Abstract
Purpose
This study was undertaken to evaluate the incidence of pulmonary disease among patients treated with radiation therapy (RT) for pulmonary metastases (PM) from Wilms tumor (WT).
Patients and Methods
We reviewed records of 6,449 patients treated on National Wilms Tumor Studies -1, -2, -3, and -4 whose flow sheets or annual status reports documented one of several pulmonary conditions. Cases were fully evaluable if pulmonary function test (PFT) results were available, pulmonary fibrosis was identified on a chest radiograph or was listed as the primary or a contributing factor to death. Partially evaluable cases were those for whom PFT results could not be obtained. We evaluated the relationship between RT factors and the occurrence of pulmonary disease using hazard ratios (HR) and cumulative incidence, treating death as a competing risk.
Results
Sixty-four fully evaluable and 16 partially evaluable cases of pulmonary disease were identified. The cumulative incidence of pulmonary disease at 15 years since WT diagnosis was 4.0% (95% confidence interval (CI) 2.6-5.4%) among fully evaluable and 4.8% (95% CI 3.3-6.4%) among fully and partially evaluable patients who received lung RT for PM at initial diagnosis. Rates of pulmonary disease were substantially higher among those who received lung RT for PM present at initial diagnosis or relapse compared to those who received no RT or only abdominal RT (hazard ratio (HR) 30.2, 95% CI 16.9-53.9).
Conclusion
The risk of pulmonary disease must be considered in evaluating the risk:benefit ratio of lung RT for the management of PM from WT.
Keywords: Wilms tumor, pulmonary disease, radiation therapy, actinomycin D, doxorubicin
Introduction
Approximately 90% of all children with Wilms tumor (WT) survive for five years [1]. Although the majority is treated with chemotherapy with or without abdominal radiation therapy (RT), a minority receive whole lung RT, either for pulmonary metastases identified at the time of initial diagnosis or as a site of relapse following initial treatment for localized disease.
Lung function has been evaluated in several studies of survivors of WT. Wohl et al. reported decreased total lung capacity and vital capacity among children who received only bilateral whole lung RT and greater impairment among those who were treated with both thoracotomy and lung RT[2]. Their results are supported by several other studies in similar populations [3-5].
We undertook the present study to determine the frequency of pulmonary disease in survivors of Wilms tumor treated on National Wilms Tumor Studies 1 – 4 who were enrolled in the Long-Term Follow-Up Study (LTFUS).
Patients and Methods
The original consent documents for NWTS -1, -2, -3 and -4, which were approved by the Institutional Review Boards of all participating institutions, included consent for follow-up of surviving patients for evaluation of long-term outcomes. Patients became eligible for the LTFUS on the second anniversary of their date of diagnosis of WT.
NWTS flank RT protocols specified that the lateral, superior, and inferior limits of the treatment portals were to encompass the site of the kidney and associated tumor as visualized on the preoperative excretory urogram or abdominal computed tomography scan with a 1 cm margin using an anteroposterior-posteroanterior (AP-PA) technique. The field was extended across the midline to include the vertebral bodies. NWTS whole lung RT portals included bilateral lungs and the costophrenic recesses with a 1 cm margin using an AP-PA technique. The flank RT doses were age-adjusted in NWTS-1 and -2 [6, 7]. In NWTS-3 and -4, lower RT doses were employed [8, 9]. Whole lung RT doses were specified to be 1400 cGy in NWTS-1 [6]. The dose was decreased to 1200 cGy during the course of NWTS − 2 [7] and remained at that dose in NWTS - 3 and − 4 [8-10].
Pulmonary Outcome
Pulmonary conditions were abstracted from flowsheets, Annual Status Reports, physical examination reports, death certificates, and other submitted documents and coded using ICD-9. We excluded patients with several pulmonary conditions from further review (Table I). These conditions in general specified an etiology that could not be related to radiation therapy, did not involve the lung parenchyma and/or were of clear infectious etiology. We attempted to obtain additional documentation regarding pulmonary conditions for those patients who reported one of several other pulmonary conditions (Table I) after obtaining a signed medical record release from the patient.
Table I. Included and Excluded Conditions.
| Included Conditions | |||||
|---|---|---|---|---|---|
| ICD Code | Pulmonary condition | Reviewed | Cohort | Pulmonary group | Included In analysis |
| 49280 | Other emphysema | 2 | 0 | ||
| 49400 | Bronchiectasis | 1 | 0 | ||
| 49600 | Chronic airway obstruction, NEC | 3 | One | Two | 1 |
| 50800 | Acute pulmonary manifestations due to radiation | 1 | 0 | ||
| 50810 | Pulmonary fibrosis due to radiation | 20 | Three | One | 9 |
| Four | One | 2 | |||
| 51500 | Other pulmonary fibrosis | 37 | One | One | 1 |
| Two | One | 1 | |||
| Three | One | 3 | |||
| Four | One | 6 | |||
| 51800 | Pulmonary collapse | 9 | Two | One | 1 |
| 51881 | Acute respiratory failure | 1 | 0 | ||
| 51884 | Lung lesion | 13 | Two | One | 2 |
| 51885 | Lung nodule | 16 | 0 | ||
| 51889 | Other diseases of lung, NEC | 22 | One | One | 1 |
| One | Two | 1 | |||
| Three | One | 6 | |||
| Four | One | 3 | |||
| Four | Two | 1 | |||
| 51940 | Disorders of the diaphragm | 0 | 0 | ||
| 51990 | Respiratory disease (chronic), NOS | 63 | Two | One | 3 |
| Two | Two | 3 | |||
| Three | One | 17 | |||
| Three | Two | 4 | |||
| Four | One | 8 | |||
| Four | Two | 2 | |||
| 78600 | Respiratory abnormality, NOS | 11 | 0 | ||
| 78606 | Tachypnea | 3 | 0 | ||
| 79310 | Nonspecific findings on examination of lung | 4 | Three | Two | 1 |
| Excluded Conditions | |||||
|---|---|---|---|---|---|
| ICD Code | Pulmonary condition | Total | |||
| 11505 | Histoplasmosis, lung | 0 | |||
| 46590 | Acute URIs, unspecified sites | 0 | |||
| 48100 | Lobar pneumonia | 1 | |||
| 48290 | Bacterial pneumonia | 0 | |||
| 48500 | Bronchial pneumonia | 0 | |||
| 48600 | Pneumonia, NOS | 11 | |||
| 49000 | Bronchitis, not specified as acute or chronic | 1 | |||
| 49120 | Obstructive chronic bronchitis | 0 | |||
| 49180 | Other chronic bronchitis | 1 | |||
| 49300 | Extrinsic asthma | 13 | |||
| 51100 | Pleurisy w/o effusion or current tuberculosis | 1 | |||
| 51190 | Pleural effusion | 0 | |||
| 51280 | Other spontaneous pneumothorax | 1 | |||
| 51679 | Endogenous lipid pneumonia | 0 | |||
| 51780 | Lung sarcoidosis | 1 | |||
| 51850 | Pulmonary insufficiency following trauma & surgery | 0 | |||
NEC – Not Elsewhere Classified; NOS – Not Otherwise Specified
We graded pulmonary toxicity using the Common Terminology Criteria for Adverse Events v 3.0 (CTCAE) [11]. We considered two different definitions of pulmonary disease based on more or less restrictive criteria. The stricter criterion (definition 1) included patients with records of one or more abnormal pulmonary function test results [forced expiratory volume in one second (FEV1), vital capacity (VC), diffusing capacity of the lung for carbon monoxide corrected for hemoglobin (DLCOcorr)], CTCAE grade 1 or greater fibrosis on a chest radiograph, CTCAE grade 1 or greater hypoxia and/or pulmonary fibrosis listed as a primary or contributing cause of death. The grading system did not distinguish between reductions in lung function due to restrictive or obstructive defects since total lung capacity or bronchodilator response were not included, but for this population reduced values are likely to be due to restrictive defects. The grade distributions of CTCAE are shown in Supplementary Table I. Fifteen patients without any pulmonary function test results had CTCAE grade 1 or higher fibrosis on a chest radiograph and one patient, whose medical record was lost by the treating institution, died from well documented pulmonary fibrosis. The less strict criterion (definition 2) also included patients with physician notes indicating restrictive pulmonary disease/pulmonary fibrosis but who lacked supporting pulmonary function test records.
Radiation Therapy Cohorts
We defined four cohorts of patients with the following lung RT histories: 1) No RT for initial treatment (lung or otherwise); 2) Abdominal RT only for initial treatment; 3) Lung RT for initial treatment; and, 4) Lung RT for relapse treatment. Patients were moved from cohorts 1 and 2 into cohort 4 at the time they received lung RT for relapse.
Radiation Doses
We considered both the cumulative whole lung RT dose (WLRT) and the maximum of cumulative RT doses to left or right lungs considered separately (MAXRT). Calculations for MAXRT did not assume that the lung RT occurred in overlapping sites. Time-dependent versions of MAXRT and WLRT were constructed for each individual by recalculating the totals each time additional whole or partial lung RT was administered. MAXRT and WLRT were considered as ordered categorical variables, with categories of 0 to 1200, 1201 to 1500, and greater than 1501 cGy.
Statistical Methods
The cumulative incidence of pulmonary disease was estimated by time since WT diagnosis in cohorts 1- 3 and by time since initiation of lung RT for relapse in cohort 4, treating death as a competing risk [12]. We assessed the relationship between pulmonary RT and rates of pulmonary disease in cohorts 3 and 4 with Cox proportional hazards models, using time-dependent RT variables as predictors. The time scale for these analyses was the same as with the cumulative incidence calculations, that is, starting at WT diagnosis for cohort 3 and at initiation of lung RT for cohort 4.
Missing lung RT dose data
Dose was not recorded for 2 of 783 patients who received lung RT for treatment of PM at WT diagnosis and these patients were assigned the standard protocol dose. There were 960 records for pulmonary relapse in 696 patients, of which 833 were considered compete. Only 22 of the 288 complete records for patients from cohort 3 indicated that lung RT was administered at relapse as opposed to 388 of 545 records for patients from cohort 4. Doses were known for all of these episodes. The remaining 127 records with missing RT dose information included 43 with unknown lung RT treatment status, 18 for which no whole lung RT but a partial lung dose of unknown magnitude was delivered and 66 for which both field and dose were unknown.
We accounted for the missing data by multiple imputation, constructing 20 datasets by stochastically “filling-in” missing values based on predictor variables related to the missing variable or the probability it was known, performing the statistical analysis on each of the imputed datasets, and combining inferences across the 20 analyses [13]. An approximate Bayesian bootstrap hot-deck method was used to impute missing values [14]. As a sensitivity analysis, we fit the same models to “complete case” data in which incomplete dose records were simply removed.
Results
The study sample consisted of 6,499 patients from NWTS-1-4 from US or Canadian institutions. The median and Interquartile Range (IQR) of follow-up for those alive at last contact was 17.9 years (IQR 9.05 years, 25.0 years). Demographic characteristics are shown in Table II. There were 80 total pulmonary disease cases, 64 under definition 1 and 16 additional cases under definition 2. Pulmonary disease was more frequent among females and those diagnosed with WT at older ages
Table II. Demographic characteristics of study population, by pulmonary disease status (wide definition).
| Pulmonary Disease | No | Yes | Total | |||
|---|---|---|---|---|---|---|
| Sex | No. | % | No. | % | No. | % |
| Male | 3,057 | 98.9 | 35 | 1.1 | 3,092 | 100 |
| Female | 3,362 | 98.7 | 45 | 1.3 | 3,407 | 100 |
| Ethnicity | ||||||
| White | 4,670 | 98.5 | 69 | 1.5 | 4,739 | 100 |
| Black | 1,094 | 99.4 | 7 | 0.6 | 1,101 | 100 |
| Hispanic | 518 | 99.2 | 4 | 0.8 | 522 | 100 |
| Other | 137 | 100.0 | 0 | 0.0 | 137 | 100 |
| Age at WT dx (months) | ||||||
| 0 to < 24 | 1,994 | 99.6 | 8 | 0.4 | 2,001 | 100 |
| ≥ 24 to < 48 | 2,085 | 98.6 | 29 | 1.4 | 2,114 | 100 |
| ≥ 48 | 2,340 | 98.2 | 43 | 1.8 | 2,383 | 100 |
| NWTS Study | ||||||
| 1 | 546 | 97.3 | 15 | 2.7 | 561 | 100 |
| 2 | 772 | 98.8 | 9 | 1.2 | 781 | 100 |
| 3 | 2,219 | 98.4 | 35 | 1.6 | 2,254 | 100 |
| 4 | 2,882 | 99.3 | 21 | 0.7 | 2,903 | 100 |
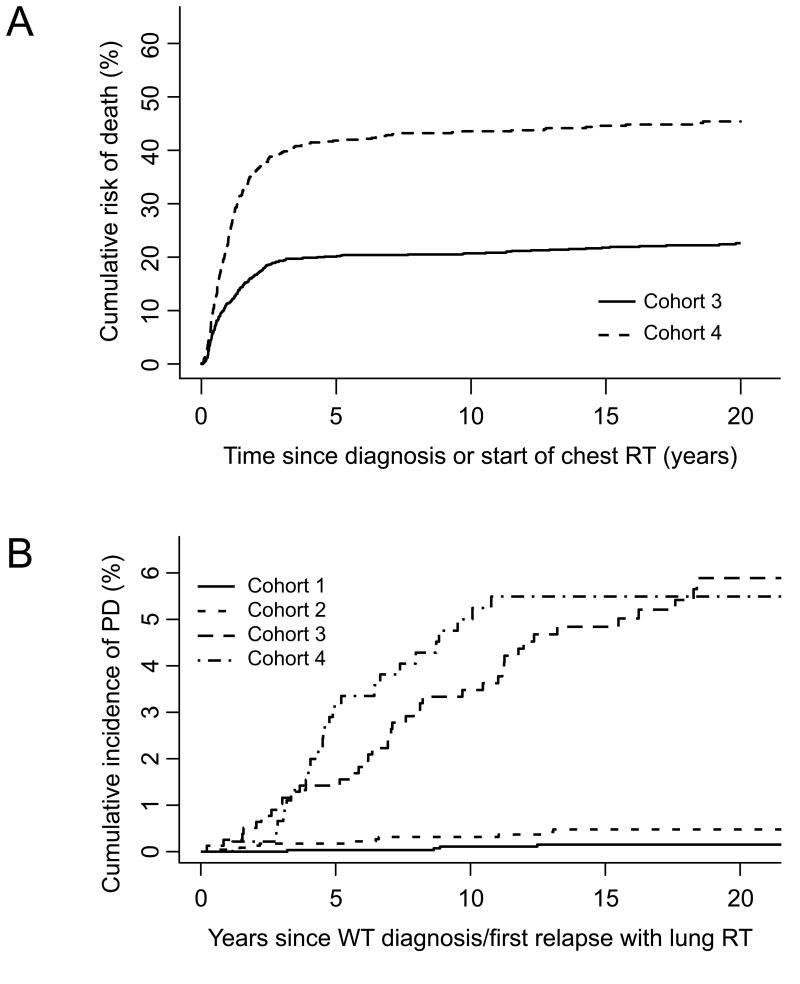
The numbers of patients in each of the four cohorts are shown in Table III. As expected, patients from cohorts 1 and 2 who moved to cohort 4 after developing PM had much higher mortality thereafter than those who did not (Tables III and IV). As shown in Figure 1A, the cumulative risk of death after five years from start of lung RT was twice as high for patients who first received lung RT to treat relapse (cohort 4) compared to those who first received lung RT at WT diagnosis (cohort 3). The excess mortality in cohort 4 was due primarily to progressive WT (Table IV). This meant that fewer patients survived long enough to develop pulmonary disease, which affected the cumulative incidence comparisons. All seven deaths ascribed to pulmonary disease occurred in cohorts 3 and 4 (Table IV).
Table III.
Numbers of deaths and cumulative incidence of pulmonary disease at 15 years since WT diagnosis (Cohorts 1-3) or start of chest RT (Cohort 4), by Cohort.
| Cumulative Incidence of Pulmonary Disease | ||||||||
|---|---|---|---|---|---|---|---|---|
| Narrow Definition | Wide Definition | |||||||
| Cohort | Number of Patients* | Number of Deaths* | Number of. Cases | Percent at 15 years | 95% CI | Number of Cases | Percent at 15 years | 95% CI |
| 1 | 3,198 (188) | 300 (90) | 2 | 0.1 | [0.0, 0.2] | 4 | 0.2 | [0 0.3] |
| 2 | 2,518 (273) | 541 (197) | 7 | 0.3 | [0.1,0.6] | 10 | 0.5 | [0.2, 0.8] |
| 3 | 783 | 244 | 35 | 4.0 | [2.6, 5.4] | 42 | 4.8 | [3.3, 6.4] |
| 4 | 461 | 287 | 20 | 4.6 | [2.6, 6.5] | 24 | 5.5 | [3.4, 7.6] |
| Total | 6,499 | 1,085 | 64 | 0.9 | [0.7, 1.2] | 80 | 1.2 | [0.9, 1.5] |
Numbers in parentheses are numbers of patients who transferred to Cohort 4 after chest RT for pulmonary relapse, and the number of deaths that occurred subsequently among these patients. CI – Confidence Interval
Table IV. Causes of death by cohort.
| Cause of death | Cohort 1* | Cohort 2* | Cohort 3 | Cohort 4 | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Alive | 2,800 | 93.0 | 1,901 | 84.7 | 539 | 68.8 | 174 | 37.7 | 5,414 | 83.3 |
| WT | 145 | 4.8 | 253 | 11.3 | 214 | 27.3 | 262 | 56.8 | 874 | 13.4 |
| SMN | 9 | 0.3 | 31 | 1.4 | 12 | 1.5 | 5 | 1.1 | 57 | 0.9 |
| CHF | 1 | 0.0 | 9 | 0.4 | 7 | 0.9 | 8 | 1.8 | 25 | 0.4 |
| ESRD | 16 | 0.5 | 11 | 0.5 | 0 | 0.0 | 2 | 0.4 | 29 | 0.4 |
| PD | 0 | 0.0 | 0 | 0.0 | 4 | 0.5 | 3 | 0.7 | 7 | 0.1 |
| Other | 39 | 1.3 | 40 | 1.8 | 7 | 0.9 | 7 | 1.5 | 93 | 1.4 |
| Totals | 3,010 | 100.0 | 2,245 | 100.0 | 783 | 100.0 | 461 | 100.0 | 6,499 | 100.0 |
For Cohorts 1 and 2, counts and percentages pertain to deaths that occurred prior to onset of chest RT for pulmonary relapse, or to patients who remained alive without ever receiving chest RT. WT = progressive WT and/or toxicity; SMN=secondary malignant neoplasm; CHF=congestive heart failure; ESRD=end stage renal disease; PD=pulmonary disease
Table V. Hazard ratios for pulmonary disease by time-dependent lung RT exposure in Cohorts 3 and 4.
| Total† | Pulmonary Disease: Definition 1 | Pulmonary Disease: Definition 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WLRT | Cases† | HR | 95% CI | p‡ | Cases† | HR | 95% CI | p‡ | |
| 0-1200 (ref) | 903.1 | 39.0 | .39 | 46.8 | .29 | ||||
| 1201-1500 | 371.9 | 13.0 | 1.10 | 0.59, 2.06 | .77 | 16.2 | 1.23 | 0.69, 2.19 | .48 |
| 1501+ | 71.9 | 3.0 | 2.01 | 0.60, 6.77 | .26 | 3.0 | 1.80 | 0.53, 6.08 | .34 |
| MAXRT | |||||||||
| 0-1200 (ref) | 745.9 | 32.0 | .12 | 37.2 | .03 | ||||
| 1201-1500 | 298.9 | 12.0 | 1.12 | 0.58, 2.18 | .73 | 14.3 | 1.16 | 0.85, 1.59 | .64 |
| 1501+ | 302.0 | 11.0 | 1.84 | 0.92, 3.67 | .08 | 14.5 | 2.11 | 1.54, 2.89 | .02 |
averages over multiple imputations of dose;
p-values for reference category based on trend tests
Figure 1.
A) The cumulative incidence of death in the two cohorts treated with lung RT. WT – Wilms tumor, RT – radiation therapy; B) The cumulative incidence of pulmonary disease in each of the four cohorts. PD – pulmonary disease, WT – Wilms tumor, RT – radiation therapy
The cumulative incidence of pulmonary disease at 15 years from the start of lung RT was < 0.5% for those who remained in cohorts 1 and 2, but was 4.0 to 5.5% in cohorts 3 and 4 (Figure 1B), with slight variations depending on the definition of pulmonary disease (Table III). Using definition 2, rates of pulmonary disease per unit time were very much higher in cohorts 3 and 4 compared to cohorts 1 and 2 (Hazard Ratio (HR) 30.2, 95% Confidence Interval (CI), 16.9-53.9, p<.001). Those in Cohort 2 had higher rates of pulmonary disease than those in cohort 1 (HR 3.5, 95% CI, 1.1-11.1, p=.035) and those in cohort 4 had higher rates than those in cohort 3 (HR 1.7, 95% CI 1.0-2.7, p=0.052). Similar differences were found using definition 1. In spite of the somewhat higher rates of pulmonary disease observed among survivors in cohort 4 compared to cohort 3, the cumulative incidences were comparable (Figure 1B) due to the higher burden of intervening mortality in cohort 4.
The analysis of time dependent RT doses on rates of pulmonary disease (Table V) provided no evidence for an increase in risk with increasing dose to the whole lung (WLRT). By contrast, there was moderate evidence for a roughly two-fold increase in risk between the lowest and highest categories of dose to portions of the lung (MAXRT) and for a trend with ordered category. The failure to identify an increase for WLRT is not surprising since these doses were heavily concentrated in the range of 1200-1400 cGy and thus showed little variation. The standard errors and confidence limits were little affected by the missing data, since the component of variability stemming from differences between imputed data sets was small (< 4%) in comparison to the estimated variability of the regression coefficients within data sets. Results were comparable, but subject to substantially greater statistical uncertainty, in the sensitivity analysis using the complete dose records.
Discussion
This analysis demonstrated that, among WT patients who received lung RT (cohorts 3 and 4), there was approximately a 5% cumulative incidence of pulmonary disease based on available records. In contrast, among those who did not receive lung RT, the 15-year cumulative incidence of pulmonary disease was less than 0.5%. It is possible that we have not ascertained all cases of pulmonary disease, in which case the actual cumulative incidence would be higher. However, the cumulative incidence estimates were not greatly changed when we considered a diagnosis of pulmonary disease based on physician notes and not just abnormal pulmonary function tests. Pulmonary disease was more frequent among females and those diagnosed with WT at older ages. This is probably the result of differences in age at WT diagnosis among those who develop pulmonary metastasis. In addition, participants in earlier studies were more likely to be diagnosed with pulmonary disease, due in part to the more frequent and intense administration of radiation therapy and the longer period of follow-up.
Lung function has been evaluated in several studies of survivors of WT. Wohl et al. evaluated lung function in six children with WT treated with whole lung RT only 6.9 to 12.5 years after completing lung RT and six children with WT treated with whole lung RT plus boost RT or thoracotomy 6.0 to 16.4 years after complete of treatment. TLC was 71% and VC 72% of predicted among children who received only bilateral whole lung RT and 58% and 60% of predicted among those who received both thoracotomy and lung RT [2]. Their results are supported by several other studies in similar populations. Littman et al. reported reductions in TLC and VC in ten patients evaluated four to 12 years (median - six years) after treatment for pulmonary metastases with whole lung RT of 1200‐1370 cGy [3]. Benoist et al. also reported significant reductions in TLC and VC among 48 children evaluated two to 17 years following 2,000 cGy of whole lung RT and actinomycin D for metastatic WT. Eleven of these children had additional reductions in TLC and VC between 18 and 48 months after therapy [4]. Weiner et al. evaluated pulmonary function of 30 childhood cancer survivors zero to 13.7 years (median – 2.79 years) after completion of treatment with whole lung RT (median dose 1,200 (1,050 – 1,760) cGy). Twenty percent of those tested (6/30) had either moderately or severely reduced FVC and/or FEV1. TLC was moderately or severely reduced in 43% (10/23) and DLCO in 43% (9/21) of those evaluated [5]. None of these studies included a sufficiently large number of patients followed for many years after completion of treatment to allow estimates of cumulative risk and relative risk to be made.
Previous estimates of the prevalence of pulmonary dysfunction among survivors of childhood cancer (CCSs) have been based largely on self-report. Mertens et al. reported that the cumulative incidence of lung fibrosis among participants in the Childhood Cancer Survivor Study who were treated with chest RT was 3.5% 20 years after diagnosis [15], a finding that is in close agreement with the NWTS LTFU data for the occurrence of pulmonary disease. Mulder et al. evaluated pulmonary function in 193 of 220 five-year CCSs (median time since diagnosis 17.9; range, 5.6 to 36.8 years) at risk for abnormal pulmonary function because of treatment exposures [16]. Four CCSs (2.1%) had obstructive pulmonary disease. Disease severity according to the GOLD criteria [17] in these patients was moderate (grade 2). None had grades 3 to 5 impairment and two CCSs (1.0%) had grade 1 impairment. Thirty-four CCSs (17.6%) had restrictive pulmonary disease according to CTCAE v4.0 criteria [18] . The severity was moderate (grade 2) in 31 patients (16.1%) and severe (grade 3) in 3 patients (1.6%). None had grade 4 or 5 impairment and 78 CCSs (40.4%) had grade 1 impairment. The prevalence of a decreased DLCO was 39.9% (75 CCSs). In 73 CCSs (38.8%) DLCO was moderately decreased (grade 2) and in 2 CCSs (1.1%) it was severely decreased (grade 3). None had grade 4 or 5 impairment and 78 patients (41.5%) had grade 1 impairment. Thoracic surgery and pulmonary radiation therapy were the most significant risk factors for restrictive pulmonary function impairment.
This study has several strengths, including documentation of outcomes based on medical reports. A weakness of the study was the need to rely on imputation, due to missing lung RT records, to estimate the dose-response relationship between lung RT levels and higher rates of pulmonary disease. Multiple-imputation based estimates of the dose-response relationship suggested a two-fold increase in pulmonary disease rates for MAXRT>1500 cGy compared to 0-1200 cGy. Pulmonary disease was not included as a predictor in the missing data models, since to do so would generate strata too small for “hot deck” imputation, and this may have biased the hazard ratios (HRs) towards the null [19]. If both RT records and pulmonary disease records were unavailable for a common reason, such as poor correspondence with the hospital, imputing RT data but failing to acknowledge under-ascertainment of the pulmonary disease outcome would also result in reduction in HRs.
Conclusion
Abnormal pulmonary function is a frequent long-term complication of lung RT administered to treat pulmonary metastases from WT. Long-term survivors of such therapy should be monitored carefully and encouraged to adopt lifestyles that protect their remaining cardiac and pulmonary function, such as obtaining adequate physical exercise and avoiding cigarette smoke. Future research may identify WT patients whose response to initial chemotherapy obviates the need for whole lung RT.
Supplementary Material
Acknowledgments
Supported in part by United States Public Health Service grant nos. CA-54498 (N.E. Breslow, Principal Investigator), CA-42326 (D.M. Green, Principal Investigator). The authors thank the investigators of the Pediatric Oncology Group and the Children's Cancer Group and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered on the National Wilms' Tumor Studies,
Footnotes
CONFLICT OF INTEREST STATEMENT: Drs. Green, Qu, Kalapurakal, Stokes, Gregoriev, Friedman and Breslow and Mss. Lange, Peterson, Takashima and Norkool have no affiliations that they consider to be relevant and important with any organization that to any author's knowledge has a direct interest, particularly a financial interest, in the subject matter discussed.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008. [Google Scholar]
- 2.Wohl MEB, Griscom NT, Traggis DG, Jaffe N. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics. 1975;55:507–516. [PubMed] [Google Scholar]
- 3.Littman P, Meadows AT, Polgar G, et al. Pulmonary function in survivors of Wilms' tumor. Cancer. 1976;37:2773–2776. doi: 10.1002/1097-0142(197606)37:6<2773::aid-cncr2820370631>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Benoist MR, Lemerle J, Jean R, et al. Effects on pulmonary function of whole lung irradiation for Wilms' tumour in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner DJ, Maity A, Carlson CA, Ginsberg JP. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer. 2006;46:222–227. doi: 10.1002/pbc.20457. [DOI] [PubMed] [Google Scholar]
- 6.D'Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms' tumor: Results of the National Wilms' Tumor Study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.D'Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms' tumor: Results of the Second National Wilms' Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.D'Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms' tumor: Results of the Third National Wilms' Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 10.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:3744–3751. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006. [Google Scholar]
- 12.Gooley T, Leisenring W, Crowley J, Storer B. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 14.Demirtas H, Arguelles LM, Chung H, Hedeker D. On the performance of bias-reduction techniques for variance estimation in approximate Bayesian bootstrap imputation. Comput Stat Data An. 2007;51:4064–4068. [Google Scholar]
- 15.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 16.Mulder RL, Thonissen NM, van der Pal HJ, et al. Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax. 2011;66:1065–1071. doi: 10.1136/thoraxjnl-2011-200618. [DOI] [PubMed] [Google Scholar]
- 17.Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2008. [Google Scholar]
- 18.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 (CTCAE) Bethesda: National Institutes of Health; 2009. [Google Scholar]
- 19.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.