The active intervention of salvage treatment and supporting care may also offset the detrimental effect of longer TTT and shorter DT on outcome. Despite this, in view of the findings of pre-treatment disease progression within a 2 month period, it seems prudent to follow recommendations by the Joint Council for Clinical Oncology that potentially curative treatment should ideally start within 2 weeks and no longer than 4 weeks after decision of treat [26].
We acknowledge several limitations in our study. Firstly, the estimation of the doubling times was based on only two pre-treatment PET/CT images rather than multiple serial scans, therefore the calculations would be more susceptible variation due to technical factors in volumetric and metabolic measurements. Another limitation was that CT-GTV in our study was delineated on the CT portion of integrated PET/CT rather than IV-contrast enhanced CT, which could lead to substantial uncertainty for target identification, though our protocol did not allow for an IV-contrast CT in addition to the PET/CT for our patient. Further investigations on a larger number of patients with multiple serial measurements would be required to better understand the natural growth of lung cancer, however due to ethical considerations this would not be acceptable as a clinical study design.
5. Conclusions
The natural history of NSCLC diagnosed at routine clinical care based on FDG-PET/CT is that of rapid growth in tumor volume with relatively stable tumor metabolic activity (NSUVmean and NSUVmax). Longer waiting time before treatment is associated with higher risk of pre-treatment disease progression. For treatment delays of longer than 2 months after initial PET/CT examination, repeated staging workup is recommended.
Acknowledgements
We sincerely thank Yue Cao, Ph.D., Randall K Ten Haken, Ph.D., and Marc Kessler Ph.D. for the establishment and maintenance of FIAT work station.
Funding
This work was funded in part by R21CA127057 and R01 CA142840.
Footnotes
Disclosures: This work was presented as a poster presentation at the 52nd Annual Meeting of American Society for Therapeutic Radiology and Oncology (ASTRO) held in San Diego, California, October 31–November 4, 2010.
Conflict of interest statement
None declared.
References
- 1.Mohammed N, Kestin LL, Grills IS, Battu M, Fitch DL, Wong CY, et al. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:466–472. doi: 10.1016/j.ijrobp.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Schultz EM, Sanders GD, Trotter PR, Patz EF, Jr, Silvestri GA, Owens DK, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63:335–341. doi: 10.1136/thx.2007.084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65:284–297. doi: 10.1002/(sici)1096-9098(199708)65:4<284::aid-jso11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Usuda K, Saito Y, Sagawa M, Sato M, Kanma K, Takahashi S, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer. 1994;74:2239–2244. doi: 10.1002/1097-0142(19941015)74:8<2239::aid-cncr2820740806>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Yankelevitz DF, Kostis WJ, Henschke CI, Heelan RT, Libby DM, Pasmantier MW, et al. Overdiagnosis in chest radiographic screening for lung carcinoma: frequency. Cancer. 2003;97:1271–1275. doi: 10.1002/cncr.11185. [DOI] [PubMed] [Google Scholar]
- 6.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, et al. Fiveyear lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242:555–562. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 7.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Mand rekar JN. 5-year lung cancer screening experience: growth curves of 18 lung cancers compared to histologic type, CT attenuation, stage, survival, and size. Chest. 2009;136:1586–1595. doi: 10.1378/chest.09-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings SG, Winer-Muram HT, Tann M, Ying J, Dowdeswell I. Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements. Radiology. 2006;241:554–563. doi: 10.1148/radiol.2412051185. [DOI] [PubMed] [Google Scholar]
- 9.Kong FM, Frey K, Quint L, Ten Haken R, Hayman J, Kessler M, et al. A pilot study of 18F.fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 10.Kong FM, Mahasittiwat P, Yuan S, Xie C, Ritter T, Shen Z, et al. ITART 2010 Imaging for Treatment Assessment in Radiation Therapy. National Harbor, MD: 2010. Jun 21–22, Define tumor volume during radiotherapy to individulize adaptive radiation dose escalation in non-small cell lung cancer. [Google Scholar]
- 11.Everitt S, Herschtal A, Callahan J, Plumridge N, Ball D, Kron T, et al. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer. 2010;116:5030–5037. doi: 10.1002/cncr.25392. [DOI] [PubMed] [Google Scholar]
- 12.Tann M, Sandrasegaran K, Winer-Muram HT, Jennings SG, Welling ME, Fletcher JW. Can FDG-PET be used to predict growth of stage I lung cancer? Clin Radiol. 2008;63:856–863. doi: 10.1016/j.crad.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Quint LE, Cheng J, Schipper M, Chang AC, Kalemkerian G. Lung lesion doubling times: values and variability based on method of volume determination. Clin Radiol. 2008;63:41–48. doi: 10.1016/j.crad.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol. 2008;3:781–792. doi: 10.1097/JTO.0b013e31817c9230. [DOI] [PubMed] [Google Scholar]
- 15.Winer-Muram HT, Jennings SG, Tarver RD, Aisen AM, Tann M, Conces DJ, et al. Volumetric growth rate of stage I lung cancer prior to treatment: serial CT scanning. Radiology. 2002;223:798–805. doi: 10.1148/radiol.2233011026. [DOI] [PubMed] [Google Scholar]
- 16.Vriens D, de Geus-Oei LF, van Laarhoven HW, Timmer-Bonte JN, Krabbe PF, Visser EP, et al. Evaluation of different normalization procedures for the calculation of the standardized uptake value in therapy response monitoring studies. Nucl Med Commun. 2009;30:550–557. doi: 10.1097/MNM.0b013e32832bdc80. [DOI] [PubMed] [Google Scholar]
- 17.Feng M, Kong FM, Gross M, Fernando S, Hayman JA, Ten Haken RK. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;73:1228–1234. doi: 10.1016/j.ijrobp.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Loon J, Grutters JP, Wanders R, Boersma L, Dingemans AM, Bootsma G, et al. 18FDG-PET-CT in the follow-up of non-small cell lung cancer patients after radical radiotherapy with or without chemotherapy: an economic evaluation. Eur J Cancer. 2010;46:110–119. doi: 10.1016/j.ejca.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Frings V, de Langen AJ, Smit EF, van Velden FH, Hoekstra OS, van Tinteren H, et al. Repeatability of metabolically active volume measurements with 18FFDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med. 2010;51:1870–1877. doi: 10.2967/jnumed.110.077255. [DOI] [PubMed] [Google Scholar]
- 20.Eastham DV, Weerasuriya D, Wakelee H, Quon A, Maxim P, Le Q, et al. Quantification of progression of non-small cell lung cancer in the interval between diagnosis and radiotherapy treatment planning PET scans. Int J Radiat Oncol Biol Phys. 2007;69:S520–S521. [Google Scholar]
- 21.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;(Suppl. 50)(1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackillop WJ, Bates JH, O’Sullivan B, Withers HR. The effect of delay in treatment on local control by radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:243–250. doi: 10.1016/0360-3016(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Correa CR, Hayman JA, Zhao L, Cease K, Brenner D, et al. Time to treatment in patients with stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:790–795. doi: 10.1016/j.ijrobp.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson E, Mohilever J, Zidan J, Sapir D. Delay in diagnosis of cancer. Possible effects on the stage of disease and survival. Cancer. 1984;54:1454–1460. doi: 10.1002/1097-0142(19841001)54:7<1454::aid-cncr2820540739>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Williams MV, Drinkwater KJ, Jones A, O’Sullivan B, Tait D. Waiting times for systemic cancer therapy in the United Kingdom in 2006. Br J Cancer. 2008;99:695–703. doi: 10.1038/sj.bjc.6604529. [DOI] [PMC free article] [PubMed] [Google Scholar]