Abstract
We sought to explore the roles of the hippocampal subregions and adjacent medial temporal lobe regions in pattern separation and any differential contributions based on sequential or spatial information. Young adults performed an incidental encoding task on a sequence of four objects presented on the screen in one of 8 locations while we collected high-resolution functional MRI brain scans. We employed 5 trials of interest: first presentations, exact repetitions, lures in which the same objects were repeated in different locations (spatial lures), lures in which the same objects were presented in a different sequential order (sequential lures), and lures in which both the spatial location and sequence were changed (both lures). We found no evidence for spatial or sequential specialization in the hippocampal subfields, consistent with the hypothesis that the dentate gyrus acts as a universal pattern separator. Likewise, we did not observe specialization for the perirhinal or parahippocampal cortices for spatial or sequential information, though both regions show evidence for associative processing in this task.
Keywords: hippocampus, medial temporal lobe, CA1, CA3, sequence learning
Introduction
Episodic memory refers to memory for specific events along their spatiotemporal context (Tulving, 1972) and requires, at the very least, a combination of what, where, and when components (Eichenbaum, Sauvage, Fortin, Komorowski, & Lipton, 2012). The neural mechanisms that underlie spatial and temporal representations thought to be components of episodic memory have been related to place cells, and more recently to time cells, in the hippocampus (O’Keefe & Dostrosky, 1971; MacDonald, Lepage, Eden, & Eichenbaum, 2011). The hippocampus itself can be divided into subregions (Amaral & Witter, 1989) that may be differentially involved in these memory processes. The current study aims to explore the contributions of the hippocampal subregions to the spatial and temporal dimensions of episodic memory by assessing subregional activity consistent with pattern separation along these two dimensions.
Computational modeling, electrophysiological, and immediate early gene (IEG) studies have demonstrated that the dentate gyrus (DG) subregion of the hippocampus is preferentially involved in pattern separation – transforming similar input representations into highly dissimilar output representations (Treves & Rolls, 1994; Guzowski, Knierim, & Moser, 2004a; Leutgeb, Leutgeb, Treves, Moser, & Moser, 2004; Leutgeb et al., 2005a; Leutgeb, Leutgeb, Moser, & Moser, 2007; Leutgeb et al., 2005b; Leutgeb & Leutgeb, 2007; Vazdarjanova & Guzowski, 2004). The granule cells of the dentate gyrus are capable of performing strong pattern separation on overlapping/distributed representations arriving from the entorhinal cortex (Marr, 1971), which is then projected onto the CA3 subfield of the hippocampus. This pattern separation ability is often proposed to be a critical component of episodic memory (Norman, 2010).
Recent studies utilizing high-resolution functional magnetic resonance imaging (fMRI) have added credence to the pivotal role of the DG in pattern separation in humans (Bakker, Kirwan, Miller, & Stark, 2008; Lacy, Yassa, Stark, Muftuler, & Stark, 2011).Bakker et al. (2008) reported that only the DG/CA3 region (combined due to limitations in the resolution that prevent isolating them) exhibited a response consistent with a strong pattern separation signal by treating similar lure items much like new items. In an extension of this work, the transfer function (relationship between similarity of the input and similarity of the output) was shown to be highly non-linear and sensitive to small changes in input in the human DG/CA3, again consistent with pattern separation (Lacy et al., 2011).
In contrast to the DG/CA3, IEG (Guzowski, Knierim, & Moser, 2004b; Vazdarjanova & Guzowski, 2004) and electrophysiological recording studies in rodents (Leutgeb et al., 2004; Leutgeb et al., 2005a; Leutgeb et al., 2005b; Leutgeb & Leutgeb, 2007) and imaging studies in humans (Lacy et al., 2011; Duncan, Ketz, Inati, & Davachi, 2011) have demonstrated that the CA1 often responds linearly to the amount of change in input, whereas the CA3 responds in a sigmoidal or thresholded manner. Thus, the transfer function that maps the degree of mismatch between the inputs onto output representations differs across CA1, CA3, and DG in pattern separation responses (Chen, Olsen, Preston, Glover, & Wagner, 2011; Yassa & Stark, 2011).
Beyond differences in pattern separation, the hippocampal subfields may also demonstrate preferential specialization for specific information. For example, some rodent studies have shown that the CA1 and DG preferentially respond to temporal and spatial information, respectively (see Rolls & Kesner, 2006; Gilbert, Kesner, & Lee, 2001; Kesner, Lee, & Gilbert, 2004). Lesion studies have shown that the CA3 is critical for remembering sequences of spatial locations (Hunsaker, Lee, & Kesner, 2008), but not sequences of nonspatial events (Hoge & Kesner, 2007). However, this spatial vs. temporal or sequential differentiation is not universally observed. Other studies suggest that CA3 is not limited to spatial information, but is critical for sequence memory in general as well (Farovik, Dupont, & Eichenbaum, 2010). Likewise, the DG may be critical for sequence learning in collaboration with the CA3 (Lisman, Talamini, & Raffone, 2005). Thus, there is conflicting evidence regarding specificity for the subfields concerning spatial and temporal specialization. It may be that the DG and CA3 are domain-agnostic with the DG being a universal pattern separation device that operates along any dimension rather than a device that only separates along a spatial dimension.
We sought to determine whether there was differential sensitivity to small changes along sequential or spatial dimensions in hippocampal subregions and adjacent medial temporal lobe regions using a paradigm similar to that inBakker et al. (2008). Participants engaged in an incidental-encoding task during a high-resolution fMRI scan, wherein we manipulated the temporal and spatial components of the task. We chose to vary the type of change independently to produce a parametric scale of the total amount of change in the input. Thus, we included trials in which the number of changes that constitute a lure increases from 0 (an exact repetition) to 1 (a spatial or a sequential lure) to 2 (both a spatial and sequential lure).
Assessing pattern separation using fMRI or behavior can currently only be done in an indirect manner as we cannot observe the actual transformation of neural representations. Here, we took advantage of the repetition suppression effect, in which the second presentation of an item (an exact repetition) will often elicit less fMRI activity than a novel item (Grill-Spector & Malach, 2001). Utilizing this approach allows us to assess activity consistent with pattern separation by comparing the activity for similar lure items to the activity for exact repeats and novel foils (Bakker et al., 2008; Lacy et al., 2011). If activity for similar lure items resembles activity for novel items and not activity for repeated items, we have evidence that this region is highly sensitive to the change and treats the similar lures as if they were independent of the prior version – an effect consistent with pattern separation.
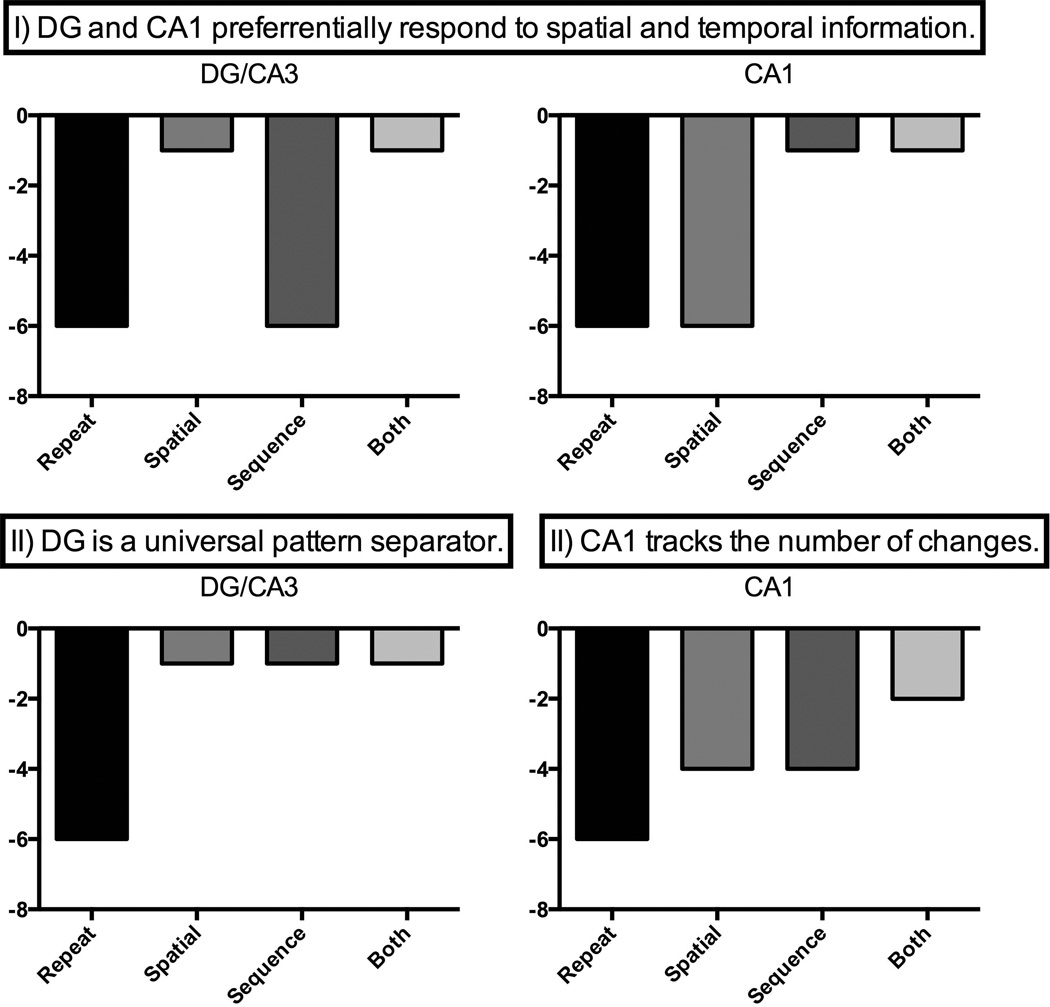
We sought to test two possible predictions: (I) DG/CA3 will exhibit a domain-general sensitivity to all similar lures (spatial, sequential and both lures). Alternatively, (II) the CA1 and DG/CA3 might preferentially respond to sequential and spatial lures, respectively. Specifically, this prediction states that the CA1 would exhibit the least amount of repetition suppression for similar temporal lures and DG/CA3 for similar spatial lures compared to the other conditions. Consistent with some of the aforementioned studies, the CA1 may also exhibit a linear transfer function that tracks the number of changes (from repeat-0 to sequence/spatial-1 to both-2; Figure 1). In addition, we acquired high-resolution functional neuroimaging data from the entire MTL region, which affords the opportunity to contrast the activity in the MTL cortical regions, specifically the perirhinal (PRC) and parahippocampal (PHC) cortices.
Figure 1.
Alternative predictions for the DG and CA1 hippocampal subfields for the sequential, spatial, and both lures.
Materials and Methods
Participants
Twenty-two participants (10 females; mean age = 21.79 years, range from 18–28) were recruited from the University of California, Irvine and were compensated for their time. All participants had normal or corrected-to-normal vision and no notable neurological or psychiatric history. Written consent was obtained in compliance with the local Institutional Review Board. Two participants were excluded because of excessive head movement and one participant terminated their session early due to discomfort in the MRI scanner, resulting in a total of 19 participants. All participants were right-handed and fluent in English.
Experimental design
Task stimuli consisted of 1,389 pictures of common objects, presented with MATLAB 7.4. Each trial consisted of four consecutively presented pictures, each located on one of eight locations on the screen. Each stimulus was presented for 900 ms with an inter-stimulus interval of 400 ms. Each trial was followed by one of three possible encoding questions presented for 1.9 seconds: 1) Were there more living than non-living items?; 2) Were there more living items in the first two items of the sequence versus the last two items?; or 3) Were there more living items than non-living item presented on the left and top part of the screen than the right and bottom parts? Participants were instructed to group the top three locations and left middle position together and the bottom three locations and right middle location for this latter judgment. Participants pressed buttons using their right hand, indicating if the answer was ‘yes’, ‘no’, or ‘equal’. Subjects were encouraged to respond to each trial during the presentation of the encoding questions. If they succeeded in responding during the allowable time, the word ‘OK’ was presented on the screen. Training on the task using novel items was provided for each subject prior to data collection to ensure an adequate understanding of the instructions and compliance with the task.
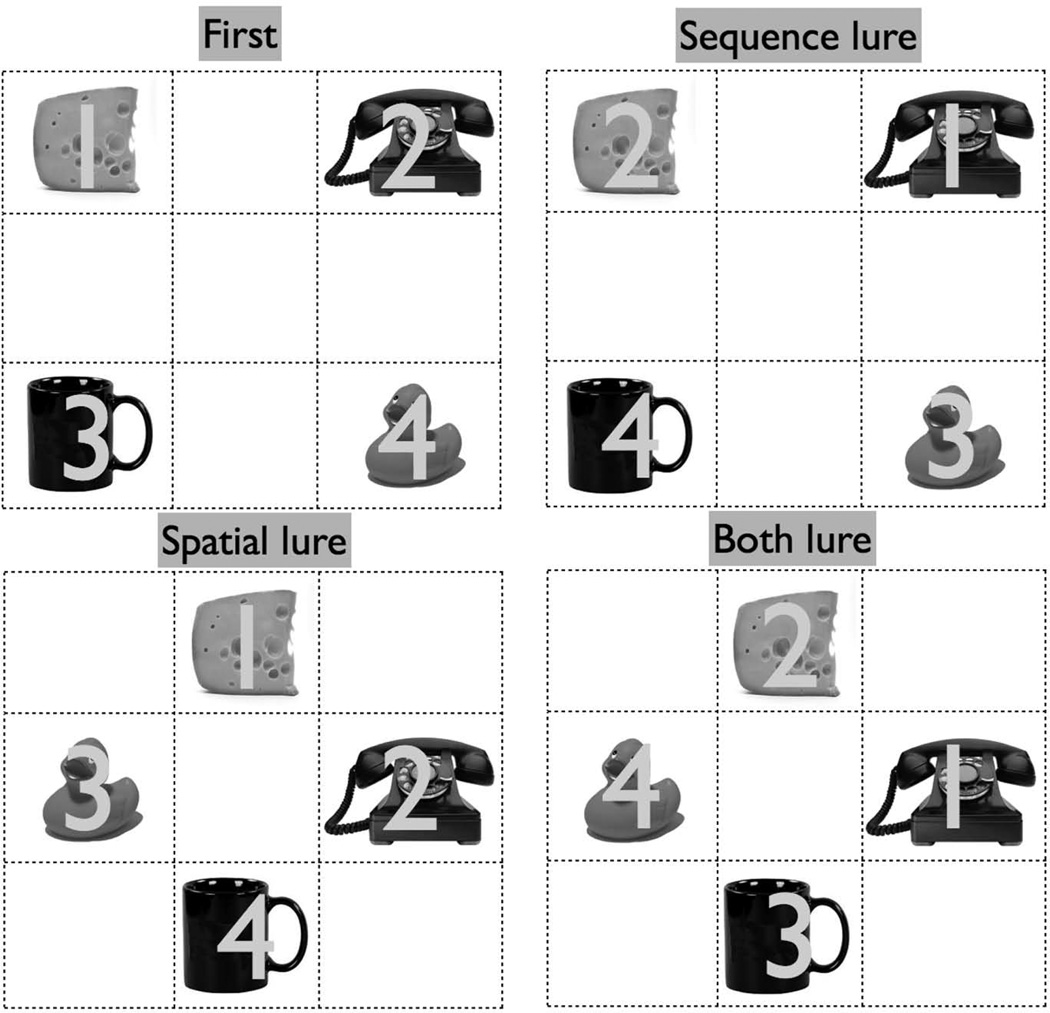
Each trial belonged to one of six conditions (Figure 2): (1) the singleton condition was defined as first presentation trials that were not repeated in any form later. (2) first presentations for which there was a second iteration (either an exact repeat or a lure): (3) exact repetitions of earlier trials (repeats), (4) repetitions of earlier trials presented in the original sequence but with all items presented in novel locations on the screen that were not occupied in the earlier matching first presentation trial (spatial lures) (5) repetitions of earlier trials but presented in novel sequences in which none of the ordinal positions matched the earlier first presentation trials (sequence lures), or (6) repetitions in which both the sequence and spatial locations differed from the matching earlier first presentation trial (both lures). There were 64 trials of each condition, resulting in a total of 576 trials. The gap between any first presentation and its matching second presentation was 2, 3, or 4 trials.
Figure 2.
Schematic of the experimental paradigm. The numbers 1–4 indicate the ordinal position of the stimulus presentation. Only one stimulus was presented on the screen at a time, and actual stimuli were presented in color.
The stimuli were presented on a 3×3 grid (the grid lines were not visible during the task), but no stimulus was ever presented in the center of the screen, resulting in 8 possible locations. In a first presentation, stimuli were randomly presented in 4 of the 8 possible locations. During spatial and both lure trials, stimuli were then randomly assigned to the other 4 previously unused locations. For the sequential and both lures, the 4 stimuli were randomly presented in an ordinal position (1–4) during the first presentation and then randomly mixed such that none of the four stimuli occupied the same ordinal position that they occupied previously.
All trials that were related to previous trials (repetitions or lures – see below) were followed by the default living/non-living encoding questions; only ‘singleton’ trials (see below) were followed by an equal number of default, sequence, or spatial instructions. We included the sequence and spatial instructions on the singleton trials in order to ensure that participants were attending to and encoding these spatial and sequential aspects as, to the participant, they might be asked about either dimension on any given trial. However, we did not want this decision process and the variability associated with different decisions to affect the main trial types of interest. Given the slow nature of the BOLD response, estimates of activity during the later aspects of the trial would be contaminated by these. By using only the living/non-living judgment following the trial types of interest, we could avoid these problems.
Prior to the neuroimaging study, we conducted a behavioral study (n=17) to examine whether our spatial and sequential conditions were of similar difficulty and that participants would remember the spatial and temporal aspects of trials in our paradigm. This study used the same behavioral parameters, but included 12 extra living/non-living singleton trials at the end of the task using the default living/nonliving judgment. These extra trials were then used in a surprise subsequent memory test. This test assessed how well participants had incidentally encoded the spatial and sequential information by randomly showing four repetitions, four spatial lures, and four sequence lures, and asking participants to identify whether that exact trial had been shown.
Scanning Session and Imaging Parameters
MRI data were collected on a 3T Philips scanner using an 8-channel SENSE (SENSitivity Encoding) head coil. The trials were divided among 16 functional runs, each taking 4.2 minutes. fMRI data were collected using an EPI pulse sequence with a repetition time (TR) of 1500 ms, an echo time (TE) of 26 ms, a flip angle of 70 degrees, a field of view (FOV) of 120X120, and a SENSE reduction factor of 2 to yield coverage of the MTL at 1.5 mm isotropic resolution. For anatomical localization and segmentation, we acquired a 0.75 mm isotropic MPRAGE structural scan.
Distortions of the EPI signal were controlled by: 1) higher-order shims (which can directly compensate for local field distortions), 2) SENSE parallel imaging (which uses multiple surface coils to under-sample k-space with fewer phase encoding steps (Pruessmann, Weiger, Scheidegger, & Boesiger, 1999), and 3) the use of thin slices as artifacts are a function of number of slices from the boundary that causes inhomogeneity in the magnetic field rather than absolute distance from this boundary (Buxton, 2001). Before data analysis, images were first co-registered to correct for within- and across-scan head motion. Acquisitions in which a significant motion events occurred were excluded. We used Advanced Normalization Tools (ANTs; Avants et al., 2008) for cross-participant alignments.
fMRI data analysis
The fMRI data were first subjected to a traditional general linear model analysis using multiple regression in AFNI (Cox, 1996). In addition to nuisance vectors coding for low-frequency drift in the signal, four vectors of interest were specified: exact repetitions (repeat) and second presentations of either sequence lures (sequence), spatial lures (spatial) or both sequence and spatial lures (both). First presentation trials (including singles) were not modeled and served as an implicit non-zero baseline condition, against which each of the other four conditions was compared (Stark & Squire, 2001). These vectors were used to individually model each participant’s functional data utilizing a deconvolution approach built into AFNI's 3dDeconvolve (Ward, 2001). We used 15 tent functions to estimate the hemodynamic response to each condition. The resultant fit coefficients (β coefficients) represent activity versus baseline for each condition of interest at a given time point in each voxel. The sum of the resultant fit coefficients over the bulk of the expected hemodynamic response (3–13.5 seconds after trial onset) was taken as the model’s estimate of the response for each trial type and passed on to group-level analyses.
To investigate pattern separation, we first isolated voxels in which a repetition of the stimulus results in a change in the activity (often called a "repetition suppression " effect, although the change in activity is not limited to reductions here). The activity for similar lure items in voxels showing repetition-related changes can be used to infer whether a region is biased towards exhibiting pattern separation-like or completion-like signals (e.g. Bakker et al., 2008; Lacy et al. 2011). Specifically, if a voxel displays similar responses to first presentation and lure items, this would be consistent with the voxel reflecting pattern separation related activity for this particular form of lure.
To identify these signals, group-level analyses began by warping the model estimates into template space using the vector field derived with ANTs for structural scan normalization. In the process, the activity estimates were resampled to 0.75 mm isotropic voxels. In selecting repetition-sensitive voxels, a somewhat liberal threshold was used (p<.05, 50 contiguous voxels) in this first pass. This threshold is consistent with our prior related work (Lacy et al., 2011) and is designed to reduce voxel selection biases in the initial filtering step (Baker et al., 2007). Activity for each condition was then collapsed within each functional ROI so that the critical comparisons with the lure conditions could be made. The alpha for these t-tests and F-tests was set at p<0.05 representing the final alpha in the group analyses.
A second analysis of the fMRI data examined differences in the functional connectivity between brain regions as a function of trial type. The term "functional connectivity" refers simply to the temporal correlation between regions or voxels (Friston, Frith, Fletcher, Liddle, & Frackowiak, 1996). Here, we used a psychophysiological interaction (PPI) approach (Friston et al., 1997) to assess how this correlation is modulated by trial type. In particular, we focused on how the seed regions of interest (DG/CA3 and CA1) correlated with the rest of MTL regions change as a function of the spatial versus sequence contrast. The analysis began with a deconvolution protocol that modeled all trials including baseline (first presentations) trials for all scanned voxels. To assess the basic functional connectivity, we included a regressor representing the time series activity from our seed region. To assess the modulation, we also included a second regressor representing the interaction between our critical spatial/sequence contrast and the time series from our seed region. The isolated interaction correlation coefficients (r) between the seed region and each voxel in the MTL were Fisher’s z-transformed and applied to the group level. Given our coding, a positive correlation would indicate that the functional coupling between the seed region and a voxel is greater for spatial lure trials relative to sequential lure trials, whereas a negative correlation would indicate the converse.
Behavioral Results
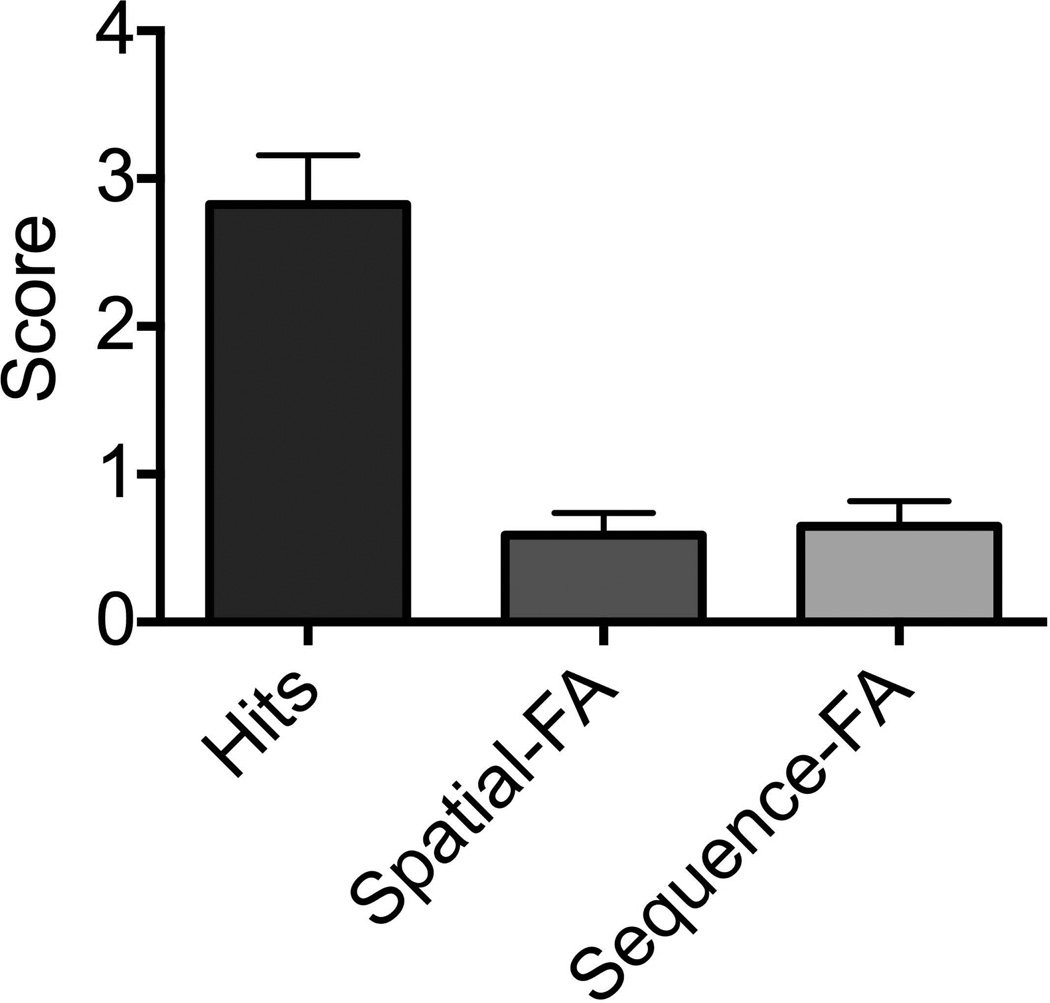
Our initial behavioral experiment (Figure 3) indicated that participants were successfully encoding both the spatial and sequential aspects of the task. In a 12-item recognition post-test, participants endorsed an average of 2.8 of the exact repetitions (70% correct) and had false alarms to spatial and sequential lures an average of 0.59 and 0.64 trials respectively (85% and 84% correct; Mann Whitney U=140, p=0.97). Thus, even when the post-stimulus instructions were not overtly spatial or sequential (simply living/non-living) and even though the lag between study and test here was twice as long as the lags during the main experiment, these aspects were encoded to a similar degree (see Van Asselen et al., 2006, for similar results). Though we were limited in the number of trials in which we could assess memory using the surprise recognition test, these results demonstrate that the spatial and sequential elements were both attended to in a comparable way.
Figure 3.
Behavioral test results indicate comparable memory performance for the spatial and temporal lures.
Spatial vs. Sequential Changes in Hippocampal Subfields
In this paradigm, we first identified repetition-sensitive voxels (first presentations – exact repeats: p<0.05, 50 contiguous voxels). As expected (Bakker et al., 2008; Lacy et al., 2011), repetition-suppression sensitive voxels were found throughout the MTL. Here, we focused on the regions pertinent to our pre-experimentally determined hypotheses, the PRC, PHC, CA1, and DG/CA3. We calculated the mean beta coefficients for each trial type of interest, each of these regions, and each participant. Critically, we searched for any differential response to sequence and spatial lures.
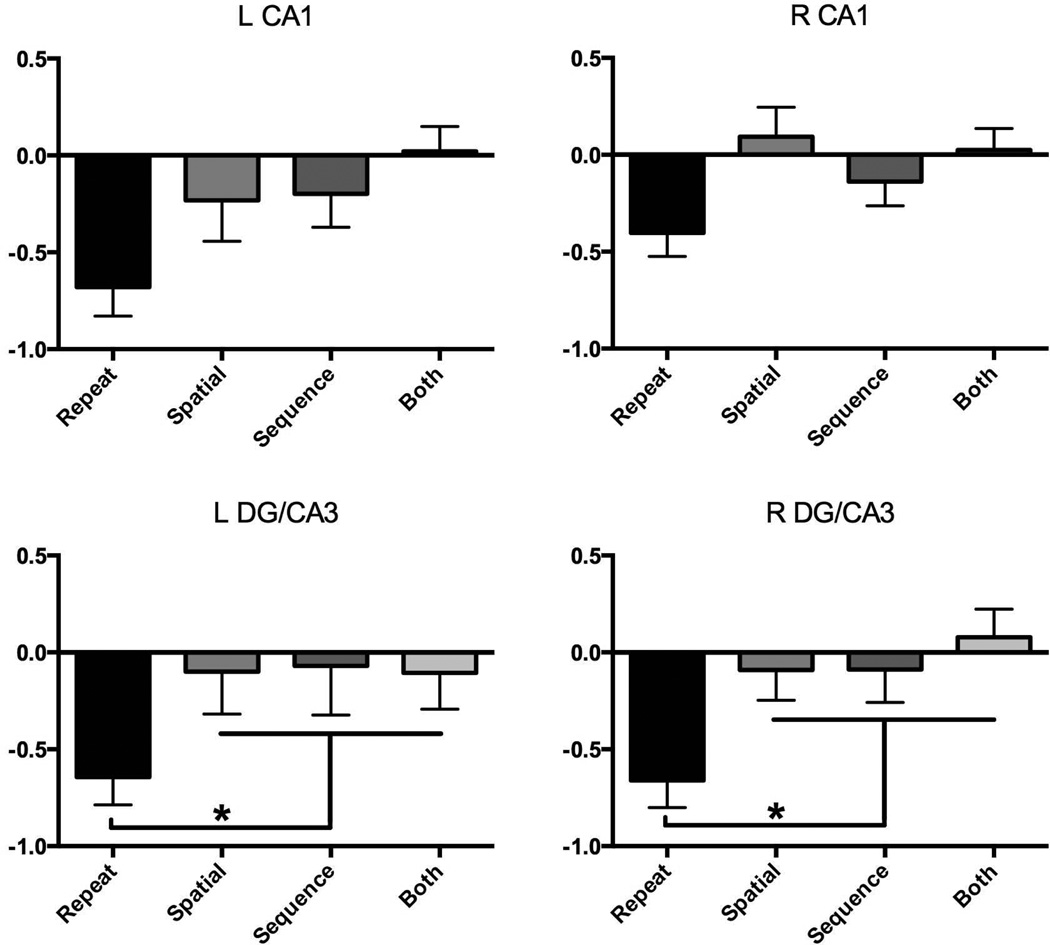
We first assessed whether any of the hippocampal regions exhibited differential activity for any of the lure conditions (Figure 4). Using a set of four repeated-measures one-way ANOVAs including only the three lure conditions, we found no evidence for differential activity across the lure conditions in the repetition-sensitive regions of left or right DG/CA3 and CA1 (all F-values < 0.9, all p’s > 0.4). Collapsing across lure conditions, all regions showed lure activity differing from repetitions (all p’s < .05 via paired t-tests) and not differing from zero (aka first presentations; all one-sample t-values < 1.0, all p’s > 0.2), consistent with a pattern separation response. Breaking this down by individual lure type, paired t-tests revealed that virtually all of the individual lure conditions exhibited activity differing from repetitions (all p’s < .05 except left CA1 spatial vs. repeat p=0.11 and right CA1 sequential vs. repeat p = .17). The lack of reliable differences in activity in these specific comparisons tempers the conclusions that can be drawn and it may be the case that a hemispheric interaction with stimulus type is present in the CA1. It may also be the case that, given a potentially graded response in CA1 to the amount of change in the input, we were underpowered to detect intermediate changes. Importantly though, one-way t-tests revealed that none of the individual lure conditions showed activity differing from zero (i.e. first presentations; all p’s > 0.25). No difference between these conditions is again consistent with a pattern separation response, but one that is agnostic to the type of lure.
Figure 4.
Parameter estimates in arbitrary units for R/L DG and CA1 extracted for each of the four conditions. A similar pattern was observed for each ROI: greater activity for repeats than lures, with no differences between sequential, spatial, or both lure conditions.
Spatial vs. Sequence Functional Connectivity in the Hippocampus
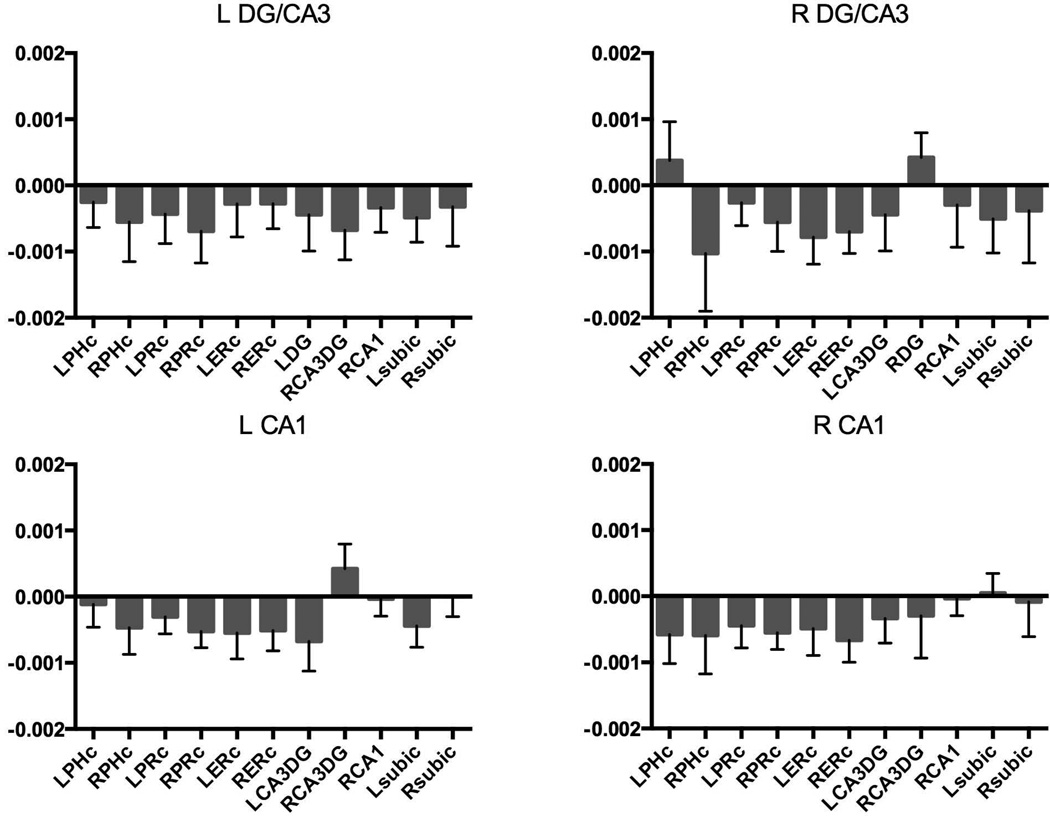
Although there were no hints of any preferential responses to spatial and sequence lures revealed by the traditional fMRI analyses, we performed functional connectivity analyses that assess the correlation of activity over time between regions. The critical test was to examine whether the connectivity varied as a function of lure condition. Here, we used a psychophysiological interaction (PPI) style of analysis to measure the difference in functional connectivity in spatial vs. sequential lure trials (Figure 5).
Figure 5.
Functional coupling between left DG/CA3, right DG/CA3, left CA1, right CA1 and other MTL regions as a function of spatial-sequence contrast. No evidence for spatial or sequence specialization for any of the subfields.
If there is a specialization for the DG/CA3 in spatial pattern separation and in the CA1 for temporal pattern separation, then one might expect to see differences in the pattern of connectivity. However, none were observed. The spatial vs. sequential connectivity for each of the four hippocampal seed regions showed no main effect of target region using separate one-way ANOVAs (all p’s > 0.25). Using separate 2 (DG/CA3 and CA1)×6 (bilateral PHC, PRC, subiculum) ANOVAs for each hemisphere, we asked whether the CA1 vs. DG/CA3 spatial-vs-sequential connectivity pattern differed. We failed to find any main effects or interactions with DG/CA3 vs. CA1 (all p’s > 0.1).
Spatial and Sequence Changes in Cortical MTL Regions
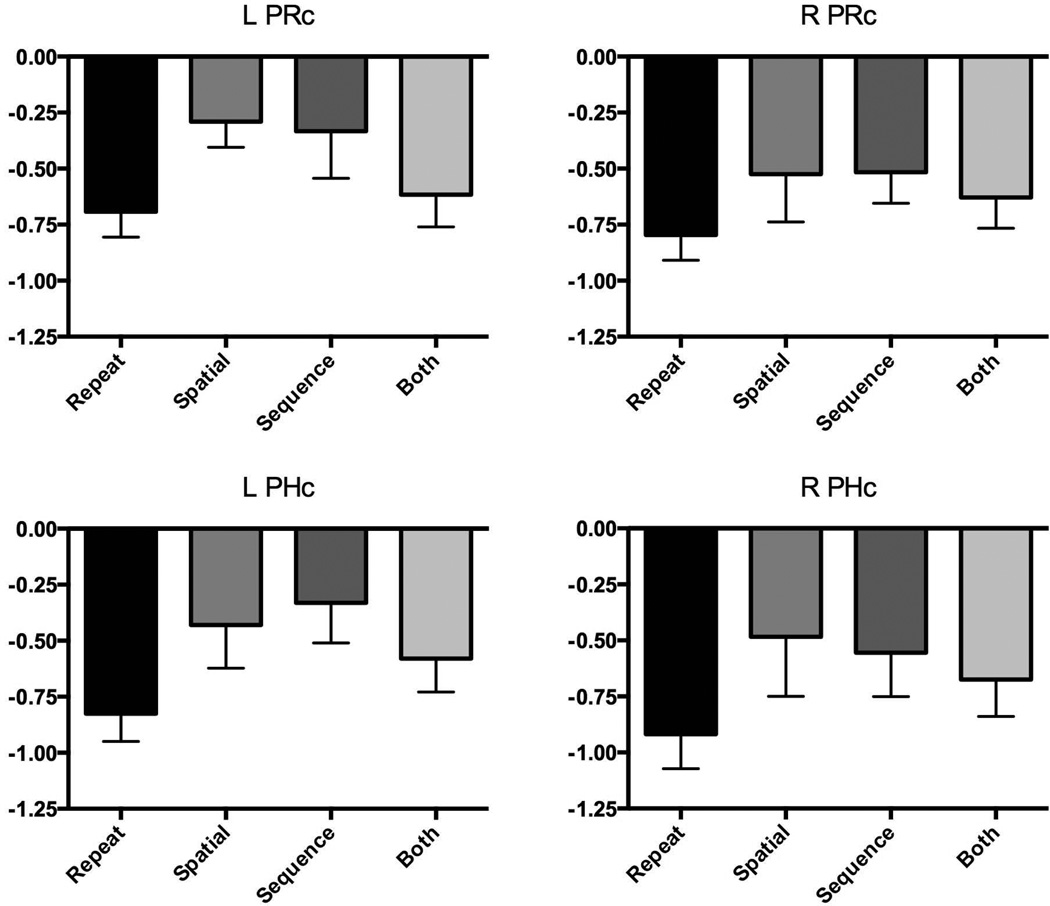
We next turned to investigating activity in the cortical regions of the MTL that had exhibited repetition-suppression signals (regions of bilateral PRC and PHC). Using the same one-way repeated-measures ANOVAs on activity in the three lure conditions, we found no differences between spatial and sequential lures (all p’s > 0.25). When collapsing across lure conditions using paired t-tests, activity in the left PRC and PHC differed reliably from repetitions (p’s < .05). In the right hemisphere, the apparent difference was not reliable (R PRC p = 0.2, R PHC p = .08). Unlike regions in the hippocampus, however, one-sample t-tests revealed that activity for the lures differed from zero (activity associated with first presentations) in all MTL cortical regions (all p’s < .005). Taken together, these results show MTL cortical activity for lures is different from both the activity associated with first presentations and the activity associated with repetitions, yet show no selectivity for spatial or sequential lures.
Discussion
In the current experiment, we asked whether signals associated with pattern separation in the hippocampal subfields differed for spatial versus sequential domains. We tested two hypotheses: 1) that the CA1 and DG/CA3 subfields might be preferentially involved in spatial and temporal pattern separation, respectively (e.g. Rolls & Kesner, 2006); and 2) that the dentate gyrus might be a universal pattern separator that is domain-agnostic (here, spatial or temporal), while the CA1 might be more sensitive to the degree of change, but not the type of change per se.
Our results are largely consistent with a domain-general role of the dentate gyrus. It was sensitive to both spatial and sequential changes, exhibiting activity on par with first presentations and thereby consistent with pattern separation for either change individually or combined. The DG/CA3 and CA1 also showed a similar patterns of functional connectivity related to sequence vs. spatial trials. Thus, both traditional univariate fMRI and PPI functional connectivity analyses support the hypothesis that the dentate gyrus is agnostic to the type of change. This finding is consistent with findings from other pattern separation tasks utilizing changes in objects for lures instead of temporal or spatial lures (Baaker et al., 2008; Lacy et al., 2011), as well as electrophysiological studies and computational models which emphasize the importance of the dentate gyrus for pattern separation processing (Treves & Rolls, 1994; Guzowski, Knierim, & Moser, 2004a; Leutgeb, Leutgeb, Treves, Moser, & Moser, 2004; Leutgeb et al., 2005a; Leutgeb, Leutgeb, Moser, & Moser, 2007; Leutgeb et al., 2005b; Leutgeb & Leutgeb, 2007; Vazdarjanova & Guzowski, 2004). It is important to note that while we did not find evidence consistent with the hypothesis that the DG is limited to spatial pattern separation here, due to resolution limitations in our ability to separate the DG from CA3, these effects might be masked by having to group these two regions together.
We also observed a similar pattern of activation in the CA1, which showed activity consistent with separation for both spatial and sequential lures that did not respond parametrically. Changing both sequential and spatial aspects exhibited similar levels of activity as changing either one. This finding is seemingly at odds withLacy et al. (2011), which reported a smaller change in activity for lures with a high degree of visual overlap compared to low-overlap lures. Likewise, theoretical models predict a linear increase in CA1 activity as the degree of change in the input increases (see Yassa & Stark, 2011 for a review). Perhaps the task did not include a sufficient range of changes in input to afford the opportunity to detect a more graded response function in the CA1. We had hypothesized that the conjoint lure types might comprise a similar parametric manipulation to the number of changes. However, it appears that these changes are not necessarily additive since the “both” condition did not vary from the single conditions. Future studies should parametrically manipulate the range of similarity or overlap between the target and its’ lure within a single dimensions (e.g. spatial or temporal) in an effort to determine if, using these sorts of changes to stimuli, a gradual or linear trend is observed in the CA1. For the present purposes, the critical finding was that activity in the CA1 does not differ for spatial and temporal lures, indicating a lack of specialization in this region for either type of processing.
It is possible that the experimental paradigm stressed discrimination or pattern separation enough to push both the DG and CA1 to display patterns of activations consistent with that signal. Likewise, as the circuitry of the hippocampus relies on signals from the DG and CA3 to exit the hippocampus via the CA1, it is possible that the strong pattern separation signal displayed by the DG was propagated to CA1, reducing our ability to observe any parametric changes. Why this pattern of activity in the CA1 is observed at times (here and Kirwan & Stark, 2007) and not at others (Bakker et al., 2008; Lacy et al., 2011; Duncan et al., 2011) is not clear. However, the fact that the DG/CA3 responded in a separation-like fashion to sequential lures argues strongly against the idea that it is limited to spatial pattern separation. This finding is in contrast to some of the rodent literature, in which CA1 appears to be preferentially involved in sequence memory while the DG is preferentially involved in spatial pattern separation (Rolls & Kesner, 2006). It is certainly possible that this specialization is more pronounced in rodents, in which spatial processing might be more prominent (O’Keefe, 1999). However, here, no clear differentiation was observed.
Our results are consistent with other neuroimaging studies that have linked the hippocampus to spatial and temporal processing (Brown, Ross, Keller, Hasselmo, & Stern, 2010; Staresina & Davachi, 2009; Lehn, Steffenach, van Strien, Witter, & Haberg, 2009; Kumaran & Maguire, 2006). However, we failed to find any differences in hippocampal subfield contributions to spatial versus temporal lures. To our knowledge, high-resolution fMRI studies exploring activity in the hippocampal subfields for temporal and spatial memory have not been explored prior to this study. However, the animal literature remains diverse in the role of the hippocampal subfields in spatial and temporal processing. Lesion studies have identified a specific role for the CA1 for temporal information and the DG for sequential memory (Gilbert et al., 2001). The CA3 has been linked to sequence memory for both spatial and nonspatial material (Farovik et al., 2010). However, using hippocampal recording, MacDonald et al. (MacDonald et al., 2011) have argued that most of the hippocampal cells fire during both the spatial and sequential tasks with little specificity. Perhaps some of the differences in the animal literature can be attributed to methodological and experimental design differences. The specific contributions of these subfields continue to be a topic for further research.
Interestingly, activity in the adjacent cortices of the MTL was not the same as activity in the hippocampus. While activity in the hippocampus was akin to first presentations and different for repeats vs. lures in both the temporal and spatial conditions, the level of activity for lures in the parahippocampal and perirhinal cortices was greater than first presentation and less than that for repeated trials. One interpretation of this data is somewhat consistent with a number of fMRI studies advancing the view that MTL cortical activity is consistent with item-only or simple associations (Diana, Yonelinas, & Ranganath, 2007 for review;). Here, each of the lure trial types maintained the same items, but varied the form of association that was disrupted. In showing activity that differed from first presentations (zero) and that was constant across lure conditions, these results are consistent with the item-representation hypothesis. However, as there was also evidence for a difference between exact repetitions and lures, the results are not fully consistent with this view, suggesting that some form of associative activity is present.
We did not detect a difference between spatial and sequential lures in the medial temporal cortices in this task. This finding is consistent with other reports of hippocampal activity for spatial and temporal information (Ekstrom & Bookheimer, 2007; Ekstrom et al., 2011). However, whenever there is a lack of difference between two conditions, the issue of power or effect size to detect such a difference becomes an issue of importance. Estimating power in fMRI designs is rarely straightforward and the present design is particularly challenging with its two-step nature (selecting voxels based on one contrast and then interrogating their activity in another). It is certainly possible that with different task demands (e.g. an explicit encoding task or activity during a recall or recognition task) might be more sensitive to region specificity for spatial and temporal pattern separation. Nevertheless, the data presented here is consistent in both hemispheres (indicating a degree of reliability) and the effects were strong without borderline trends that might have benefitted from a greater sample size.
In conclusion, the present study found activity in the DG/CA3 and in the CA1 that treated similar lures like novel items rather than repetitions. This pattern was consistent regardless of whether the lures were altered along spatial or sequential dimensions and when task demands were matched across conditions. In contrast to the prediction that the DG is engaged in pattern separation only along spatial dimensions, these results are consistent with it operating along sequential or temporal dimensions as well, supporting the view that it is domain-agnostic and may perform pattern separation processes along any available dimension.
Figure 6.
Parameter estimates extracted for the right and left PRC and PHC for each of the four conditions. Though each condition is different from a first presentation, there is no evidence for sequential or spatial specialization for either ROI.
Acknowledgements
This research was supported in part by a grant from the National Institutes on Aging R01 AG034613.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neurscience. 1989;3(571):591–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Avants B, Duda JT, Kim J, Zhang HY, Pluta J, Gee JC, Whyte J. Multivariate analysis of structural and diffusion imaing in traumatic brain injury. Academic Radiology. 2008;15:1360–1375. doi: 10.1016/j.acra.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Hutchison TL, Kanwisher N. Does the fusiform face area contain subregions highly selective for nonfaces? Nature Neuroscience. 2007;10:3–4. doi: 10.1038/nn0107-3. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller MI, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. Journal of Neuroscience. 2010;30(21):7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18(8):523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal Comput.Biomed.Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: A high-resolution fMRI study of the human hippocampus. Hippocampus. 2011;22(3) doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning & Memory. 2007;14(10):645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP. Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage. 2011;56(3):1803–1813. doi: 10.1016/j.neuroimage.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem. 2010;17:12–17. doi: 10.1101/lm.1616209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink GR, Morris JC, Rolls ET, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston K, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: Multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6(2):156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004a;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004b;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hoge JA, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, Kesner RP. Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behav Brain Res. 2008;188:310–315. doi: 10.1016/j.bbr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the neurosciences Rev.Neurosci. 2004;15(5):333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal acivation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach H, van Strien NM, Witter MP, Haberg AK. A specific role of the hippocampus in recall of temporal sequences. Journal of Neuroscience. 2009;29(11):3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science (New York, N.Y.) Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser EI. Progressive transformation of hippocampal neuronal representations in morphed environments. Neuron Neuron. 2005a;48(2):345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & memory (Cold Spring Harbor, N.Y.) Learn.Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science (New York, N.Y.) Science. 2005b;309(5734):619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Talamini LM, Raffone A. Recall of memory by interaction of the dentate and the CA3: A revised model of the phase precession. Neural Networks. 2005;18:1191–1201. doi: 10.1016/j.neunet.2005.08.008. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philosophical transactions of the Royal Society of London.Series B, Biological sciences Philos.Trans.R.Soc.Lond.B.Biol.Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contributes to recognition memory: Revisiting the Complementary Learningn Systems model. Hippocampus. 2010;20(11):1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrosky J. The hippocampus as a spatial map: Preliminary data from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus. 1999;9(4):352–364. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity Encoding for Fast MRI. Magnetic Resonance in Medicine. 1999;42:952–962. [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Mind the gap: Binding experiences aross space and time in the human hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- van Asselen M, Van der Lubbe RHJ, Postma A. Are space and time automatically integrated in episodic memory? Memory. 2006;14(2):232–240. doi: 10.1080/09658210500172839. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data. 2001 [Google Scholar]