Abstract
Background
As people diagnosed with HIV and receiving combination antiretroviral therapy are now living longer, they are likely to acquire chronic conditions related to normal ageing and the effects of HIV and its treatment. Comordidities for people with HIV have not previously been described from a representative population perspective.
Methods
We used linked health administrative data from Ontario, Canada. We applied a validated algorithm to identify people with HIV among all residents aged 18 years or older between April 1, 1992 and March 31, 2009. We randomly selected 5 Ontario adults who were not identified with HIV for each person with HIV for comparison. Previously validated case definitions were used to identify persons with mental health disorders and any of the following physical chronic diseases: diabetes, congestive heart failure, acute myocardial infarction, stroke, hypertension, asthma, chronic obstructive lung disease, peripheral vascular disease and end-stage renal failure. We examined multimorbidity prevalence as the presence of at least two physical chronic conditions, or as combined physical-mental health multimorbidity. Direct age-sex standardized rates were calculated for both cohorts for comparison.
Results
34.4% (95% confidence interval (CI) 33.6% to 35.2%) of people with HIV had at least one other physical condition. Prevalence was especially high for mental health conditions (38.6%), hypertension (14.9%) and asthma (12.7%). After accounting for age and sex differences, people with HIV had significantly higher prevalence of all chronic conditions except myocardial infarction and hypertension, as well as substantially higher multimorbidity (prevalence ratio 1.30, 95% CI 1.18 to 1.44) and combined physical-mental health multimorbidity (1.79, 95% CI 1.65 to 1.94). Prevalence of multimorbidity among people with HIV increased with age. The difference in prevalence of multimorbidity between the two cohorts was more pronounced among women.
Conclusion
People living with HIV in Ontario, especially women, had higher prevalence of comorbidity and multimorbidity than the general population. Quantifying this morbidity at the population level can help inform healthcare delivery requirements for this complex population.
Keywords: Human immunodeficiency syndrome, Primary health care, Chronic disease, Comorbidity, Multimorbidity
Background
The accumulation of chronic conditions over the lifespan is a significant and rising burden on individuals and healthcare systems. In Canada, 33% of community dwelling individuals report having at least one of 7 common chronic conditions [1]. There is strong evidence that the management of chronic diseases is most effectively and economically provided in well-supported primary care settings [2,3]. The community-based management of these chronic conditions consumes substantial healthcare resources [4], and the bulk of this management occurs in primary care [5,6].
As people with Human immunodeficiency syndrome (HIV) on combination antiretroviral therapy (ART) are now living longer, they are likely to acquire additional chronic conditions related to normal aging as well as from the effects of HIV and its treatment [7,8]. While the literature regarding what factors contribute to the prevalence of specific conditions is evolving, it is clear that multimorbidity, the presence of several of these conditions, is increasingly the norm for people with HIV [9]. Early in the era of HIV, it was considered a deadly, acute condition, requiring a specialist focus on care [10-12]. However, with ART HIV becomes a chronic condition, and a generalist perspective may now have a more major role as comorbidities increase [13-17]. In order to determine the best way to integrate primary and specialist care [18], we require a full understanding of the complex health needs of this population.
The specific objectives of this study are to describe the prevalence of comorbidities and their multimorbidity for people living with HIV and to compare this prevalence to an age and sex adjusted general population. In Ontario, Canada, administrative data for over 13 million multiethnic residents is routinely collected at the time of care in a single-payer health system. These data can be used to measure the prevalence of chronic disease [19]. To our knowledge, this is the first study to use a population-based approach to measure the types and numbers of chronic diseases associated with HIV. As a result, this study will inform the complex healthcare needs of this population.
Methods
Study design
We conducted a retrospective observational study to examine the demographic and clinical characteristics of people living with HIV in Ontario compared with those in the general Ontario population. We analyzed the administrative databases held at the Institute for Clinical Evaluative Sciences (ICES) from the province of Ontario, Canada comprising data on almost 13 million individuals (2008). These data are made available to accredited researchers through a data sharing agreement with the Ontario Ministry of Health and Long-Term Care and are individually linked using an anonymous identification number in accordance with the provincial Personal Health Information Protection Act. The study was approved by the Ottawa Hospital Research Ethics Board and the Sunnybrook Health Sciences Centre Research Ethics Board.
Study population
We identified eligible individuals from the Registered Persons Database (RPDB), an electronic registry of all Ontarians eligible for health coverage that captures patient demographic information, including age, sex, postal code and mortality data (see flow diagram Figure 1). We used data from the Ontario Health Insurance Plan (OHIP) billing claims system, which records claims for about 95% of physician services conducted in the province, to obtain an HIV cohort. We applied a previously validated algorithm to people 18 years of age and older and living in Ontario between April 1, 1992 and April 1, 2009 [20]. Briefly, this algorithm requires 3 physician claims (International Classification of Diseases, Ninth Revision (ICD-9) code for HIV infection (042, 043, 044)) over a 3-year period and has a sensitivity and specificity of 96.2% (95% CI 95.2% - 97.9%) and 99.6% (95% CI 99.1% - 99.8%), respectively for identifying people living with HIV and receiving HIV care. As a comparison, a cohort of individuals 18 years or older, living in Ontario on April 1, 2009, and not in the HIV cohort was created using a computerized random number generator in a 5:1 ratio to the HIV cohort. For both cohorts, individuals without a valid health card, age or sex value and those without valid postal codes were excluded.
Figure 1.
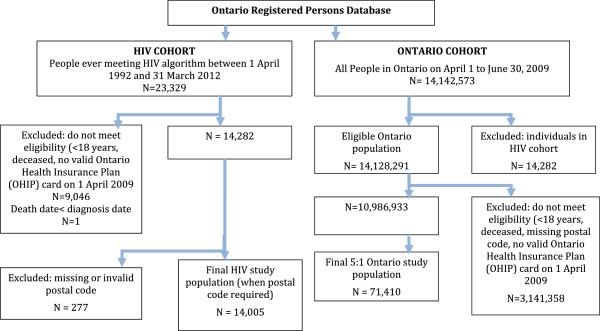
Flow diagram for study cohorts.
Variables
All variables for patients in the HIV and Ontario cohorts were captured using identical methods. Age, sex and postal code on April 1, 2009 were obtained from the RPDB. To broadly describe the populations, we used postal codes at the neighborhood level linked to 2006 Statistics Canada census data to assign income quintiles, marginalization quintiles and rurality scores. We used Matheson’s Canadian Marginalization Index [21] to assign marginalization quintiles for four components of marginalization (1 lowest to 5 highest): dependency, residential instability, material deprivation, and ethnic concentration and present these as a summary score. Rurality was assigned categorically into major urban areas (score 0 to 9), non-major urban areas (10 to 44), and rural areas (45 or higher) according to the Rurality Index of Ontario [22].
Using OHIP billing claims data, the Canadian Institute for Health Information (CIHI) Discharge Abstract Database, which supplies information on acute care hospitalizations and the CIHI National Ambulatory Care Reporting System, which captures information on visits to emergency departments, we applied additional previously validated algorithms in both cohorts to identify the status (i.e. presence or absence) of the following physical comorbidities on April 1, 2009: diabetes, congestive heart failure, acute myocardial infarction, hypertension, asthma, chronic obstructive lung disease, stroke, end stage renal disease and peripheral vascular disease [19,23-26]. People with mental health conditions between April 1, 2007 and April 1, 2009 were broadly captured using an algorithm previously validated to identify people receiving mental health services in the primary care setting [27].
Disease count was used to measure the prevalence of multimorbidity [28]. Multimorbidity was defined as the presence of two or more listed physical chronic conditions, and physical-mental health comorbidity was defined as a combination of mental health condition and any physical chronic health condition. In patients in the HIV cohort, this multimorbidity is in addition to HIV as their index condition.
We used the Ontario Drug Benefits claims database to identify individuals in the cohorts who were prescribed drugs paid for by the public system which covers those aged 65 and older and those receiving social assistance (Ontario Works, Ontario Disability Support Program, or the subsidized Trillium program).
Statistical analysis
We used descriptive statistics to describe the demographic characteristics of the two cohorts. For our descriptive analyses, age was treated as both a continuous variable and a categorical variable. Sex, age group, income quintiles, rurality categories and use of Ontario Drug Benefits were described as categorical variables, and the marginalization composite score as a continuous variable. These characteristics were compared between the cohorts using two-sample t-tests for continuous variables and chi-squared tests for categorical variables. We calculated the prevalence of individual physical and mental health comorbidities, multimorbidity, and physical-mental health comorbidity in the HIV cohort, together with 95% confidence intervals (CI).
We used direct standardization to calculate age and sex standardized prevalence rates for both populations. The Canadian 1991 population was used as the reference standard [29]. We present the comparative ratio of these rates together with 95% confidence interval, calculated using the formula provided by Breslow and Day [30]. We also compare the prevalence of comorbidity and multimorbidity with age between the HIV and Ontario cohorts for both men and women. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results
Table 1 provides for descriptive purposes the demographic characteristics of people in the HIV cohort compared to the Ontario cohort. The sex and age distributions differed substantially between the HIV and Ontario populations; people with HIV were more often male (80.5% vs. 48.8%, p < 0.001) and less likely to be in the youngest (18–35 years) or oldest (>65 years) age groups (16.8% vs. 30.2% and 4.1% vs. 15.9% respectively, p < 0.001). Overall, people with HIV were poorer (lowest income quintile 31.7% vs 19.5%, p < 0.001), lived in more marginalized neighborhoods (marginalization index 3.35 (SD 0.74) vs. 2.97 (SD 0.7), p < 0.001), were more likely to live in urban settings (89.7% vs. 72.8%, p < 0.001) and were more likely to have obtained provincial drug benefits than those in the Ontario cohort (62.8% vs. 30.1%, p < 0.001).
Table 1.
Demographic characteristics of HIV and Ontario cohorts (April 1, 2009)
| |
|
HIV cohort |
Ontario cohort |
p-value |
|---|---|---|---|---|
| n = 14,005 | n = 71410 | |||
|
Age (years) |
Mean (SD) |
45.36 (10.77) |
46.65 (17.79) |
<.001 |
|
Age group (n, %) |
18-35 years |
2,355 (16.8%) |
21,600 (30.2%) |
<.001 |
| |
36-50 years |
7,798 (55.7%) |
21,895 (30.7%) |
|
| |
51-65 years |
3,283 (23.4%) |
16,575 (23.2%) |
|
| |
>65 years |
569 (4.1%) |
11,340 (15.9%) |
|
|
Sex (n, %) |
Male |
11,270 (80.5%) |
34,862 (48.8%) |
<.001 |
|
Income quintile (1 lowest, 5 highest) |
Missing |
131 (0.9%) |
302 (0.4%) |
<.001 |
| |
1 |
4,441 (31.7%) |
13,918 (19.5%) |
|
| |
2 |
2,875 (20.5%) |
14,224 (19.9%) |
|
| |
3 |
2,280 (16.3%) |
14,053 (19.7%) |
|
| |
4 |
2,085 (14.9%) |
14,554 (20.4%) |
|
| |
5 |
2,193 (15.7%) |
14,359 (20.1%) |
|
|
Marginalization index |
Mean (SD) |
3.35 (0.74) |
2.97 (0.78) |
<.001 |
|
Rurality group |
Missing |
41 (0.3%) |
596 (0.8%) |
|
| |
Major urban |
41 (0.3%) |
596 (0.8%) |
|
| |
Non-major urban |
12,563 (89.7%) |
52,020 (72.8%) |
<.001 |
| |
Rural |
1,006 (7.2%) |
13,341 (18.7%) |
|
|
ODB claim |
Yes |
395 (2.8%) |
5,453 (7.6%) |
|
| 8,789 (62.8%) | 21,509 (30.1%) | <.001 |
The prevalence of individual comorbidities and of multimorbidity among individuals in the HIV cohort are presented in Table 2. More than one-third (38.6%, 95% CI 37.8% to 39.4%) of people with HIV had a mental health condition diagnosed within the previous 2 years. One-third (34.4%, 95% CI 33.6% to 35.2%) had at least one physical chronic condition in addition to their HIV. Comorbidities with especially high prevalence included hypertension (14.9%), asthma (12.7%), diabetes (8.3%) and chronic obstructive pulmonary disease (7.9%). The prevalence of multimorbidity (at least two physical conditions) was 10.8% (95% CI 10.3% to 11.3%), and of physical-mental health comorbidity was 15.5% (95% CI 14.9% to 16.1%).
Table 2.
Comorbidities and multimorbidity prevalence of people living with HIV in Ontario (April 1, 2009)
|
Comorbidity |
Prevalence |
||
|---|---|---|---|
| N = 14,005 | (n) | (%) | 95% CI |
|
Mental health condition |
5,401 |
38.6% |
(37.8% to 39.4%) |
|
Physical conditions: |
|
|
|
|
Myocardial infarction |
174 |
1.2% |
(1.1% to 1.4%) |
|
Congestive heart disease |
233 |
1.7% |
(1.5% to 1.9%) |
|
Chronic obstructive pulmonary disease |
1,106 |
7.9% |
(7.5% to 8.3%) |
|
End stage renal disease |
223 |
1.6% |
(1.4% to 1.8%) |
|
Peripheral vascular disease |
72 |
0.5% |
(0.4% to 0.6%) |
|
Asthma |
1,780 |
12.7% |
(12.2% to 13.3%) |
|
Diabetes |
1,167 |
8.3% |
(7.9% to 8.8%) |
|
Hypertension |
2,093 |
14.9% |
(14.4% to 15.5%) |
|
Stroke |
173 |
1.2% |
(1.1% to 1.4%) |
|
At least one physical condition |
4820 |
34.4% |
(33.6% to 35.2%) |
|
Physical-mental health multimorbidity |
2,174 |
15.5% |
(14.9% to16.1%) |
| Multimorbidity (at least two physical conditions) | 1,508 | 10.8% | (10.3% to 11.3%) |
Table 3 presents the age and sex standardized prevalence rates for the two cohorts (standardized to the 1991 Canadian population), along with the prevalence rate ratios and 95% confidence intervals. After accounting for age and sex differences, people in the HIV cohort were more likely to have at least one chronic condition than those in the Ontario cohort (prevalence ratio 1.13, 95% CI 1.07 to 1.20). People with HIV had significantly higher prevalence of all chronic conditions except myocardial infarction and hypertension, which were not significantly different from the general population. Individuals with HIV had substantially higher multimorbidity (prevalence ratio 1.30, 95% CI 1.18 to 1.44) and physical-mental health comorbidity (1.79, 95% CI 1.65 to 1.94) than the Ontario population.
Table 3.
Comparison of comorbidities and multimorbidity prevalence between HIV and Ontario cohorts (standardized by age and sex to Canadian 1991 population)
| |
HIV cohort |
Ontario cohort |
HIV:ON |
|||
|---|---|---|---|---|---|---|
| |
N = 14,005 |
N = 71,410 |
|
|
||
| |
n |
Prevalence |
n |
Prevalence |
Prevalence ratio |
(95% CI) |
| (%) | (%) | |||||
|
Mental health condition |
5401 |
40.55 |
15935 |
21.99 |
1.84 |
(1.75,1.94) |
|
Physical conditions: |
|
|
|
|
|
|
|
Acute myocardial infarction |
174 |
1.19 |
949 |
1.06 |
1.12 |
(0.78,1.60) |
|
Congestive heart failure |
233 |
3.35 |
1428 |
1.49 |
2.26 |
(1.74,2.92) |
|
Chronic obstructive lung disease |
1106 |
8.33 |
4621 |
5.33 |
1.56 |
(1.39,1.76) |
|
End stage renal disease |
223 |
1.96 |
631 |
0.76 |
2.57 |
(1.92,3.44) |
|
Peripheral vascular disease |
72 |
0.63 |
248 |
0.29 |
2.15 |
(1.35,3.40) |
|
Asthma |
1780 |
15.90 |
8448 |
12.13 |
1.31 |
(1.20,1.43) |
|
Diabetes |
1167 |
9.65 |
6730 |
8.13 |
1.19 |
(1.06,1.33) |
|
Hypertension |
2093 |
19.33 |
16886 |
20.29 |
0.95 |
(0.88,1.04) |
|
Stroke |
173 |
1.55 |
891 |
1.01 |
1.53 |
(1.15,2.03) |
|
At least one physical condition |
4820 |
38.72 |
26907 |
34.23 |
1.13 |
(1.07,1.20) |
|
Physical-mental health multimorbidity |
2174 |
16.98 |
7443 |
9.49 |
1.79 |
(1.65,1.94) |
| Multimorbidity (at least two physical conditions) | 1508 | 14.49 | 9417 | 11.13 | 1.30 | (1.18,1.44) |
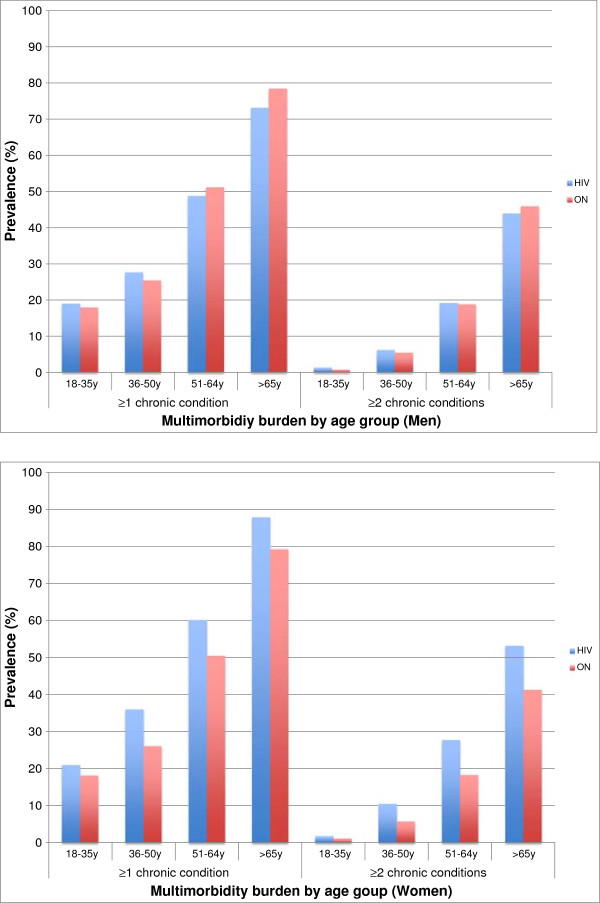
Figure 2 shows the prevalence of comorbidity and multimorbidity, for men and women respectively, by age group for the HIV and Ontario cohorts. Prevalence of multimorbidity increased with age in all groups and was higher for women than men. Men in the HIV cohort only had higher prevalence of at least one chronic condition and multimorbidity than men in the Ontario cohort in the younger age groups. In contrast, multimorbidity prevalence among women with HIV was consistently higher than among Ontario women in all age groups, and this gap appeared to widen slightly with age.
Figure 2.
Morbidity prevalence by age group among men and women with HIV versus the Ontario general population.
Discussion
Our study quantifies the substantial comorbidity prevalence among people living with HIV in Ontario. Mental health conditions and individual physical comorbidities were more prevalent among people with HIV than among the general population, as were multimorbidity and physical-mental health comorbidity. In addition, as is seen in the general population, our study confirms the accumulation of multiple chronic conditions with age for both men and women with HIV. Women had higher multimorbidity than men across all groups, which is consistent with the Canadian general population [1]. Furthermore, while men with HIV had slightly lower comorbidity and multimorbidity than Ontario men in most age groups, women with HIV had consistently higher comorbidity and multimorbidity prevalence than women without HIV.
To our knowledge, this is the first study to use a population-based approach to measure the types and numbers of chronic diseases associated with HIV; most studies have been conducted in clinical cohorts of people living with HIV. While the prevalence of comorbidity in people with HIV is clearly high, there have been inconsistencies regarding which conditions are more or less common in people with HIV. For instance, Butt et al. [31] found that HIV was associated with a decreased risk of diabetes, but that increasing age had a greater effect on diabetes rates in those with HIV than in the general population. Crothers et al. [32] found HIV to be an independent risk factor for COPD even after adjusting for smoking status and other risk factors. In their very large, almost exclusively male study comparing HIV positive with non HIV positive veterans, Goulet et al. [33] found that those with HIV had lower risk of hypertension, diabetes, vascular disease and psychiatric disorders, and higher rates of renal and liver disease than their HIV negative cohort. In their study of ART-experienced patients, Guaraldi et al. [8] found that those who were HIV positive had higher rates of diabetes, bone fracture and renal failure, but no difference in cardiovascular disease and hypertension. It is likely that the prevalence of individual comorbidities is a result of the complex interplay of aging, behavioral risk factors such as smoking (known to be higher among those with HIV), genetic risk factors, HIV severity, and ART history [8,9,34-36].
This study offers the unique strength of presenting the multimorbidity of people with HIV from a broad, multiethnic population of men and women from a variety of socioeconomic groups. Our findings are consistent with the literature highlighting that multimorbidity is common for people with HIV [7,8,33,34]. However, previously published prevalences of multimorbidity vary depending on the population base of the study, as clinical cohorts of people with HIV are often limited to certain demographic populations or at-risk groups. For example, our study showed that women with HIV have excess multimorbidity, which is consistent with Salter’s study of HIV positive injection drug users, but not with Guaraldi’s study of polypathology in ART-experienced people with HIV enrolled in a metabolic clinic. As men have historically represented a larger but decreasing proportion of people living with HIV, it is possible that Ontario men who were infected earlier in the epidemic were sicker and had higher mortality, resulting in a healthier cohort of older HIV-positive men within our population. However, because our population base isn’t limited by the definition of any high risk cohort and presents the clinical experience of people currently living with HIV our data is likely to be a robust estimate of the prevalence of comorbidities in the population.
In addition, previous studies have varied with respect to definitions for multimorbidity, such as specific diseases included, methods of clustering conditions, and numbers of conditions required to meet multimorbidity criteria. [5,37-40]. It is likely that broadening our comorbidity measures would have resulted in higher prevalence of multimorbidity [7,33]. Finally, in finding that almost 50% of people with HIV who have at least one comorbidity also have a mental health diagnosis, our study is the first to our knowledge to quantify the relationship between these conditions. Barnett et al. [37] found that those living in the most deprived areas had the highest prevalence of physical-mental health comorbidity at 11.0%, which is lower than the prevalence found in our HIV population, despite their higher deprivation compared to the Ontario general population.
There are several limitations to our study. First, we did not identify those who were unaware of their HIV status, estimated to be 26% of prevalent infections in Canada [41], or those not accessing health care. Furthermore, there are some settings in Ontario, most notably community health centres, that are not included in administrative data and so HIV patients in these setting where not included in the study. Community health centres are estimated to provide primary care for about 1% of the Ontario population [42], thus our findings are unlikely to be substantially affected by missing these individuals. Second, we were initially concerned that providers, especially those who have high-volume HIV practices or those who receive incentives for providing HIV care, would have preferentially identified HIV diagnosis codes for services received over other codes for chronic conditions. This potential bias would result in under ascertainment of chronic conditions in the HIV population compared to the general population. In light of this ascertainment issue, we are confident in our finding that, compared to the general Ontario population, the prevalence of almost all chronic conditions among those with HIV is higher.
Conclusion
This population-based study quantifies the substantially higher comorbidity and multimorbidity prevalence among people living with HIV relative to the general population, and the high contribution of mental health diagnoses to these conditions. This additional burden of disease increasingly defines the care needs of this population [43,44]. Strategies for chronic disease management, including for people with HIV, must be expanded with a view to multimorbidity [18,37,43,45,46]. Particular attention should be paid to the complex care needs defined by the comorbidity burden on women with HIV. By contributing to quantifying this increasing burden with age, our results can inform policy direction around HIV health services delivery. Future work will be directed at understanding how these complex patients access the healthcare system, and how providers are meeting the healthcare needs of this population.
Abbreviations
HIV: Human immunodeficiency syndrome; RPDB: Registered Persons Database; OHIP: Ontario Health Insurance Program; SAS: Statistical analysis system.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CK designed the study and oversaw its implementation, performed the analysis, was the primary author and approved the final version of the manuscript. JW and JY were involved in the data analysis, participated in the editing of the manuscript and approved the final version of the manuscript. DGM, MT, RG and WH contributed to the concept of the study, oversaw its implementation, helped guide the analysis and participated in the writing and approved the final version of the manuscript. All authors read and approved the final manuscript.
Authors’ information
CK is a PhD candidate in Epidemology at the University of Ottawa. She is a Clinician Investigator and Associate Professor at the C.T. Lamont Primary Health Care Research Centre and Bruyère Research Institute and Department of Family Medicine, University of Ottawa, Canada and a research fellow at the Institute for Clinical Evaluative Sciences. She holds a Canadian Institutes for Health Research (CIHR) Fellowship in the Area of Health Services/Population Health HIV/AIDS Research. JW was an analyst at the Institute for Clinical Evaluative Sciences at the time this research was completed. JY was an analyst at the Institute for Clinical Evaluative Sciences at the time this research was completed. MT is an Assistant Professor Department of Epidemiology and Community Medicine, at the University of Ottawa, Ottawa, Ontario, Canada and a Scientist at the Ottawa Hospital Research Institute, Clinical Epidemiology Program, Ottawa, Ontario, Canada. WH is Professor and Senior Research Advisor at the Department of Family Medicine, U of O, and the C.T. Lamont Primary Health Care Research Centre and Bruyère Research Institute. He holds the research Chair in Primary Health Care with a focus on Healthy Living. RG is a Senior Scientist at the Institute for Clinical Evaluative Sciences, a Scientist in the Centre for Research on Inner City Health at St. Michael’s Hospital in Toronto, and a Professor of Family and Community Medicine at the University of Toronto and St. Michael’s Hospital. DGM is a Senior Scientist, Ottawa Hospital Research Institute, Adjunct Scientist, Institute for Clinical Evaluative Sciences, Associate Professor, University of Ottawa and University of Toronto, and Associate Scientist, C.T. Lamont Primary Health Care Research Centre and Bruyère Research Institute.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Claire E Kendall, Email: ckendall@uottawa.ca.
Jenna Wong, Email: Jenna.wong@gmail.com.
Monica Taljaard, Email: mtaljaard@ohri.ca.
Richard H Glazier, Email: rick.glazier@ices.on.ca.
William Hogg, Email: whogg@uottawa.ca.
Jaime Younger, Email: jyounger@ohri.ca.
Douglas G Manuel, Email: dmanuel@ohri.ca.
Acknowledgements
This research was funded in part by the Ontario Ministry of Health and Long Term Care (MOHLTC). This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
CK holds a Canadian Institutes for Health Research (CIHR) Fellowship in the Area of Health Services/Population Health HIV/AIDS Research. RG is supported as a Clinician Scientist in the Department of Family and Community Medicine at the University of Toronto. DGM holds a chair in Applied Public Health Sciences, CIHR/PHAC.
With appreciation to Tony Antoniou for his guidance in use of the HIV algorithm and to Hannah Irving for assistance in editing this manuscript.
References
- Broemeling A-M, Watson DE, Prebtani F. Health Outcomes. Population patterns of chronic health conditions, co-morbidity and healthcare use in Canada: implications for policy and practice. Heal Q. 2008;11:70–76. doi: 10.12927/hcq.2008.19859. [DOI] [PubMed] [Google Scholar]
- Rothman AA, Wagner EH. Future of primary care chronic illness management: what is the role of primary care? Ann Int Med. 2003;138:256–262. doi: 10.7326/0003-4819-138-3-200302040-00034. [DOI] [PubMed] [Google Scholar]
- Dahrouge S, Devline RA, Hogg B, Russell G, Coyle D, Fergusson D. The economic impact of improvements in primary healthcare performance. Ottawa: Canadian Health Services Research Foundation; 2012. [Google Scholar]
- Terner M, Reason B, McKeag AM, Tipper B, Webster G. Chronic conditions more than age drive health system use in Canadian seniors. Heal Q. 2011;14:19–22. doi: 10.12927/hcq.2011.22485. [DOI] [PubMed] [Google Scholar]
- Fortin M, Hudon C, Haggerty JL, van den Akker M, Almirall J. Prevalence estimates of multimorbidity: a comparative study of two sources. BMC Heal Serv Res. 2010;10:111. doi: 10.1186/1472-6963-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggah E, Graves E, Bennett C, Manuel DG. The impact of multiple chronic diseases on ambulatory care use; a population based study in Ontario, Canada. BMC Heal Serv Res. 2012;12:452. doi: 10.1186/1472-6963-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R. Swiss HIVCS. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- Rackal JM, Tynan AM, Handford CD, Rzeznikiewiz D, Agha A, Glazier RH. Provider training and experience for people living with HIV/AIDS. Cochrane Database Syst Rev. 2011;15(6):Art. No: CD003938. doi: 10.1002/14651858.CD003938.pub2. doi:10.1002/14651858.CD003938.pub2. [DOI] [PubMed] [Google Scholar]
- Handford CD, Rackal JM, Tynan A-M, Rzeznikiewiz D, Glazier RH. The association of hospital, clinic and provider volume with HIV/AIDS care and mortality: systematic review and meta-analysis. AIDS Care. 2011;24:267–282. doi: 10.1080/09540121.2011.608419. [DOI] [PubMed] [Google Scholar]
- Handford C, Am T, Jm R, Glazier R. Setting and organization of care for persons living with HIV / AIDS. Cochrane Database Syst Rev. 2009. p. Article No: CD004348. doi:10.1002/14651858.CD004348.pub2. [DOI] [PMC free article] [PubMed]
- Fultz SL, Goulet JL, Weissman S, Rimland D, Leaf D, Gibert C, Rodriguez-Barradas MC, Justice AC. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis. 2005;41:738–743. doi: 10.1086/432621. [DOI] [PubMed] [Google Scholar]
- Duffus WA, Barragan M, Metsch L, Krawczyk CS, Loughlin AM, Gardner LI, Anderson-Mahoney P, Dickinson G, del Rio C, Antiretroviral T. Access Studies Study G. Effect of physician specialty on counseling practices and medical referral patterns among physicians caring for disadvantaged human immunodeficiency virus-infected populations. Clin Infect Dis. 2003;36:1577–1584. doi: 10.1086/375070. [DOI] [PubMed] [Google Scholar]
- Sheth AN, Moore RD, Gebo KA. Provision of general and HIV-specific health maintenance in middle aged and older patients in an urban HIV clinic. AIDS Patient Care STDS. 2006;20:318–325. doi: 10.1089/apc.2006.20.318. [DOI] [PubMed] [Google Scholar]
- Reinhold JP, Moon M, Tenner CT, Poles MA, Bini EJ. Colorectal cancer screening in HIV-infected patients 50 years of age and older: missed opportunities for prevention. Am J Gastroenterol. 2005;100:1805–1812. doi: 10.1111/j.1572-0241.2005.50038.x. [DOI] [PubMed] [Google Scholar]
- Leece P, Kendall C, Touchie C, Pottie K, Angel JB, Jaffey J. Cervical cancer screening among HIV-positive women. Retrospective cohort study from a tertiary care HIV clinic. Can Fam Physician. 2010;56:e425–e431. [PMC free article] [PubMed] [Google Scholar]
- Chu C, Selwyn PA. An epidemic in evolution: the need for new models of HIV care in the chronic disease era. J Urban Heal. 2011;88:556–566. doi: 10.1007/s11524-011-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iron K, Lu H, Manuel D, Henry D, Gershon A. Using linked health administrative data to assess the clinical and healthcare system impact of chronic diseases in Ontario. Healthc Q. 2011;14:23–27. doi: 10.12927/hcq.2011.22486. [DOI] [PubMed] [Google Scholar]
- Antoniou T, Zagorski B, Loutfy MR, Strike C, Glazier RH. Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. PLoS One. 2011;6:e21748. doi: 10.1371/journal.pone.0021748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Canadian marginalization index user guide. Toronto, ON: Canadian Journal of Public Health; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj B. Measuring “rurality” for purposes of health care planning: an empirical measure for Ontario. Ont Med Rev. 2000;67:33–52. [Google Scholar]
- Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16:183–188. doi: 10.1155/2009/963098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu K, Campbell NR, Chen Z-L, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- Tu JV, Naylor CD, Austin P. Temporal changes in the outcomes of acute myocardial infarction in Ontario, 1992–1996. CMAJ. 1999;161:1257–1261. [PMC free article] [PubMed] [Google Scholar]
- Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- Steele LS, Glazier RH, Lin E, Evans M. Using administrative data to measure ambulatory mental health service provision in primary care. Med Care. 2004;42:960–965. doi: 10.1097/00005650-200410000-00004. [DOI] [PubMed] [Google Scholar]
- Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10:134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institue for Health Information. Making sense of health indicators: statisical considerations. Ottawa, ON: CIHI; 2010. [Google Scholar]
- Breslow NE, Day NE. Statistical methods in cancer research, volume II: the design and analysis of cohort studies. New York: Oxford University Press; 1987. pp. 61–64. [PubMed] [Google Scholar]
- Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, Leaf D, Justice AC. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, Bryant K, Justice AC. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53:1256–1264. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röling J, Schmid H, Fischereder M, Draenert R, Goebel FD. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. 2006;42:1488–1495. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22:17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Broemeling A, Watson D, Black C. Chronic conditions and co-morbidity among residents of British Columbia. Vancouver, BC, Canada: Centre for Health Services and Policy Research; 2005. [Google Scholar]
- Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health. 2012;12:201. doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A, Winbald B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Heal. 2008;98:1198–1200. doi: 10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada. HIV/AIDS epi update: national HIV prevalence and incidence estimates in Canada for 2008. Ottawa, Ontario, Canada: Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control; 2010. [Google Scholar]
- Glazier RH, Zagorski BM, Rayner J. Comparison of primary care models in Ontario by demographics, case mix and emergency department use, 2008/09 to 2009/10. ICES investigative report. Toronto, Ontario: ICES Investigative Report. Toronto: Institute for Clinical Evaluative Sciences; 2012; 2012. [Google Scholar]
- Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS. 2012;26(Suppl 1):S11–S18. doi: 10.1097/QAD.0b013e3283558500. December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe AK, Chander G, Moore RD. Control of medical comorbidities in individuals with HIV. J Acquir Immune Defic Syndr. 2011;58:458–462. doi: 10.1097/QAI.0b013e31823801c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2014;58(1):e1–e34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9. doi: 10.1016/S0140-6736(12)60482-6. [DOI] [PubMed] [Google Scholar]