Abstract
The diagnosis of acute Kawasaki disease (KD) is based on characteristic clinical signs and not on a specific diagnostic test. The authors performed a comprehensive evaluation of acute-phase reactants in KD to determine which of the acute-phase reactants would most accurately distinguish KD from other febrile illnesses. Blood was collected from 218 cases of febrile children with KD (64 cases); bacterial pneumonia (74 cases); hand, foot, and mouth disease (31 cases); and upper respiratory tract infection (49 cases) in acute-stage illness before any therapy. The demographics, body temperature, and laboratory markers including white blood cell count, red blood cell count, and levels of hemoglobin, platelets, C-reactive protein, haptoglobin, apolipoprotein A-I, and apolipoprotein B were evaluated. Using post hoc analysis, the platelet count (103/μl) and haptoglobin/apolipoprotein A-I ratio were significantly higher for the KD patients (404.64 ± 161.68, P = 0.004; 4.74 ± 2.73, P < 0.001) than for the other groups including patients with pneumonia (272.76 ± 115.07, 2.03 ± 1.88); hand, foot, and mouth disease (274 ± 105.9, 2.24 ± 1.19); and upper respiratory tract infection (b282.06 ± 107.72, 1.4 ± 0.98). The best cutoff value of the haptoglobin/apolipoprotein A-I ratio obtained from receiver operating characteristics (ROC) curves for KD was 2 (area under the ROC curve, 0.88; 95% confidence interval, 0.801–0.955), with a sensitivity of 89.7% and a specificity of 85.6% for detecting KD. Our data indicate that the serum haptoglobin/apolipoprotein A-I ratio could be a useful supplemental laboratory marker for the acute phase of KD.
Keywords: Acute-phase reactants, Apolipoprotein A-I, Apolipoprotein B, Haptoglobin, Haptoglobin/apolipoprotein A-I ratio, Kawasaki disease
The etiology of Kawasaki disease (KD) currently is not known, and the diagnosis of typical KD is made by clinical features, as first described by Dr. Tomisaku Kawasaki [6], and diagnostic criteria have been established for clinical use [8]. Currently, no specific diagnostic test is available for diagnosing KD.
Most cases of KD are typical and meet the diagnostic criteria, but some cases are atypical or incomplete [20], which not only can make the diagnosis challenging but also can cause a delay in the diagnosis. Supplemental diagnostic guidelines were added by the American Heart Association [16] to aid in the diagnosis of incomplete or atypical KD. Because both typical and atypical KD need early diagnosis and early therapy with intravenous immunoglobulin for the prevention of coronary artery aneurysms [16], a need remains for early and easy diagnosis.
Marked polyclonal immune activation and a significant inflammatory response in KD results from an increase in the acute-phase reactants [4, 10]. In most cases of KD, blood, urine, and spinal fluid cultures remain negative, and acute-phase reactants serve as the supplemental laboratory tests [16].
For this study, we evaluated various acute-phase reactants including C-reactive protein (CRP), platelets, haptoglobin (Hp), apolipoprotein A-I (apoA-I), and apolipoprotein B (apoB) in the acute phase of KD and compared them with levels in other childhood febrile illnesses including bacterial illness (pneumonia), viral illness (hand, foot, and mouth disease), and mixed infection (upper respiratory infection). Findings have shown that Hp binds apoA-I and inhibits the apoA-I-dependent activity of the enzyme (lecithin cholesterol acyltransferase), thus impairing the high-density lipoprotein (HDL) function [5, 17].
We also evaluated the profile of the haptoglobin/apolipoprotein A-I ratio (HAR) in this study. We aimed to determine which of the acute-phase reactants would most accurately distinguish KD from other febrile illnesses in children.
Materials and Methods
Study Population
The study was approved by the institutional review board of Kaohsiung Medical University Hospital. All the patients in the pediatric department of Kaohsiung Medical University Hospital were evaluated. This prospective study from 1 January 2002 to 31 December 2008 enrolled 218 pediatric cases including 64 cases of KD; 74 cases of bacterial pneumonia; 31 cases of hand, foot, and mouth disease (HFMD); and 49 cases of upper respiratory tract infection (URI). Fever was defined as an axillary temperature of 37.5°C or higher. An inclusion criterion was the presence of fever for 3 days or more.
Kawasaki disease was diagnosed according to the diagnostic guidelines [16] as complete or typical KD fulfilling at least five of the six clinical criteria required for diagnosis. Peripheral blood was collected from each of the enrolled patients on the day of admission before they received any antibiotics, steroid, acetylsalicylic acid, or immunoglobulin therapy.
We used the Beckman Image Immunochemistry System Autoanalyzer (Beckman IMAGE, Beckman Coulter, Brea, CA, USA) to analyze the serum CRP, Hp, apoA-I, HAR, and apoB. The white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), and platelet count were measured by the Coulter JT counter.
Statistical Analysis
To assess whether the groups studied differed in terms of baseline characteristics, analysis of variance was used for continuous variables, and the Tukey’s method (honestly significant difference (HSD) factor) of post hoc testing was used for categorical variables. Values are reported as mean ± standard deviation or as percentages, with 95% confidence intervals (CIs) provided when appropriate. Multiple logistic regression analysis was used to assess the association of the dependable variable with the various independent variables studied. The receiver operating characteristics (ROC) curve was used to acquire appropriate sensitivity and specificity of variables for diagnosing KD. Overall performance levels were summarized with a conventionally used probability of correct discrimination or, equivalently, with the area under the ROC curve (AUC), which under a binary scale is related to Youden’s Index. All tests were two-sided, with significance defined as a p value less than 0.05.
Results
Clinical Characteristics of the Study Population
The patients’ characteristics are depicted in Table 1. The children with KD were younger than the other groups. The gender and body temperature variables did not differ significantly among the groups, as shown in Table 1. Regarding laboratory data, the platelet count, apoB, Hp, and HAR were significantly higher for the KD patients than for the other groups (Table 1). The ApoA-I level was relatively lower for the KD patients than for the pneumonia and URI groups (Table 1).
Table 1.
Clinical and laboratory characteristics of the study populations
| Variables | KD mean (range) | Pneumonia mean (range) | HFMD mean (range) | URI mean (range) | P value |
|---|---|---|---|---|---|
| n | 64 | 74 | 31 | 49 | |
| Gender | 0.439 | ||||
| Male | 42 | 45 | 14 | 29 | |
| Female | 22 | 29 | 17 | 20 | |
| Age (years) | 2.49 ± 2.26 (0.5–10) | 3.61 ± 2.81 (0.17–12) | 2.59 ± 3.53 (0.67–18) | 3.56 ± 3.43 (0.08–14) | 0.019a |
| BT (°C) | 38.58 ± 0.71 (37–40) | 38.41 ± 1.01 (37–40) | 38.04 ± 0.98 (37–39.8) | 38.37 ± 1.15 (36–41.1) | 0.453 |
| WBC (103/μl) | 13.16 ± 8.09 (3.10–59.0) | 10.45 ± 6.10 (2.2–33.03) | 10.15 ± 4.34 (3.3–21.4) | 9.28 ± 3.97 (2.75–20.86) | 0.195 |
| RBC (103/μl) | 3.98 ± 0.51 (2.81–5.74) | 4.2 ± 0.54 (3–5.91) | 4.3 ± 0.42 (3.78–5.36) | 4.4 ± 0.46 (3.19–6.03) | 0.319 |
| Hb (g/dl) | 10.65 ± 1.34 (7–14.6) | 11.43 ± 1.57 (9.7–19.3) | 11.37 ± 1.03 (9.28–13.6) | 11.57 ± 1.28 (8.3–14.3) | 0.050 |
| PLT (103/μl) | 404.64 ± 161.68 (182–827) | 272.76 ± 115.07 (2.58–510) | 274 ± 105.9 (101–531) | 282.06 ± 107.72 (14–545) | 0.004a |
| CRP (μg/ml) | 78.05 ± 67.45 (3.19–305) | 32 ± 58.05 (1.24–282) | 18.54 ± 18.96 (1.52–68.6) | 16.13 ± 40.33 (1.24–237) | 0.070 |
| Hp (mg/dl) | 325.73 ± 107.63 (114–577) | 175.75 ± 112.46 (5.83–520) | 218.54 ± 79.82 (56.5–370) | 142.47 ± 78.57 (5.83–321) | <0.001a |
| apoA-I (mg/dl) | 108.99 ± 28.49 (55.1–171) | 110.25 ± 37.32 (25–193) | 102.59 ± 36.25 (25–263) | 114.34 ± 28.07 (53.4–211) | 0.008a |
| apoB (mg/dl) | 97.93 ± 32.62 (44–211) | 74.05 ± 24.85 (16.5–133) | 96.16 ± 28.73 (35–165) | 67.98 ± 20.53 (35–116) | <0.001a |
| HAR | 4.74 ± 2.73 (0.98–13.54) | 2.03 ± 1.88 (0.03–9.67) | 2.24 ± 1.19 (0.55–5.39) | 1.4 ± 0.98 (0.04–4.69) | <0.001a |
Data are presented as mean ± standard deviation. The P value compares the KD group with the other groups using multiple logistic regression analysis
Statistically significant
KD Kawasaki disease, HFMD hand, foot, and mouth disease, URI upper respiratory tract infection, BT body temperature, WBC white blood cell count, RBC red blood cell count, Hb hemoglobin, PLT platelets, CRP C-reactive protein, Hp haptoglobin, apoA-I apolipoprotein A-I, apoB apolipoprotein B, HAR haptoglobin/apolipoprotein A-I ratio
Table 2 compares the KD group with the other three groups (pneumonia, HFMD, and URI) according to the post hoc Turkey method (HSD factor), which could show significant differences by considering a two-sided P value less than 0.05. The post hoc Turkey method indicated that the mean values for CRP, Hp, apoA-I, apoB, and HAR in the KD groups were significantly different from those for the pneumonia, HFMD, and URI groups according to multiple comparison analysis. The mean values for CRP, Hp, apoB, and HAR in the KD groups were significantly higher than for the other three groups (P < 0.05), whereas the mean value for apoA-I in the KD groups was significantly lower than for the other three groups (P < 0.05).
Table 2.
Mean difference (I–J) according to the post hoc Tukey test
| I
|
KD | Pneumonia | HFMD | URI |
|---|---|---|---|---|
| J | ||||
| CRP | ||||
| KD | – | 0.54 (0.000a) | 0.56 (0.000a) | 0.76 (0.000a) |
| Pneumonia | −0.54 (0.000a) | – | 0.01 (0.999) | 0.22 (0.05) |
| HFMD | −0.56 (0.000a) | −0.01 (0.999) | – | 0.20 (0.21) |
| URI | −0.76 (0.000a) | −0.22 (0.05) | −0.20 (0.21) | – |
| Hp | ||||
| KD | – | 152.3 (0.000a) | 109.5 (0.000a) | 185.6 (0.000a) |
| Pneumonia | −152.3 (0.000a) | – | −42.8 (0.173) | 33.3 (0.252) |
| HFMD | −109.5 (0.000a) | 42.8 (0.173) | – | 76.1 (0.004a) |
| URI | −185.6 (0.000a) | −33.3 (0.252) | −76.1 (0.004a) | – |
| apoA-I | ||||
| KD | – | −26.5 (0.000a) | −25.2 (0.002a) | −30.5 (0.000a) |
| Pneumonia | 26.5 (0.000a) | – | 1.3 (0.998) | −4.1 (0.913) |
| HFMD | 25.2 (0.002a) | −1.3 (0.998) | – | −5.3 (0.902) |
| URI | 30.5 (0.000a) | 4.1 (0.913) | 5.3 (0.902) | – |
| apoB | ||||
| KD | – | 19.36 (0.000a) | 4.04 (0.902) | 25.43 (0.000a) |
| Pneumonia | −0.32 (0.000a) | – | –15.32 (0.050) | 6.07 (0.577) |
| HFMD | −4.04 (0.902) | 15.32 (0.050) | – | 21.39 (0.004a) |
| URI | −25.43 (0.000a) | −6.07 (0.577) | −21.39 (0.004a) | – |
| HAR | ||||
| KD | – | 0.32 (0.000a) | 0.23 (0.000a) | 0.37 (0.000a) |
| Pneumonia | −0.32 (0.000a) | – | −0.09 (0.193) | 0.05 (0.554) |
| HFMD | −0.23 (0.000a) | 0.09 (0.193) | – | 0.15 (0.019a) |
| URI | −0.37 (0.000a) | −0.05 (0.554) | −0.15 (0.019a) | – |
The mean difference is significant at the 0.05 level by the post hoc Tukey test
KD Kawasaki disease, HFMD hand, foot, and mouth disease, URI upper respiratory tract infection, CRP C-reactive protein, Hp haptoglobin, apoA-I apolipoprotein A-I, apoB apolipoprotein B, HAR haptoglobin/apolipoprotein A-I ratio
ROC and Cutoff Values
The areas under the receiver operating characteristic curves (AUC-ROC) were calculated to assess the sensitivity–specificity relationship of each marker and to compare marker accuracy. The maximum Youden’s indices were used to determine marker thresholds that could produce the best overall diagnostic information. As a summary measure of diagnostic accuracy, AUC-ROC values are commonly used [11].
In this study, the potential diagnostic value of each marker was estimated using an AUC-ROC. We calculated the AUC-ROC values for all nine markers to estimate the potential use of each identified marker (Hp, HAR, PLT, and apoB) to discriminate KD patients from the other three groups (Fig. 1). The AUC-ROC values for the measured markers varied from the lowest for Hb (0.42 Hb) to the highest for HAR (0.88). The measured AUC-ROC values for the other markers were as follows: 0.77 for Hp, 0.74 for CRP, 0.71 for platelets, 0.69 for apoA-I, 0.62 for apoB, 0.60 for WBC, 0.43 for RBC, and 0.42 for Hb. The highest AUC-ROC value is considered to have excellent diagnostic accuracy [13]. In this study, the AUC-ROC value for HAR was the highest (Fig. 1).
Fig. 1.
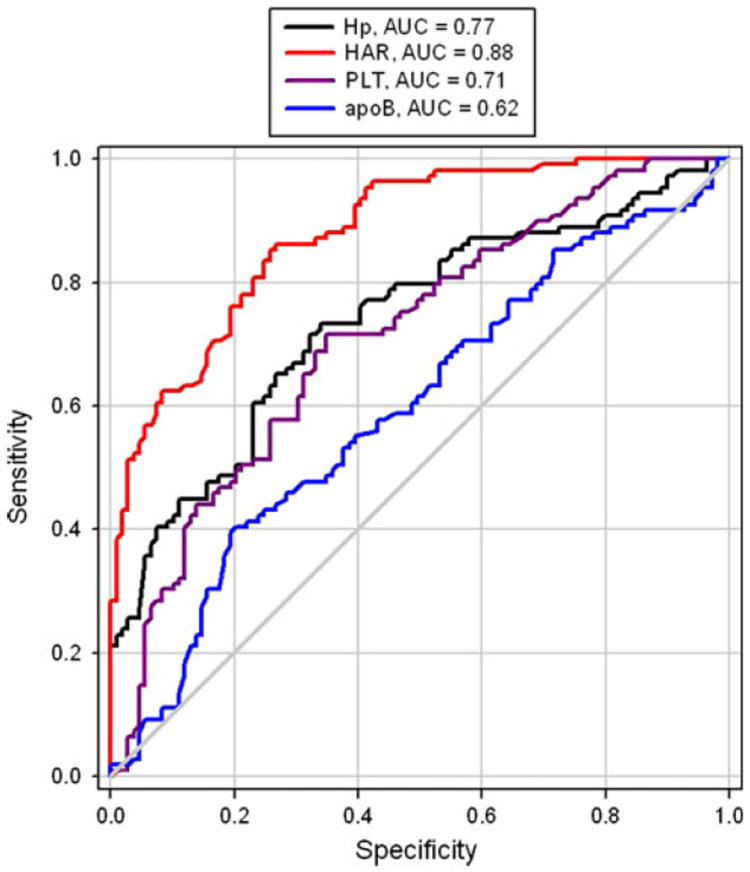
Receiver operating characteristics (ROC) of the haptoglobin/apolipoprotein A-I ratio (HAR), platelets (PLT), apolipoprotein B (apoB), and haptoglobin (Hp) show that HAR has sensitivity of 0.897 and specificity of 0.856 under its best cutoff value of 2 in the acute phase of Kawasaki disease (KD)
For comparison of different cutoff values, the AUC-ROC was used. According to the ROC curve, the best HAR cutoff value for diagnosing KD with the highest accuracy was 2 (sensitivity 89.7%, specificity 85.6%) (Table 3). The cutoff values for the other markers were 3 for Hp (sensitivity 76.5%, specificity 80.3%), 5.6 for CRP (sensitivity 81.1%, specificity 70.6%), and 450 × 109/l for platelets (sensitivity 73.9%, specificity 75.1%).
Table 3.
The cutoff values of the haptoglobin/apolipoprotein A-I ratio (HAR) obtained from receiver operating characteristics (ROC) curves
| Cutoff point | Sensitivity | Specificity | Youden’s index |
|---|---|---|---|
| 3 | 0.649 | 0.979 | 0.648 |
| 2.5 | 0.732 | 0.911 | 0.643 |
| 2.00a | 0.897 | 0.856 | 0.753 |
| 1.50 | 0.903 | 0.774 | 0.677 |
| 1 | 0.958 | 0.612 | 0.570 |
HAR’s area under the curve (AUC) = 0.88. The cutoff value with the best sensitivity and specificity was 2 for HAR (sensitivity, 89.7%; specificity, 85.6%)
The highest accuracy point, Youden’s Index = maximum (sensitivity + specificity) − 1
Discussion
Acute-phase reactants increase in inflammatory diseases such as autoimmune diseases, bacterial infections, toxin-mediated diseases, and viral infections and thus are used during the course of illness as measures of inflammation. A significantly elevated serum CRP level in the acute phase of KD has been reported by many researchers [10, 15, 18]. Thrombocytosis reflects another acute-phase reactant that occurs in KD [12]. Our study showed that the platelet level in the acute stage of KD was significantly higher than in the other three febrile diseases. Haptoglobin, an acute-phase protein synthesized by the liver in response to inflammatory cytokines, has been seen in association with vascular disease [2, 3]. The haptoglobin 2–1 phenotype has been reported in association with incomplete KD [9].
In our study, that the serum Hp level in KD was significantly higher than in other febrile illnesses. ApoA-I is the major protein component of the serum HDL particles and like other lipoproteins is significantly decreased during acute stress or illness, including KD [7]. Low levels of HDL have been reported in the acute phase of KD [1, 7, 14, 19]. Our study showed a significantly lower serum apoA-I level in acute KD than in other febrile illnesses.
Thus, in our study, the levels of Hp, apoA-I, and platelets differed significantly between KD and the other three febrile diseases. Changes in Hp and HAR in the acute phase of KD have not been reported previously. Determining the serum levels of Hp and apoA-I as well as their ratio (HAR) may be a useful supplemental diagnostic aid for KD. Using an HAR cutoff value of 2 (sensitivity, 89.7%; specificity, 85.6%) may be a useful diagnostic aid for detecting KD.
This study had a small number of study subjects from a limited number of febrile diseases. The study findings need to be confirmed with a larger sample using a variety of other febrile diseases in childhood. In addition, a supplemental test such as the HAR needs to be analyzed in a study population different from that used in this study. We did not evaluate the degree of coronary artery involvement in this study, so its relationship with the acute-phase reactants needs further investigation.
Conclusion
Early diagnosis and treatment of KD are critical for a better prognosis and better survival rates for children. In the acute phase of KD, a haptoglobin/apolipoprotein A-I ratio greater than 2 may be a useful supplemental biologic marker for KD.
Contributor Information
Ming-Yii Huang, Department of Radiation Oncology, Cancer Center, Kaohsiung Medical University Hospital, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, No. 100 Tzyou 1st Road, Kaohsiung 807, Taiwan.
Monesha Gupta-Malhotra, Department of Pediatrics, Division of Pediatric Cardiology, Children’s Memorial Hermann Hospital, University of Texas-Houston Medical School, 6411 Fannin Houston, Texas 77030, USA.
Joh-Jong Huang, Department of Family Medicine, Yuan’s General Hospital, No. 162 Cheng Kung 1st Road, Kaohsiung 80249, Taiwan.
Fei-Kai Syu, The Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, 615 N. Wolfe Street, Baltimore, MD 21205, USA.
Teh-Yang Huang, Email: huang190814@gmail.com, Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, 100 Shih-Chuan 1st Road, Kaohsiung 807, Taiwan.
References
- 1.Cabana VG, Gidding SS, Getz GS, Chapman J, Shulman ST. Serum amyloid A and high-density lipoprotein participate in the acute-phase response of Kawasaki disease. Pediatr Res. 1997;42:651–655. doi: 10.1203/00006450-199711000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007;29:92–110. doi: 10.1111/j.1751-553X.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 3.Fiotti N, Giansante C, Ponte E, Delbello C, Calabrese S, Zacchi T, Dobrina A, Guarnieri G. Atherosclerosis and inflammation: patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis. 1999;145:51–60. doi: 10.1016/s0021-9150(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 4.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21:193–199. doi: 10.1023/a:1011039216251. [DOI] [PubMed] [Google Scholar]
- 5.Henderson RJ, Wasan KM, Leon CG. Haptoglobin inhibits phospholipid transfer protein activity in hyperlipidemic human plasma. Lipids Health Dis. 2008;8:27–35. doi: 10.1186/1476-511X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 7.Kim H, Yamaguchi H, Inamo K, Okada T, Harada K. Changes in apolipoproteins during the acute phase of Kawasaki disease. Acta Paediatr Jpn. 1995;37:72–676. doi: 10.1111/j.1442-200x.1995.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 8.Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson textbook of pediatrics. 18. Saunders Publishers; UK: 2010. pp. 1036–1042. [Google Scholar]
- 9.Lee WC, Hwang KP, King YT, Chen HC, Chiou SS, Yang RC, Huang TY. Late diagnosis of Kawasaki disease is associated with haptoglobin phenotype. Eur J Clin Invest. 2000;30:379–382. doi: 10.1046/j.1365-2362.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin CY, Hwang B. Serial immunologic studies in patients with mucocutaneous lymph node syndrome (Kawasaki disease) Ann Allergy. 1987;59:91–297. [PubMed] [Google Scholar]
- 11.Loy CT, Irwig L. Accuracy of diagnostic tests read with and without clinical information: a systematic review. JAMA. 2004;292:1602–1609. doi: 10.1001/jama.292.13.1602. [DOI] [PubMed] [Google Scholar]
- 12.Melish ME. Kawasaki syndrome: a new infectious disease? J Infect Dis. 1981;143:17–324. doi: 10.1093/infdis/143.3.317. [DOI] [PubMed] [Google Scholar]
- 13.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 14.Newburger JW, Burns JC, Beiser AS, Loscalzo J. Altered lipid profile after Kawasaki syndrome. Circulation. 1991;84:625–631. doi: 10.1161/01.cir.84.2.625. [DOI] [PubMed] [Google Scholar]
- 15.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 16.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 17.Spagnuolo MS, Cigliano L, D’Andrea LD, Pedone C, Abrescia P. Assignment of the binding site for haptoglobin on apolipoprotein A-I. J Biol Chem. 2005;280:1193–1198. doi: 10.1074/jbc.M411390200. [DOI] [PubMed] [Google Scholar]
- 18.Ueno Y, Takano N, Kanegane H, Yokoi T, Yachie A, Miyawaki T, Taniguchi N. The acute-phase nature of interleukin 6: studies in Kawasaki disease and other febrile illnesses. Clin Exp Immunol. 1989;76:337–342. [PMC free article] [PubMed] [Google Scholar]
- 19.Weng KP, Hsieh KS, Huang SH, Lin CC, Huang DC. Serum HDL level at acute stage of Kawasaki disease. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1998;39:28–32. [PubMed] [Google Scholar]
- 20.Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999;104:e10. doi: 10.1542/peds.104.1.e10. [DOI] [PubMed] [Google Scholar]


