Abstract
BACKGROUND
Intra-operative tumor spill increases the risk of local recurrence of Wilms tumor, and adversely impacts relapse-free (RFS) and overall survival (OS) rates.
METHODS
Surgical checklists, operative notes, institutional pathology reports, central pathology review and flow sheets of 602 patients registered between August 1986 and September 1994 on National Wilms Tumor Study – 4 as randomized, followed or switched and coded as Final Stage II, favorable histology (FH) were reviewed. RFS and OS were estimated using the Kaplan-Meier method. Hazard ratios (HRs) were estimated using the Cox model and tested for statistical significance by the log-rank test.
RESULTS
Four hundred ninety-nine patients were found after review to have stage II, FH Wilms tumor. The eight-year RFS percentages were 85.0% (95% confidence interval (CI) – 81.1%, 88.1%) for those with no spill compared to 75.7% (65.8%, 83.2%) for those with spill. The eight-year OS percentages were 95.6% (93.1%, 97.3%) for those with no spill compared to 90.3% (82.2%, 94.9%) for those with spill. The HR for relapse among those with spill was 1.55 ((95%CI – 0.97,2.51), p = 0.067) and the HR for death was 1.94 ((0.92,4.09), p = 0.077).
CONCLUSIONS
RFS and OS were lower for patients who had intra-operative tumor spill. The majority of NWTS stage II, FH patients with intra-operative tumor spill have an overall excellent outcome when treated with two drug chemotherapy (vincristine and actinomycin D) and no abdominal irradiation.
INTRODUCTION
Intra-operative spill of favorable histology (FH) Wilms tumor cells has been thought to affect relapse-free and overall survival adversely. On National Wilms Tumor Studies (NWTS) 3–5, patients with intra-operative spill were divided into two groups: those with diffuse spillage involving the whole abdominal cavity and those with local spillage confined to the flank. Patients with diffuse spillage were treated with radiation therapy to the entire abdomen and 3-drug chemotherapy (vincristine, actinomycin-D, and doxorubicin) whereas patients with local spillage were treated with vincristine and actinomycin-D only. Based on an analysis of patients treated on NWTS- 3 and – 4 indicating that patients with stage II disease and local spillage had inferior overall survival compared to patients with stage II disease without local spillage [1], current Children’s Oncology Group studies treat patients with local spillage with doxorubicin and flank radiation. This analysis utilized Final Stage, which was the stage and histology originally submitted by the registering institution, as the determinant of tumor stage for the analysis [1]. The Final Stage may or may not represent the actual treatment stage depending on factors such as review by the NWTSG Pathologist of a complete set of histology slides that included involved lymph nodes or formal acceptance by the registering institution of a change in stage based on the central review, among other factors.
The present review was undertaken to determine the influence of intra-operative spillage on local relapse and survival among unirradiated patients with stage II, favorable histology Wilms tumor, treated with actinomycin D and vincristine only on NWTS – 4, determined by retrospective determination of stage by reviewers who were blinded to outcome.
PATIENTS AND METHODS
NWTS - 4 was a multi-institutional randomized clinical trial of different treatment regimens for patients less than 16 years of age at diagnosis with specific renal neoplasms who were diagnosed between August 6, 1986 and September 1, 1994. The required pre-operative evaluation, recommended operative procedure, central review of surgical and pathology records, staging, treatment randomization and results for the randomized trial have been published previously [2, 3]. On NWTS – 4, the Original Stage, recorded at the time of registration on study, was the stage reported by the registering institution. The Actual Stage was based on institutional acceptance of the results of the central pathology review if this differed from the Original Stage. If the central pathology review confirmed the Original Stage, Actual Stage was not entered. The Final Stage was the Original Stage when the Actual Stage was blank or the Actual Stage when Actual Stage was not blank. If the registering institution did not confirm acceptance of a change in stage as suggested by the central pathology review, the Final Stage remained the Original Stage.
Surgical checklists, operative notes, institutional pathology reports, central pathology review and flow sheets of 602 patients registered between August 1986 and September 1994 on National Wilms Tumor Study – 4 as randomized, followed or switched and coded as Final Stage II, favorable histology (FH) following central pathology review were reviewed by GJD (pediatric radiation oncologist), MHM (pediatric oncologist) or AEE (pediatric oncologist). All were reviewed by DMG (pediatric oncologist), who was blinded to the results of the other reviews. The review process included re-evaluation of the tumor stage. Stages were assigned using the NWTS – 3 staging system (Table I) [4].
Table I.
NWTS-3 Staging System
| Stage I | Tumor limited to kidney and completely excised. The surface of the renal capsule is intact. Tumor was not ruptured before or during removal. There is no residual tumor apparent beyond the margins of resection. |
| Stage II | Tumor extends beyond the kidney but is completely removed. There is regional extension of the tumor, i.e., penetration through the outer surface of the renal capsule into peri-renal soft tissues. Vessels outside the kidney substance are infiltrated or contain tumor thrombus. The tumor may have been biopsied or there has been local spillage of tumor confined to the flank. There is no residual tumor apparent at or beyond the margins of excision. |
| Stage III | Residual non-hematogenous tumor confined to abdomen. Any one or more of the following occur:
|
| Stage IV | Hematogenous metastases. Deposits beyond Stage III; e.g., lung, liver, bone, and brain. |
| Stage V | Bilateral renal involvement at diagnosis. An attempt should be made to stage each side according to the above criteria on the basis of extent of disease before biopsy. |
The stage of a tumor with thrombus present in the inferior vena cava depended upon the surgical procedure performed. When a thrombus propagating into the right atrium was removed piecemeal, the presence of residual hematogenous tumor cell spread was assumed (Stage IV). When the thrombus was transected, but removed in two pieces, one in continuity with the nephrectomy specimen and the other as a single specimen, generally accessed through the right atrium, local tumor cell contamination at the site of thrombus transection was assumed to have occurred (stage II with spill). If the thrombus was transected, but the proximal renal vein remnant, which could contain tumor, was not resected in continuity with the proximal portion of the thrombus, the surgical margin was assumed to be positive (Stage III). If the thrombus was removed as a single specimen in continuity with the nephrectomy specimen and the renal vein remnant, and local tumor spill was not otherwise noted to have occurred, the resection was assumed to have occurred without local spill (stage II without spill).
Statistical Methods
Relapse-free (RFS) and overall (OS) survival were estimated using the Kaplan-Meier method [5]. Hazard ratios (HRs) were estimated using the Cox model [6] and tested for statistical significance by the log-rank test [7]. Cumulative incidence curves and statistical tests comparing them for patients with and without spill were obtained using the R [8] cpmrsk package developed by Gray [9].
RESULTS
The records of 602 patients registered between August 1986 and September 1994 on National Wilms Tumor Study – 4 as randomized, followed or switched and coded as Final Stage II, FH following central pathology review were reviewed. Four hundred ninety-nine of the 602 were found after review to have stage II, FH Wilms tumor, and are included in the current analysis. The reasons for the differences between the Final Stage in the NWTS database and the review stage are shown in Table II. The most frequent reason for exclusion was a final down-stage to I on review. Ninety-five of these patients had spill and the remaining 404 did not have spill.
Table II.
Reasons for exclusion of NWTS-4 Final Stage II cases
| Bloody peritoneal fluid | 8 |
| Crossed ectopic kidney | 1 |
| CT pulmonary metastases | 3 |
| Doxorubicin | 1 |
| Doxorubicin + XRT | 3 |
| Extra-renal primary | 1 |
| Gross residual disease | 1 |
| Horseshoe kidney | 2 |
| Incomplete slide set – surgical margin could not be evaluated | 6 |
| IVC thrombus – no operative note | 1 |
| IVC thrombus removed piecemeal | 4 |
| IVC thrombus – renal vein remnant not resected | 3 |
| IVC thrombus removed piecemeal; renal vein remnant not resected | 3 |
| Macroscopic peritoneal metastases – not biopsied or not mentioned in pathology report | 3 |
| No operative note | 1 |
| No operative note or surgical checklist | 5 |
| Partial nephrectomy | 1 |
| Partial nephrectomy – Beckwith-Wiedemann syndrome | 1 |
| Partial nephrectomy – Simpson-Golabi-Behmel syndrome | 1 |
| Piecemeal removal of ureteral extension | 1 |
| Positive lymph nodes | 5 |
| Positive lymph node, bloody peritoneal fluid | 1 |
| Positive lymph node, positive surgical margin | 1 |
| Positive peritoneal fluid cytology | 1 |
| Positive surgical margin | 11 |
| Pre-operative biopsy – procedure not described | 2 |
| Pre-operative rupture – free retroperitoneal blood | 1 |
| Pre-operative rupture with large perirenal hematoma | 1 |
| Pre-operative rupture with gross residual disease | 1 |
| Pre-operative rupture with spill into right pleural cavity | 1 |
| Retroperitoneal hemorrhage biopsied through retroperitoneal approach | 1 |
| Solitary kidney – pre-nephrectomy chemotherapy | 1 |
| Stage I | 23 |
| Whole abdomen spill | 1 |
| XRT | 2 |
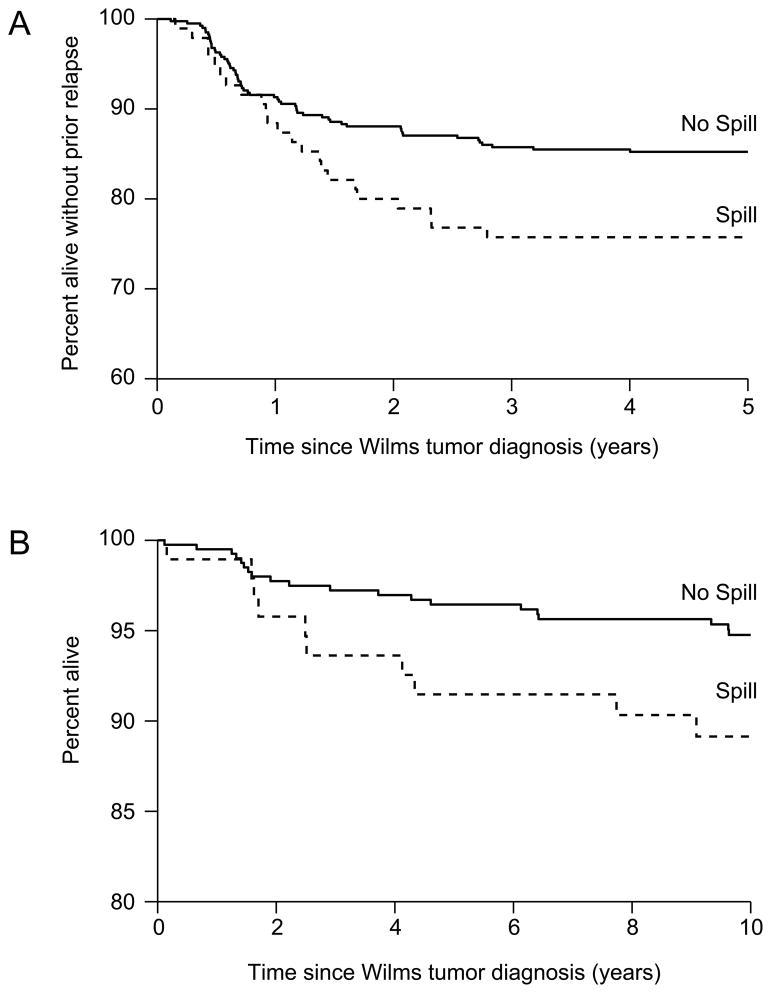
The eight-year RFS percentages were 85.0% (95% confidence interval (CI) – 81.1%, 88.1%) for those with no spill compared to 75.7% (65.8%, 83.2%) for those with spill (Figure 1A). The eight-year OS percentages were 95.6% (93.1%, 97.3%) for those with no spill compared to 90.3% (82.2%, 94.9%) for those with spill (Figure 1B).
Figure 1.
A) Relapse-free survival of NWTS-4 stage II, favorable histology patients with and without spill; B) Overall survival of NWTS-4 stage II, favorable histology patients with and without spill
Twenty-three relapses were observed among patients with spill compared to 16.3 expected (HR=1.55; 95%CI – 0.97,2.51, p = 0.067). Operative bed relapse occurred in 7.4% (7/95) of those with spill compared to 2.5% (10/404) of those without spill. Relapse in the abdomen/pelvis and the operative bed comprised a larger percentage of all relapses among those with spill compared to those without spill (Table III). Conversely the percentage of all relapses that were confined to the lung was larger among those without spill compared to those with spill.
Table III.
Distribution of site of relapse by spill category
| Site of relapse [Number (%)] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total number of patients | Total number of relapses and deaths | Abdomen or pelvis | Contralateral kidney | Liver | Lung and other | Lung only | Operative bed | Died in remission | |
| Spill | Number (%) | ||||||||
| No | 404 | 65 | 2 (3.1%) | 0 (0.0%) | 9 (13.8%) | 3 (4.6%) | 37 (56.9%) | 10 (15.4%) | 4 (6.2%) |
| Yes | 95 | 23 | 2 (8.7%) | 1 (4.3%) | 3 (13.0%) | 2 (8.7%) | 7 (30.4%) | 7 (30.4%) | 1 (4.3%) |
| Total | 499 | 88 | 4 (4.5%) | 1 (1.1%) | 12 (13.6%) | 5 (5.7%) | 44 (50.0%) | 17 (19.3%) | 5 (5.7%) |
The site of relapse within the groups of patients with and without spill by histological subtype are shown in Table IV. Local relapse was infrequent among those with epithelial or stromal predominant tumors, regardless of spill status.
Table IV.
Distribution of type of failure by histological pattern
| Histology | Total | Site | |||
|---|---|---|---|---|---|
| Censored | Local [Number (%)] | Metastatic [Number (%)] | Died | ||
| No Spill | 404 | ||||
| Blastema | 128 | 107 | 6 (4.7%) | 14 (10.9%) | 1 |
| Mixed | 238 | 204 | 6 (2.5%) | 26 (10.9%) | 2 |
| Epithelial | 25 | 18 | 0 (0.0%) | 6 (24.0%) | 1 |
| Stromal | 7 | 5 | 0 (0.0%) | 2 (28.6%) | 0 |
| Other | 6 | 5 | 0 (0.0%) | 1(16.7%) | 0 |
| Spill | 95 | ||||
| Blastema | 27 | 18 | 2 (7.4%) | 7 (25.9%) | 0 |
| Mixed | 61 | 48 | 7 (11.5%) | 5 (8.2%) | 1 |
| Epithelial | 5 | 4 | 1 (20.0%) | 0 (0.0%) | 0 |
| Stromal | 1 | 1 | 0 (0.0%) | 0 (0.0%) | 0 |
| Other | 1 | 1 | 0 (0.0%) | 0 (0.0%) | 0 |
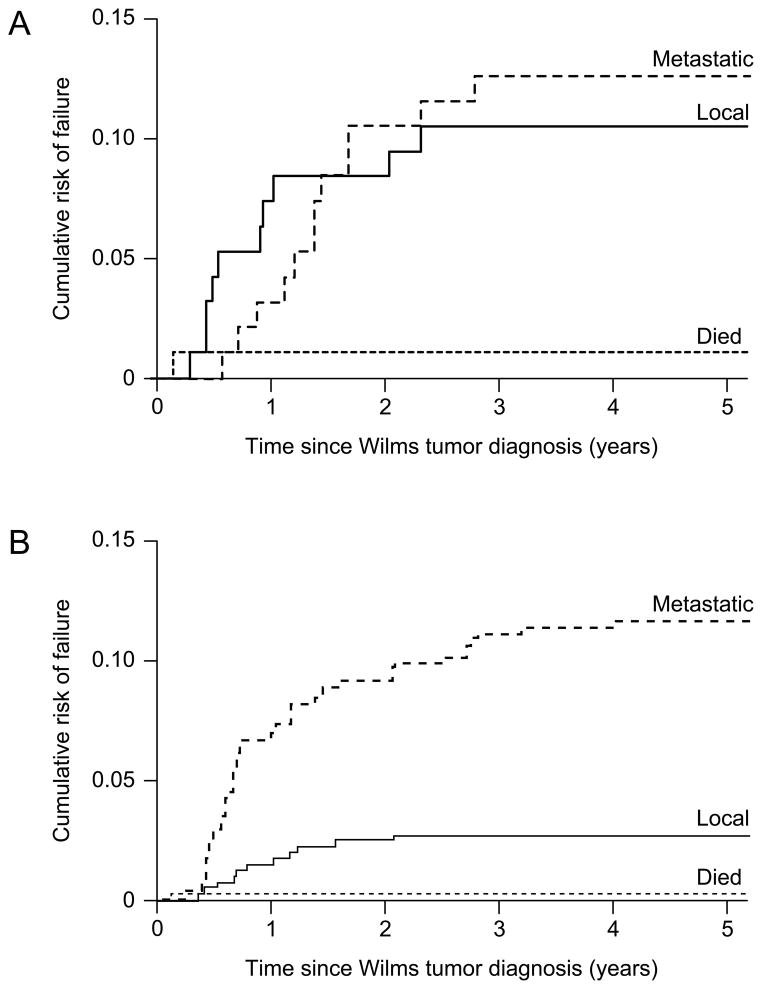
The timing of local relapse differed between those with and without spill (Figures 2A and 2B) (p = 0.001), but the timing of metastatic relapse (p = 0.942) and the timing of death (p =0.954) did not differ statistically between those with and without spill. Among those with spill, most local relapses occurred during the first year after diagnosis whereas among those without spill, local relapses occurred throughout the first two years after diagnosis. Metastatic recurrences (liver, lung) occurred throughout the first three years after diagnosis.
Figure 2.
A) Local, metastatic and death event-free survival of NWTS-4 stage II, favorable histology patients with spill; B) Local, metastatic and death event-free survival of NWTS-4 stage II, favorable histology patients without spill
Treatment of relapse differed between those with and without spill. Among those without spill, 48 (78.7%) relapsed patients were treated with doxorubicin or chest and/or abdominal irradiation, compared to 16 (72.7%) relapsed patients with spill (Odds Ratio (OR) = 1.501; 95% CI – 0.755, 2.858) (p = 0.231). Chest radiation therapy was given to 35 (57.4%) relapsed patients without spill compared to 9 (40.9%) relapsed patients with spill (OR = 1.103; 95% CI – 0.449, 2.453) (p = 0.841), whereas abdominal radiation therapy was given to 15 (24.6%) relapsed patients without spill compared to 7 (31.8%) relapsed patients with spill (OR = 2.059; 95% CI – 0.689, 5.562) (p = 0.159).
Ten deaths were observed among the patients who had tumor spill, compared to 6.1 deaths expected (HR=1.94; 95% CI - 0.92,4.09, p = 0.077). Two patients with spill died more than five years after diagnosis, one due to tumor progression and one due to congestive heart failure. There were three cases of congestive heart failure among these patients – one among those with spill and two among those without spill. Nine patients without spill died more than five years after diagnosis. Five deaths were due to tumor, one was due to end stage renal disease in a patient with focal glomerulosclerosis, Type I diabetes and hypertension, and three were due to other medical conditions, two in children with multiple congenital malformations.
DISCUSSION
This analysis has demonstrated that the relapse-free survival percentage for patients with stage II, FH Wilms tumor treated with vincristine and actinomycin D and without flank radiation therapy who experience tumor spill was 75.7% eight years after diagnosis compared to 85.0% among those who did not experience tumor spill. The eight-year overall survival rates were 90.3% and 95.6% respectively. None of these differences achieved statistical significance.
Treatment with radiation therapy to part of or the entire abdomen and administration of doxorubicin is currently recommended when pre-operative or intra-operative tumor spillage occurs, spreading tumor cells into the ipsilateral flank or entire peritoneal cavity [1]. Prior analyses based on patients entered on the National Wilms Tumor Study (NWTS) Group protocols suggested that the occurrence of spillage was an adverse prognostic factor for overall survival among patients enrolled on NWTS – 1 with stages I – III renal tumors [10], for abdominal recurrence and death among patients enrolled on NWTS – 2 with stages I – III renal tumors [11], and for abdominal recurrence and death among patients enrolled on NWTS – 3 with stages I – III favorable histology Wilms tumors [12]. Also, detailed analyses of radiation therapy parameters suggested that intra-abdominal relapse was not more frequent among those patients who received lower abdominal irradiation doses, but was significantly more frequent among those with unfavorable histology (anaplasia, rhabdoid tumor of the kidney, clear cell sarcoma of the kidney) [13].
Patients with intra-operative tumor rupture were assigned a stage III designation on NWTS – 1 and - 2. Treatment of spill originally included whole abdomen irradiation in NWTS – 1, but, as experience was gained in NWTS – 1, when spillage was confined to the flank, irradiation was confined to the flank [14]. The staging system was modified between NWTS – 2 and – 3. Patients with intra-operative spillage confined to the flank were changed from stage III (NWTS – 1 and – 2) to stage II (NWTS – 3) and those with peri-aortic lymph node involvement were changed from stage II (NWTS – 1 and – 2) to stage III (NWTS – 3) [4, 14, 15]. Radiation therapy treatment was randomized in NWTS – 3 for those with stage II disease, including those with intra-operative tumor rupture felt by the surgeon to be confined to the ipsilateral flank, between 20 Gray (Gy) and 0 Gy. There was no evidence in NWTS – 3 that the addition of either doxorubicin or radiation therapy improved the outcome for patients with stage II, FH Wilms tumor. However no subgroup analysis within stage II was performed to determine if those with spill independently benefitted from doxorubicin and/or local radiation therapy [4].
Analyses of outcome for stage I – IV patients treated on NWTS – 4 included in a case-control study suggested that the relative risk (RR) of local recurrence in a logistic regression analysis controlled for tumor histology was 4.5 (95% confidence interval (CI) – 1.4, 14.0) among stage II patients with spill [16]. A subsequent analysis of the impact of intra-operative spillage on the risk of flank relapse and overall relapse among NWTS – 3 and – 4 patients with Final Stage II and III, FH Wilms tumor suggested that 12.4% of unirradiated patients with stage II disease would develop a flank relapse compared to 0% for those treated with either 10 Gy or 20 Gy. In this analysis, treatment with three drugs (vincristine, actinomycin D and doxorubicin) did not decrease the risk of local recurrence compared to treatment with only vincristine and actinomycin D in an analysis adjusted for site of relapse and radiation therapy dose [1].
The current analysis demonstrated that relapse in the flank or any non-hematogenous abdominal site occurred in only 7.4% (7/95) and 9.5% (9/95) respectively of stage II, FH Wilms tumor patients with spill compared to 2.5% (10/404) and 3.0% (12/404) of those who did not in the same order. Thus, although the risk was higher among those with spill, the absolute risk of operative bed relapse is low. Biological prognostic markers, such as loss of heterozygosity for markers of chromosomes 1p and 16q [17, 18], were not obtained routinely on the tumor tissue of patients enrolled on NWTS-4. We thus cannot determine if these or some other marker of tumor aggressiveness might segregate with those tumors with and without spill that recurred locally or metastasized to explain the similar death rates.
Intra-operative tumor spill was a major consideration in the adoption by the International Society of Paediatric Oncology (SIOP) of the strategy of treatment of most patients with pre-nephrectomy chemotherapy. The randomization between immediate nephrectomy and pre-nephrectomy radiation therapy in SIOP-1 was discontinued due to the excess of intra-operative tumor ruptures among those randomized to immediate nephrectomy. Despite this difference in tumor rupture frequency, no difference in event-free or overall survival was reported [19]. In the United Kingdom Children’s Cancer Study Group (UKW3) randomized study between immediate nephrectomy and delayed nephrectomy after pre-nephrectomy combination chemotherapy, the frequency of intra-operative tumor rupture was 0% among those who received pre-nephrectomy chemotherapy compared to 14.6% among those randomized to immediate nephrectomy. However the frequency of local relapse was higher among those who received pre-nephrectomy chemotherapy (11.0%, 10/91) than among those who underwent immediate nephrectomy (5.4%, 5/93). There were no differences in event-free or overall survival between the two randomized treatments [20].
The current analyses indicate that the absolute risk of relapses in patients with stage II, FH with or without spillage is low. The risks of deleterious late effects associated with more aggressive therapies [21–28] than actinomycin D and vincristine without RT must all be considered in assessing the risk versus benefit of escalating treatment using toxic agents for children with stage II, FH Wilms tumor, with or without tumor cell spillage.
Acknowledgments
Supported in part by United States Public Health Service grant nos. CA-54498 (N.E. Breslow, Principal Investigator), CA-42326 (D.M. Green, Principal Investigator). The authors thank the investigators of the Children’s Oncology Group (formerly the Pediatric Oncology Group and the Children’s Cancer Group) and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered on the National Wilms’ Tumor Studies.
Footnotes
Drs. Green, Breslow, D’Angio, Malogolowkin, Ritchey, Evans, Beckwith, Perlman, Shamberger, Grundy, Dome, Thomas and Kalapurakal and Ms. Peterson affirm that they have no affiliations that they consider to be relevant and important with any organization that to any author’s knowledge has a direct interest, particularly a financial interest, in the subject matter discussed. Such affiliations include, but are not limited to, employment by an industrial concern, ownership of stock, membership on a standing advisory council or committee, a seat on the board of directors, or being publicly associated with a company or its products.
References
- 1.Kalapurakal JA, Li SM, Breslow NE, et al. Intraoperative spillage of favorable histology wilms tumor cells: influence of irradiation and chemotherapy regimens on abdominal recurrence. A report from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys. 2010;76:201–206. doi: 10.1016/j.ijrobp.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 3.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16:3744–3751. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 4.D’Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms’ tumor. Results of the Third National Wilms’ Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;33:457–481. [Google Scholar]
- 6.Cox DR. Regression models and life tables. J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 7.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc (A) 1972;135:185–206. [Google Scholar]
- 8.R Development Core Team. R. A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing; 2011. [Google Scholar]
- 9.Gray RJ. A class of X-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 10.Breslow NE, Palmer NF, Hill LR, et al. Wilms’ tumor: prognostic factors for patients without metastases at diagnosis: results of the National Wilms’ Tumor Study. Cancer. 1978;41:1577–1589. doi: 10.1002/1097-0142(197804)41:4<1577::aid-cncr2820410448>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Breslow N, Churchill G, Beckwith JB, et al. Prognosis for Wilms’ tumor patients with nonmetastatic disease at diagnosis--results of the second National Wilms’ Tumor Study. J Clin Oncol. 1985;3:521–531. doi: 10.1200/JCO.1985.3.4.521. [DOI] [PubMed] [Google Scholar]
- 12.Breslow N, Sharples K, Beckwith JB, et al. Prognostic factors in nonmetastatic, favorable histology Wilms’ tumor. Results of the Third National Wilms’ Tumor Study. Cancer. 1991;68:2345–2353. doi: 10.1002/1097-0142(19911201)68:11<2345::aid-cncr2820681103>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.D’Angio GJ, Tefft M, Breslow N, Meyer JA. Radiation therapy of Wilms’ tumor: results according to dose, field, post-operative timing and histology. Int J Radiat Oncol Biol Phys. 1978;4:769–780. doi: 10.1016/0360-3016(78)90035-4. [DOI] [PubMed] [Google Scholar]
- 14.D’Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms’ tumor: Results of the National Wilms’ Tumor Study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.D’Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms’ tumor: Results of the Second National Wilms’ Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 1999;229:292–297. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy PE, Telzerow PE, Breslow N, et al. Loss of heterozygosity for chromosomes 16q and 1p in Wilms’ tumors predicts an adverse outcome. Cancer Res. 1994;54:2331–2333. [PubMed] [Google Scholar]
- 18.Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 19.Lemerle J, Voute PA, Tournade MF, et al. Preoperative versus postoperative radiotherapy, single versus multiple courses of actinomycin D, in the treatment of Wilms’ tumor. Preliminary results of a controlled clinical trial conducted by the International Society of Paediatric Oncology (S.I.O.P) Cancer. 1976;38:647–654. doi: 10.1002/1097-0142(197608)38:2<647::aid-cncr2820380204>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell C, Pritchard-Jones K, Shannon R, et al. Immediate nephrectomy versus preoperative chemotherapy in the management of non-metastatic Wilms’ tumour: results of a randomised trial (UKW3) by the UK Children’s Cancer Study Group. Eur J Cancer. 2006;42:2554–2562. doi: 10.1016/j.ejca.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 22.Green DM, Grigoriev YA, Nan B, et al. Correction to “Congestive heart failure after treatment for Wilms’ tumor”. J Clin Oncol. 2003;21:2447–2448. doi: 10.1200/JCO.2003.99.005. [DOI] [PubMed] [Google Scholar]
- 23.Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer. 2010;127:657–666. doi: 10.1002/ijc.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow NE, Norkool PA, Olshan A, et al. Second malignant neoplasms in survivors of Wilms’ tumor: a report from the National Wilms’ Tumor Study. J Natl Cancer Inst. 1988;80:592–595. doi: 10.1093/jnci/80.8.592. [DOI] [PubMed] [Google Scholar]
- 25.Breslow NE, Takashima JR, Whitton JA, et al. Second malignant neoplasms following treatment for Wilm’s tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1995;13:1851–1859. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 26.Green DM, Peabody EM, Nan B, et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 27.Green DM, Lange JM, Peabody EM, et al. Pregnancy outcome after treatment for Wilms tumor: a report from the national Wilms tumor long-term follow-up study. J Clin Oncol. 2010;28:2824–2830. doi: 10.1200/JCO.2009.27.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cotton CA, Peterson S, Norkool PA, et al. Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol. 2009;27:1304–1309. doi: 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]