Abstract
Background
Unintentional loss of weight and muscle due to aging and disease has been associated with increased mortality. Wasting and weight loss occur in HIV infection even in the modern era of effective antiretroviral therapy.
Methods
We determined the association of MRI-measured regional and total skeletal muscle and adipose tissue with 5-year, all-cause mortality in 922 HIV-infected persons in the study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM).
Results
After 5 years of follow-up, HIV-infected participants with arm skeletal muscle in the lowest tertile had a mortality rate of 23%, compared with 11 and 8% for those in the middle and highest tertiles. After multivariable adjustment for demographics, cardiovascular risk factors, HIV-related factors, inflammatory markers, and renal disease, we found that lower arm skeletal muscle, lower leg skeletal muscle and higher visceral adipose tissue (VAT) were each independently associated with increased mortality. Those in the lowest tertile of arm or leg skeletal muscle had higher odds of death [arm: odds ratio (OR)=2.0, 95% confidence interval (CI) 0.96–4.0; leg: OR=2.4, 95% CI 1.2–4.8] compared with the highest respective tertiles. Those in the highest tertile of VAT had 2.1-fold higher odds of death (95% CI 1.1–4.0) compared with the lowest VAT tertile.
Conclusion
Lower muscle mass and central adiposity appear to be important risk factors for mortality in HIV-infected individuals. A substantial proportion of this risk may be unrecognized because of the current reliance on body mass index in clinical practice.
Keywords: body composition, cachexia, fat redistribution, HIV infection, lipoatrophy, lipodystrophy, mortality, sarcopenia
Introduction
Highly active antiretroviral therapy (HAART) has greatly reduced mortality in HIV-infected persons, but HIV infection remains associated with a higher risk of death relative to uninfected persons, even after controlling for demographic and traditional cardiovascular disease (CVD) risk factors [1]. In recent data from the Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM), we have also shown that inflammatory markers [C-reactive protein (CRP) and fibrinogen] and renal disease (as assessed by albuminuria and cystatin C) are independently associated with higher mortality risk [2,3].
Both HIV infection and aging are characterized by increased protein catabolism and decreased muscle mass [4,5], which have been associated with increased risk of mortality both in the elderly and in HIV-infected individuals. Aging is also associated with increases in visceral adipose tissue (VAT) [6], whereas subcutaneous adipose tissue (SAT) and total body fat appear to increase until age 55–64 and then decrease thereafter [6,7]. HIV infection has been complicated by loss of SAT [8,9], from which there is little recovery [10]. Although we have previously shown that HIV-associated peripheral lipoatrophy is not associated with central fat accumulation [8,9], HIV-infected individuals are known to gain VAT both with age and with restoration to health [10].
Early work by Heymsfield et al. [11] found that simple bedside estimates of arm muscle mass (using mid-arm circumference and triceps skinfold thickness) could be used in malnourished patients to predict progression to death from malnutrition. Wannamethee et al. [12] recently found that decreased mid-arm muscle circumference (MAMC) and increased waist circumference were independently associated withmortality in older men. Landi et al. [13] also reported decreased MAMC to be associated with mortality in elderly men and women. In HIV-infected individuals, it is unknown whether muscle mass loss contributes to excess mortality independently of fat changes.
No large, nationally representative study has examined the association of regional body composition with mortality in the setting of HIV infection. We therefore assessed the association of regional skeletal muscle and adipose tissue with mortality [2,3] in the FRAM study [14], a large, nationally representative, multiethnic cohort of HIV-infected men and women, which used MRI to measure body composition. We evaluated whether muscle loss, subcutaneous lipoatrophy, and/or central lipohypertrophy would predict death in HIV-infected individuals, independently of demographic and CVD risk factors, as well as inflammation and renal disease.
Methods and procedures
Study population
We determined the association of MRI-measured adipose tissue and skeletal muscle with mortality in HIV-infected persons enrolled in the FRAM study. The methods, design, and sample characteristics of the FRAM cohort have been described previously in detail [14]. Briefly, FRAM is a large, nationally representative, multicenter study of HIV infection, originally designed to evaluate lipodystrophy and metabolic abnormalities in HIV-infected persons. Between June 2000 and September 2002, 1183 HIV-infected men and women from 16 geographically diverse sites were enrolled in FRAM, with a follow-up exam conducted approximately 5 years later (FRAM-2), at which time we determined mortality status. We have described the vital status, retention, and observation time for FRAM-2 previously [1]. At the second exam, 794 HIV-infected participants were known to be alive and 128 were known to be dead. Vital status could not be determined in 261 participants in whom contact could not be re-established. Linkage to the National Death Index was not possible because of patient confidentiality constraints. The institutional review boards at all sites approved the protocols for both FRAM examinations.
MRI
Whole body MRI was performed to quantify regional and total skeletal muscle and adipose tissue volumes, as described in detail previously [8,14–16]. All scans were read by the same analyst at the Obesity Research Center, St. Luke’s Roosevelt Hospital, New York, New York, USA. Volumes were normalized by dividing by height2 with summaries back-transformed to 1.75 m of height, as in previous analyses [8,9]. We tertiled adipose tissue and skeletal muscle depots using cut-offs determined separately in men and women, using all participants with measured adipose tissue and skeletal muscle. We did not adjust to body mass index (BMI), as BMI is influenced by the phenomena being studied: quantity of fat and skeletal muscle. The skeletal muscle and adipose tissue anatomic sites considered in this analysis were: total, leg, lower trunk (abdomen and back), upper trunk (chest and back), arm, and VAT.
Anthropometric measurements
All staff members were centrally trained and certified for measurements. Anthropometric measurements were conducted according to a standard procedure [17]. Participants wore light clothing or a hospital gown and no shoes. All circumferences were made using a Gulick II measuring tape, and skinfolds were measured with a calibrated Lange caliper. Waist circumference was measured in the mid-axillary line immediately below the lowest rib. Mid-arm circumference was measured at the midpoint between the tips of the acromion process and the olecranon process perpendicular to the long axis of the upper arm. Mid-thigh circumference was measured at the midpoint between the inguinal crease and the proximal border of the patella, perpendicular to the long axis of the thigh. Triceps skinfold was measured on the posterior surface of the right upper arm, at the point previously marked for the mid-upper arm circumference. Thigh skinfold was measured on the anterior surface of the mid-arm, at the point previously marked for the mid-thigh circumference.
Mid-arm muscle circumference and mid-thigh muscle circumference (MTMC) were calculated as follows [11]:
MAMC = mid-arm circumference − π tricep skinfold
MTMC = mid-thigh circumference − π thigh skinfold
Other measurements
Standardized questionnaires that were validated in a general population were used to determine demographic characteristics; medical history; risk factors for HIV; and use of tobacco [14,18]. Research associates interviewed participants and reviewed medical charts regarding antiretroviral drug use. A diagnosis of AIDS was made by CD4 lymphocyte count less than 200 or history of opportunistic infection or malignancy.
Hepatitis C (HCV) RNA testing was performed on frozen sera using Bayer Versant 3.0 branched DNA assay (Leverkusen, Germany) in the entire cohort. CRP, fibrinogen, and cystatin C were measured using a BNII nephelometer [19]. Estimated glomerular filtration rate (eGFR) based on cystatin C was calculated using the CKD Epidemiology Collaboration Equation: [eGFR=76.7 × cystatin C−1.19] [20]. Urinalysis was performed to determine microalbuminuria [positive urine dipstick (1+ or greater) or a urine albumin-to-urine creatinine ratio greater than 30 mg/g] in real-time [21]. CD4 lymphocyte count and percentage, HIV RNA level, and other blood specimens were analyzed in a single centralized laboratory (Covance, Indianapolis, Indiana, USA). Biomarkers, plasma viremia, and all other blood specimens were measured at baseline.
Covariates
All covariates were collected at the first examination, and vital status was determined at the second examination, 5 years later. Clinical information was collected using standardized questionnaires, laboratory, and anthropometric measurement protocols. The following information was considered for inclusion in multivariable models: demographic characteristics (age, sex, and race), traditional CVD risk factors (diabetes, smoking, blood pressure, high-density lipoprotein cholesterol and total cholesterol), inflammatory markers (CRP, fibrinogen), renal markers (eGFR by cystatin C and albuminuria) and HIV-related factors [HIV RNA level, CD4 cell count, AIDS [22], and HCV infection (defined by detectable HCV RNA)]. In addition, both cumulative and past exposure to each ARV drug and class were evaluated. Multiple imputation using the Markov chain Monte Carlo method was used to impute missing covariates [23].
Statistical methods
As in previous analyses [2,3], we analyzed cumulative 5-year mortality using multivariable logistic regression analysis. Since the exact dates of death were unknown, those who died provided left-censored observations, meaning that death was only known to have occurred sometime before the contact attempt at approximately 5 years of follow-up. We therefore used logistic regression with an offset term for follow-up time, rather than Cox proportional hazards regression as our primary analysis, because this form of regression is appropriate for left-censored events. We also tested exponential regression survival models, but found that model fit was improved using logistic regression. Follow-up time was defined as elapsed time from baseline to follow-up exam or last contact. To account for those with missing vital status, we also adjusted estimates using an inverse probability weighting approach by modeling the participant’s probability of having known death status [24]. The inverse of this probability was then used as a weight applied to persons with known vital status in the logistic regression analysis of death.
To determine if MRI-measured adipose tissue and skeletal muscle depots were independently predictive of mortality, multivariable models were sequentially adjusted for demographics, traditional CVD risk factors, HIV-related factors, and inflammation and renal markers. To ensure that models were not overfit, we built parsimonious models using a backward stepwise procedure. For all models, we tested the linearity of continuous variables, and we tested the additional benefit of log transformations and quadratic terms. A comparison of tertiled depots to log-transformed depots found better model fit when depots were tertiled. Sensitivity analyses examined anthropometric measures (waist circumference, MAMC, MTMC) in place of MRI-measured depots.
Finally, in order to quantify the impact of low limb muscle and central adiposity on mortality in HIV-infected persons at a population level, we estimated the attributable risk associated with being in the lowest tertile of arm or leg skeletal muscle and in the highest tertile of VAT. The attributable risk accounts for not only the strength of the association of a risk factor with mortality, but also for the prevalence of the condition in the population of interest. The population-attributable risk percentage was calculated using incidence rates of death predicted from fully adjusted multivariable models as: 100 × [(population mortality rate − mortality rate in unexposed)/population mortality rate] [25]. All analyses were conducted using the SAS system, version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Baseline characteristics
Demographic and baseline clinical characteristics of the 922 HIV-infected participants with known vital status stratified by tertiles of arm muscle are shown in Table 1. HIV-infected participants with arm muscle in the lowest tertile at the baseline exam were more often Caucasian and nondiabetic, and had lower blood pressure, smaller waist circumferences, and lower BMI compared with those who had more arm muscle. The lowest tertile of arm muscle also had higher smoking prevalence, lower eGFR, and higher fibrinogen levels compared to the highest arm muscle tertile. Among HIV-related factors, baseline CD4 cell counts were lower and antiretroviral use was more prevalent in those with less arm muscle. Similar results were seen when we stratified by tertiles of leg muscle (Supplemental Table 1, http://links.lww.com/QAD/A147), which was positively correlated with arm muscle (Spearman r = 0.66). Results stratified by tertile of VAT are presented in Supplemental Table 2, http://links.lww.com/QAD/A147.
Table 1.
Baseline characteristics of HIV-infected FRAM participants by arm skeletal muscle tertile.a
| Arm SM tertiles (separate cut-points by sex) |
P | |||
|---|---|---|---|---|
| Tertile 1 (lowest; n = 313) |
Tertile 2 (n = 307) |
Tertile 3 (highest; n = 302) |
||
| Age (years) | 43.0 (37.0–48.0) | 42.0 (37.0–48.0) | 42.0 (37.0–47.0) | 0.51 |
| Female | 31% | 28% | 30% | 0.92 |
| Race | ||||
| African-American | 30% | 40% | 47% | <0.0001 |
| White | 57% | 46% | 40% | |
| Other | 13% | 13% | 12% | |
| Diabetes | 6% | 5% | 15% | <0.0001 |
| Smoking status | ||||
| Current | 51% | 41% | 37% | 0.0003 |
| Past | 21% | 20% | 25% | |
| Never | 28% | 39% | 38% | |
| Antihypertensive use | 22% | 18% | 24% | 0.44 |
| ACE-I use | 9% | 8% | 11% | 0.31 |
| Hyperlipidemia treatment | 13% | 16% | 17% | 0.19 |
| SBP (mmHg) | 113.0 (104.0–123.0) | 115.0 (107.0–123.0) | 118.0 (110.0–127.0) | <0.0001 |
| DBP (mmHg) | 77.0 (69.0–83.0) | 78.0 (71.0–84.0) | 80.0 (73.0–87.0) | <0.0001 |
| Waist circumference (cm) | 84.8 (76.9–92.3) | 87.9 (81.1–95.5) | 93.2 (85.8–101.0) | <0.0001 |
| BMI (kg/m2) | 22.4 (20.6–24.7) | 24.6 (22.4–26.9) | 27.1 (24.6–30.9) | <0.0001 |
| Total cholesterol (mg/dl) | 188.0 (156.0-222.0) | 194.0 (162.0–235.0) | 190.0 (159.0–219.0) | 0.98 |
| HDL (mg/dl) | 40.0 (34.0–52.0) | 43.0 (34.0–54.0) | 40.0 (33.0–52.0) | 0.70 |
| CRP (mg/l) | 1.8 (0.8–4.2) | 1.7 (0.7–3.8) | 1.9 (0.9–4.2) | 0.81 |
| Fibrinogen (mg/dl) | 379 (297–465) | 359 (302–427) | 349 (291–418) | 0.0066 |
| eGFRcys (ml/min per 1.73 m2) | 79 (67–97) | 86 (72–100) | 90 (75–106) | <0.0001 |
| Albuminuria | 22% | 21% | 20% | 0.39 |
| HIV-related factors | ||||
| Detectable HIV RNA | 51% | 45% | 53% | 0.59 |
| Current CD4 cell count (cells/µl) | 333 (169–513) | 376 (215–550) | 419 (263–566) | <0.0001 |
| HIV duration (years) | 8.1 (5.5–11.5) | 8.1 (5.3–11.8) | 7.6 (5.0–11.5) | 0.37 |
| Detectable HCV RNA | 25% | 18% | 21% | 0.15 |
| History of AIDSb | 75% | 68% | 69% | 0.090 |
| Any antiretroviral (current) | 87% | 84% | 80% | 0.0059 |
| HAART use (ever) | 90% | 89% | 85% | 0.022 |
Arm SM is adjusted for height and then tertiled, with cut-points of T1<2.7 kg for women, <3.6 kg for men. T2=2.7–3.2 kg for women, 3.6–4.3 kg for men; T3>3.2 kg for women, >4.3 kg for men. ACE-I, angiotensin-converting enzyme inhibitor; BP, blood pressure; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FRAM, Study of Fat Redistribution and Metabolic Change in HIV Infection; HDL, high-density lipoprotein; SM, skeletal muscle.
Statistics estimated using IPCW weights. Continuous data are represented as median (IQR).
AIDS defined by CD4 cell count <200 cells/µl or history of opportunistic infection or malignancy.
The number of participants lost to follow-up was fairly evenly distributed over the arm muscle tertiles: 29% in tertile 1, 37% in tertile 2, and 34% in tertile 3. Those who were lost to follow-up had levels of arm muscle, leg muscle and VAT that were similar to those who were alive (data not shown). Compared with those who were either alive or had unknown vital status at year 5, those who died were older, more often African-American, and had a higher prevalence of smoking, detectable HIV RNA, hepatitis C, history of AIDS, and more kidney disease and inflammation [3].
Association of MRI-measured skeletal muscle and adipose tissue with 5-year mortality
In unadjusted analyses, higher quantities of regional and total body skeletal muscle had protective associations with 5-year mortality risk (Table 2). Among regional skeletal muscle depots, the strongest associations were seen for arm and leg muscle. Unadjusted mortality rates for ascending tertiles of arm muscle were 23, 11, and 8%, respectively. Similarly, unadjusted mortality rates for ascending tertiles of leg muscle were 23, 12, and 7%, respectively. By contrast, associations of regional and total body adipose tissue with mortality were weak in unadjusted analysis.
Table 2.
Unadjusted associations of MRI-measured skeletal muscle and adipose tissue with 5-year mortality in HIV-infected FRAM participants.a
| SM or AT measure |
Odds of death vs. lowest tertile |
Association of SM with mortality |
Association of AT with mortality |
|---|---|---|---|
| Total | Tertile 2 vs. Tertile 1 | 0.50 (0.33, 0.76) P =0.0014 | 0.79 (0.51, 1.21) P =0.28 |
| Tertile 3 vs. Tertile 1 | 0.26 (0.16, 0.42) P < 0.0001 | 1.05 (0.71, 1.57) P =0.80 | |
| Leg | Tertile 2 vs. Tertile 1 | 0.50 (0.34, 0.75) P =0.00069 | 1.19 (0.77, 1.83) P =0.43 |
| Tertile 3 vs. Tertile 1 | 0.20 (0.12, 0.34) P < 0.0001 | 1.47 (0.96, 2.23) P =0.076 | |
| Lower trunk | Tertile 2 vs. Tertile 1 | 0.68 (0.45, 1.01) P =0.057 | 0.80 (0.53, 1.22) P =0.30 |
| Tertile 3 vs. Tertile 1 | 0.46 (0.29, 0.74) P =0.0012 | 0.99 (0.67, 1.48) P =0.98 | |
| Upper trunk | Tertile 2 vs. Tertile 1 | 0.70 (0.46, 1.04) P =0.079 | 0.57 (0.36, 0.90) P =0.016 |
| Tertile 3 vs. Tertile 1 | 0.46 (0.30, 0.70) P =0.00040 | 0.83 (0.56, 1.23) P =0.36 | |
| Arm | Tertile 2 vs. Tertile 1 | 0.41 (0.28, 0.62) P < 0.0001 | 1.04 (0.65, 1.67) P =0.86 |
| Tertile 3 vs. Tertile 1 | 0.24 (0.14, 0.42) P < 0.0001 | 1.15 (0.71, 1.87) P =0.55 | |
| VAT | Tertile 2 vs. Tertile 1 | n/a | 0.88 (0.58, 1.33) P =0.54 |
| Tertile 3 vs. Tertile 1 | n/a | 0.74 (0.48, 1.14) P =0.17 |
MRI volumes were normalized by dividing by height2 with summaries back-transformed to 1.75m of height. Tertiles were created using separate cut-points for men and women. AT, adipose tissue; FRAM, Study of Fat Redistribution and Metabolic Change in HIV Infection; n/a=not applicable; SM, skeletal muscle; VAT, visceral adipose tissue.
Results reported as odds ratio (95% confidence interval).
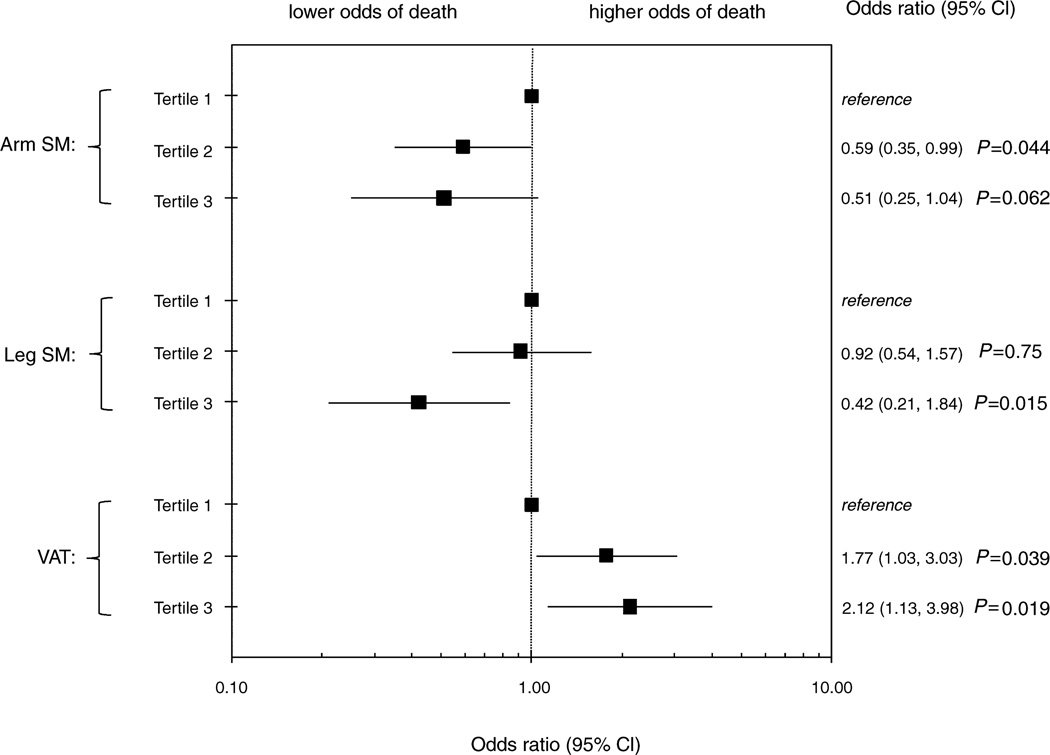
After adjustment for traditional CVD risk factors, HIV-related factors, inflammation, and renal markers, we found that decreased arm skeletal muscle, leg skeletal muscle, and total skeletal muscle were still individually associated with higher odds of death (data not shown). In the full multivariable model, with skeletal muscle and adipose tissue simultaneously included, we found that decreased arm skeletal muscle, decreased leg skeletal muscle, and increased VATwere independently associated with higher mortality risk (Fig. 1). Compared with the lowest tertile of arm skeletal muscle, the middle and highest tertiles were associated with a near halving of the odds for mortality [odds ratio (OR) = 0.59, P=0.044 for tertile 2 vs. tertile 1;OR=0.51, P=0.062 for tertile 3 vs. tertile 1). Similarly, the highest tertile of leg skeletal muscle was associated with a 58% lower odds of mortality compared with the lowest tertile of leg skeletal muscle (P=0.015), but the middle tertile appeared to confer little protection. In contrast, the middle and highest tertiles of VAT were associated with 1.8 and 2.1-fold higher odds of mortality respectively, compared to the lowest tertile. Similar results were found when we included upper trunk SAT instead of VAT in the model. We tested total skeletal muscle in place of limb skeletal muscle, and the results were similar, but the model fit was weaker. There were no statistically significant associations between lower limb fat and mortality in unadjusted or adjusted analyses.
Fig. 1. Multivariable adjusted associations of MRI-measured skeletal muscle and adipose tissue with 5-year mortality in HIV-infected FRAM participants.
The x-axis is on log10 scale. Estimates from multivariable adjusted models controlling for age, sex, race, traditional CVD risk factors, HIV-related factors, CRP, fibrinogen, eGFRcys, albuminuria, arm SM, leg SM, and VAT. Reference category is tertile 1, those with the lowest amount of muscle or adipose tissue. CI, confidence interval; SM, skeletal muscle; VAT, visceral adipose tissue.
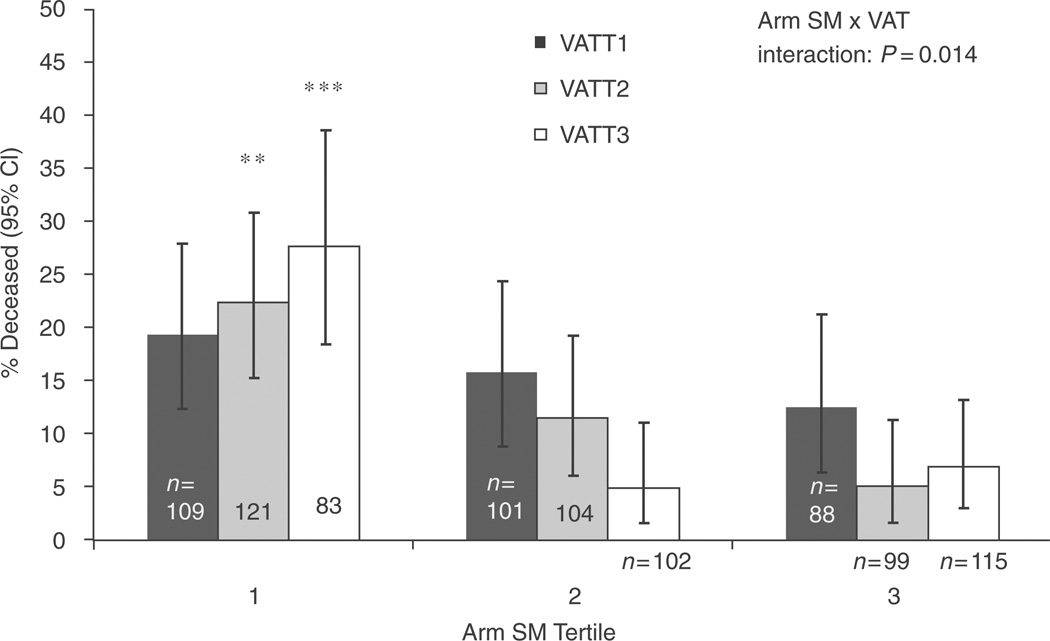
We found a statistically significant interaction of arm skeletal muscle with VAT on mortality (overall test for interaction P=0.014). Among those in the lowest tertile of arm skeletal muscle, where the mortality risk was highest, increasing VATwas also associated with a higher mortality rate [Fig. 2; adjusted OR=6.2 for VAT tertile 3 vs. 1, 95% confidence interval (CI) 2.2–17.2, P=0.0008]. By contrast, increased VAT did not appear to be associated with a higher mortality rate among those with intermediate levels of arm skeletal muscle (adjusted OR=0.92 for VAT tertile 3 vs. 1, P=0.48) or higher levels of arm skeletal muscle (adjusted OR=0.92 for VAT tertile 3 vs. 1, P=0.68). Likewise, for those with the lowest level of VAT, increasing arm skeletal muscle had little protective association in fully adjusted models. The overall test of interaction for leg skeletal muscle and VAT did not reach statistical significance (P=0.17), but a graphical inspection showed qualitatively similar results to the arm skeletal muscle by VAT interaction.
Fig. 2. Association of arm skeletal muscle and visceral adipose tissue with 5-year mortality in HIV-infected FRAM participants.
The y-axis gives unadjusted prevalence of death. Tertile 1, lowest SM or AT. **P<0.001 and ***P<0.0001 for comparison with VAT tertile 1 in fully adjusted model. CI, confidence interval; SM, skeletal muscle; VAT, visceral adipose tissue.
As reported previously, higher CD4 cell count was associated with lower odds of mortality [1]. As a sensitivity analysis, we separately analyzed those with high and low CD4 cell counts (>200 and <200) to understand the relation of these body composition measures to probable AIDS-related deaths (Supplemental Table 3, http://links.lww.com/QAD/A147). In this analysis, we found that arm skeletal muscle remained protective, and VAT remained associated with higher odds of mortality (although not all associations reached statistical significance in stratified analysis). However, leg skeletal muscle appeared to be strongly protective in those with CD4 cell count below 200, but was much weaker in those with CD4 cell count above 200 (test for CD4 by leg skeletal muscle interaction: P=0.0018).
A history of HIV-related wasting was present in 27% of our study participants. In unadjusted analysis, history of wasting was associated with 77% higher odds of mortality (95% CI 1.25–2.5, P=0.0014). In our fully adjusted model, this association was completely attenuated (OR=0.97, 95% CI 0.62–1.51, P=0.89).
African American race, injection drug use, and physical inactivity were associated with higher odds of mortality in unadjusted analysis, but the associations weakened and were no longer statistically significant in our fully adjusted model (data not shown).
Association of anthropometrically measured skeletal muscle and adipose tissue with 5-year mortality
Because most studies have used anthropometric measures such as waist circumference and MAMC as predictors of mortality, we compared these measures with our MRI-measured skeletal muscle and adipose tissue depots (Table 3). MAMC was moderately correlated with MRI-measured arm skeletal muscle (Spearman r=0.64, P<0.0001). MTMC and MRI-measured leg skeletal muscle were similarly correlated (r=0.62, P<0.0001), as were waist circumference and MRI-measured VAT (r=0.71, P<0.0001). In a model controlling for demographics, traditional CVD risk factors, HIV-related factors, inflammation, and renal disease markers, we found decreased MAMC and increased waist circumference to be independently associated with increased mortality risk. Decreased MTMC was also found to be associated with increased mortality risk. However, when we developed a multivariable model controlling simultaneously for waist circumference, MAMC, and MTMC, we found that waist circumference and MTMC were independently associated with mortality, whereas the MAMC association was no longer statistically significantly associated with mortality. The model including both waist circumference and MTMC showed similar model fit to the final MRI model.
Table 3.
Comparison of MRI and anthropometric measures of muscle and adiposity with 5-year mortality in HIV-infected FRAM participants.
| Final MRI model OR (95% CI) P |
Alternative anthropometric model 1 OR (95% CI) P |
Alternative anthropometric model 2 OR (95% CI) P |
|
|---|---|---|---|
| Arm SM: T2 vs. T1 | 0.59 (0.35, 0.99) P =0.044 | ||
| Arm SM: T3 vs. T1 | 0.51 (0.25, 1.04) P =0.062 | ||
| VAT: T2 vs. T1 | 1.77 (1.03, 3.03) P =0.039 | ||
| VAT: T3 vs. T1 | 2.12 (1.13, 3.98) P =0.019 | ||
| Leg SM: T2 vs. T1 | 0.92 (0.54, 1.57) P =0.75 | ||
| Leg SM: T3 vs. T1 | 0.42 (0.21, 0.84) P =0.015 | ||
| Mid-arm muscle circumference: T2 vs. T1 | 0.59 (0.36, 0.96) P =0.035 | 0.71 (0.42, 1.21) P =0.21 | |
| Mid-arm muscle circumference: T3 vs. T1 | 0.46 (0.26, 0.82) P =0.0080 | 0.63 (0.34, 1.16) P =0.13 | |
| Waist circumference: T2 vs. T1 | 2.32 (1.37, 3.95) P =0.0018 | 2.68 (1.56, 4.62) P =0.00038 | |
| Waist circumference: T3 vs. T1 | 2.10 (1.16, 3.80) P =0.014 | 2.73 (1.46, 5.10) P =0.0016 | |
| Thigh muscle circumference: T2 vs. T1 | 0.50 (0.31, 0.82) P =0.0055 | ||
| Thigh muscle circumference: T3 vs. T1 | 0.30 (0.16, 0.55) P =0.00012 | ||
| AIC (smaller is better) | 705.6 | 716.7 | 707.4 |
Estimates also adjust for demographics, traditional CVD risk factors, HIV-related factors, inflammation, and renal disease. Waist circumference, mid-arm muscle circumference (MAMC), and mid-thigh muscle circumference (MTMC) were tertiled using cut-offs determined separately in men and women. Italicized variables are not included in the model above, but are shown added back to the model. AIC=Akaike information criterion, which indicates a better model fit when it is smaller, but penalizes models that contain more predictors
Population-level risk for mortality attributable to skeletal muscle loss and adipose tissue gain
On the basis of the fully adjusted model presented in Fig. 1, we calculated the attributable risks associated with being in the highest tertile of VATand with being in the lowest tertiles of leg or arm skeletal muscle. For VAT, the population-attributable risk for being in the highest tertile was 6.5%, corresponding to an absolute risk of 1 death per 100 HIV-infected persons over 5 years. Similarly for leg skeletal muscle, the population-attributable risk for being in the lowest tertile was 7.2%. For arm skeletal muscle, the population-attributable risk of being in the lowest tertile was 15.1%, corresponding to an absolute risk of 2 deaths per 100 HIV-infected persons over 5 years.
Discussion
In this study of MRI-measured total body adipose tissue and skeletal muscle mass, we found that less muscle and more VAT (central adiposity) were associated with an elevated 5-year mortality risk in HIV-infected individuals. Even after accounting for inflammation, renal disease, and other factors known to increase mortality risk, decreased limb skeletal muscle and increased VAT were independently associated with increased risk of mortality. Decreased arm muscle alone accounted for 15% of the population-level mortality in HIV-infected individuals, whereas increased VAT accounted for 6.5%, suggesting that a substantial proportion of this risk may be unrecognized because of the current reliance on BMI in clinical practice.
No previous large, nationally representative study in HIV-infected patients has looked at the association of regional body composition with mortality in developed nations. A recent large study in South Africa [26] found that overweight and obese HIV-infected individuals had lower rates of mortality compared to those with normal or low BMI. A study of mortality in HIV-infected tuberculosis patients in Guinea-Bissau [27] identified low MAMC and low BMI as risk factors, but did not include a measure of central adiposity or consider other regional measures, and adjusted for a very limited set of covariates. The SMART study recently looked at AIDS and non-AIDS causes of death, including wasting syndrome, but did not include any measures of body composition other than BMI in their analysis of mortality [28].
Whereas others have found limb muscle and/or central adiposity to be associated with mortality in non-HIV infected individuals [5,12,13,29], our finding of an independent association in HIV infection may be novel. When we looked at anthropometric measures in place of MRI-measured depots, our findings were similar, although the thigh measurement (MTMC) of muscle circumference appeared to be more important than the arm (MAMC). Newman et al. [30] found muscle strength to be the most important determinant of mortality in elderly participants of the Health ABC study, with little attenuation after controlling for fat or lean mass. Dual-energy X-ray absorptiometry-measured arm and leg lean mass were only weakly associated with mortality in unadjusted analysis, and computed tomography-measured leg muscle was only associated with mortality in men.
It is noteworthy that our study found less muscle to be associated with increased risk of death even after controlling for inflammation and other factors known to be associated with mortality. Animal studies have found that administration of cytokines results in skeletal muscle catabolism [31], and it is also known that frailty increases muscle protein catabolism in the elderly [32]. It has been reported that patients with HIV wasting show inflammatory changes on muscle biopsy [33], and it has been hypothesized that inflammation may induce cachexia [34]. Associations of total muscle and limb muscle were attenuated but remained statistically significantly associated with mortality after controlling for traditional CVD, HIV-related factors, inflammation, and renal disease.
Our analyses found that increased VAT appeared to be associated with higher mortality risk primarily among those with low arm muscle. By contrast, studies in non-HIV-infected individuals [35,36] typically find that increased central obesity is a risk factor for mortality at both low and high levels of BMI. Although our study did not report cause of death, we found that those with both low arm muscle and high VAT had a number of non-HIV-related risk factors, including older age, physical inactivity, hypertension, dyslipidemia, and lower eGFR. This suggests that non-HIV-related causes of death may be dominant in this subgroup. Because we adjusted for CVD risk factors in our analysis, the associations of low muscle and excess central fat with mortality are independent of CVD risk. Since we did not have cause of death, we performed a sensitivity analysis, stratifying on high and low CD4 cell counts, in an attempt to reduce the number of AIDS-related deaths in our analysis. Of note, low arm skeletal muscle remained protective and high VAT remained associated with higher mortality in both CD4 subgroups. However, having low leg muscle showed stronger associations with increased mortality risk in those with low CD4 cell counts, which may reflect the fact that immunodeficiency-related cachexia is more prominently manifested in leg skeletal muscle.
Limitations of our study include missing vital status on 23% of our HIV-infected participants, as we have reported previously [1–3]. We used inverse probability weighting to mitigate any potential bias from those with unknown death status. Sensitivity analyses using anthropometric measures produced results that were similar to our primary modeling approach. We did not have information regarding the cause of death and were therefore unable to discern whether the independent associations of arm skeletal muscle, leg skeletal muscle, and VAT with mortality in HIV-infected individuals were due to cardiovascular or noncardiovascular deaths. We were unable to directly compare our results with our control population, because there were only six deaths in the control arm of our study. We have previously shown that baseline muscle was lower in HIV-infected vs. uninfected men, whereas HIV-infected and control women have similar muscle [37]. An additional study limitation is that markers of inflammation and other biomarkers were collected at a single time point (i.e. baseline). Development of resistance to or changes in antiretroviral therapy may result in changes in HIV viremia, which may lead to rapid changes in these biomarkers. Relying on a single time point thus limits the sensitivity of inflammatory markers as predictors of survival. Finally, there may have been incomplete or inadequate control for factors that may confound or explain the association between body composition and mortality. For example, chronic illness could result in inactivity or inadequate food intake, leading to muscle loss; however, we found that controlling for physical activity or food intake resulted in little attenuation of the association of muscle and VAT with mortality.
In conclusion, this study suggests that among treated HIV-infected patients, lower levels of arm and leg muscle and increased central fat mass are associated with worse survival. Clinicians treating HIV-infected patients should recognize that whereas HIV-wasting syndrome is a risk factor, patients with wasting and excess central adiposity may be at even higher risk. A substantial proportion of this risk may be unrecognized because of the current reliance on low BMI to assess wasting in clinical practice; BMI will underestimate the risk in those with higher VAT. Therefore, clinicians should consider measuring waist circumference and MAMC in their HIV-infected patients, and should be aware that patients with excess central fat may not necessarily have a low enough BMI to alert them to their true level of risk. Given the report in non-HIV-infected individuals of the importance of grip strength and quadriceps strength as predictors of mortality [30], future studies should evaluate muscle strength as well as body composition as clinical tools in predicting survival in HIV-infected persons.
Supplementary Material
Acknowledgements
Supported by grants from the NIH (R01-DK57508, HL74814, and HL53359; K23 AI66943 and NIH center grants M01-RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, RR00865, and UL1 RR024131), the Albert L. and Janet A. Schultz Supporting Foundation and with resources and the use of facilities of the Veterans Affairs Medical Center, San Francisco, California. The funding agencies had no role in the collection or analysis of the data.
Disclosure: R.S., S.B.H., D.L., W.G.P., P.C.T., P.B. and M.G.S. received funding from the supporting grants. C.G. has received prior research funding and/or honorarium from Merck, Bristol-Myers Squibb, Abbott, Serono and Theratechnologies.
Appendix
Sites and investigators: University Hospitals of Cleveland (Barbara Gripshover, MD); Tufts University [Abby Shevitz, MD (deceased) and Christine Wanke, MD]; Stanford University (Andrew Zolopa, MD); University of Alabama at Birmingham (Michael Saag, MD); John Hopkins University (Joseph Cofrancesco, MD and Adrian Dobs, MD); University of Colorado Health Sciences Center (Lisa Kosmiski, MD and Constance Benson, MD); University of North Carolina at Chapel Hill [David Wohl, MD and Charles van der Horst, MD(Only involved in FRAM 1 study)]; University of California at San Diego [Daniel Lee, MD and W. Christopher Mathews, MD(Only involved in FRAM 1 study)]; Washington University (E. Turner Overton, MD and William Powderly, MD); VA Medical Center, Atlanta (David Rimland, MD); University of California at Los Angeles (Judith Currier, MD); VA Medical Center, New York (Michael Simberkoff, MD); VA Medical Center, Washington DC (Cynthia Gibert, MD); St Luke’s-Roosevelt Hospital Center (Donald Kotler, MD and Ellen Engelson, PhD); Kaiser Permanente, Oakland (Stephen Sidney, MD); University of Alabama at Birmingham (Cora E. Lewis, MD); University of California at San Francisco(Only involved in FRAM 1 study) (Morris Schambelan, MD and Kathleen Mulligan, PhD); Indiana University(Only involved in FRAM 1 study) (Michael Dube, MD).
FRAM 1 Data Coordinating Center(Only involved in FRAM 1 study): University of Alabama, Birmingham (O. Dale Williams, PhD, Heather McCreath, PhD, Charles Katholi, PhD, George Howard, PhD, Tekeda Ferguson, and Anthony Goudie).
FRAM 2 Data Coordinating Center: University of Washington, Seattle (Richard A. Kronmal, PhD, Mary Louise Biggs, PhD, Joseph A.C. Delaney, PhD, and John Pearce).
Image Reading Centers: St Luke’s-Roosevelt Hospital Center: (Steven Heymsfield, MD, Jack Wang, MS and Mark Punyanitya). Tufts New England Medical Center, Boston: (Daniel H. O’Leary, MD, Joseph Polack, Anita P. Harrington).
Office of the Principal Investigator: University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development: (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Dennis Osmond, PhD(Only involved in FRAM 1 study), Michael Shlipak, MD, Rebecca Scherzer, PhD, Mae Pang, RN, MSN, Heather Southwell, MS, RD).
Footnotes
Role of the funder: The funder played no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data or preparation of the manuscript. A representative of the funding agent participated in planning the protocol.
References
- 1.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinspoon S, Mulligan K. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin Infect Dis. 2003;36:S69–S78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- 5.Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–1236. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- 6.Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457–1465. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 8.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacchetti P, Cofrancesco J, Heymsfield S, Lewis CE, Scherzer R, Shlipak M, et al. Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunfeld C, Saag M, Cofrancesco J, Jr, Lewis CE, Kronmal R, Heymsfield S, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24:1717–1726. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 12.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–1346. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 13.Landi F, Russo A, Liperoti R, Pahor M, Tosato M, Capoluongo E, et al. Midarm muscle circumference, physical performance and mortality: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study) Clin Nutr. 2010;29:441–447. doi: 10.1016/j.clnu.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 17.Lohman T, Roche A, Martorell R. In: Anthropometric standardization reference manual. Abridged ed., editor. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 19.Madden E, Lee G, Kotler DP, Wanke C, Lewis CE, Tracy R, et al. Association of antiretroviral therapy with fibrinogen levels in HIV-infection. AIDS. 2008;22:707–715. doi: 10.1097/QAD.0b013e3282f560d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, et al. Microalbuminuria in HIV infection. AIDS. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 23.Gilks WR, Richardson S, DJ S. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 24.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 25.Luepker RV, Evans A, McKeigue P, Reddy KS, et al. Cardiovascular survey methods. 3rd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 26.Hanrahan CF, Golub JE, Mohapi L, Tshabangu N, Modisenyane T, Chaisson RE, et al. Body mass index and risk of tuberculosis and death. AIDS. 2010;24:1501–1508. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustafson P, Gomes VF, Vieira CS, Samb B, Naucler A, Aaby P, et al. Clinical predictors for death in HIV-positive and HIVnegative tuberculosis patients in Guinea-Bissau. Infection. 2007;35:69–80. doi: 10.1007/s15010-007-6090-3. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason C, Craig CL, Katzmarzyk PT. Influence of central and extremity circumferences on all-cause mortality in men and women. Obesity (Silver Spring) 2008;16:2690–2695. doi: 10.1038/oby.2008.438. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 31.Ling PR, Schwartz JH, Bistrian BR. Mechanisms of host wasting induced by administration of cytokines in rats. Am J Physiol. 1997;272:E333–E339. doi: 10.1152/ajpendo.1997.272.3.E333. [DOI] [PubMed] [Google Scholar]
- 32.Chevalier S, Gougeon R, Nayar K, Morais JA. Frailty amplifies the effects of aging on protein metabolism: role of protein intake. Am J Clin Nutr. 2003;78:422–429. doi: 10.1093/ajcn/78.3.422. [DOI] [PubMed] [Google Scholar]
- 33.Miro O, Pedrol E, Cebrian M, Masanes F, Casademont J, Mallolas J, et al. Skeletal muscle studies in patients with HIV-related wasting syndrome. J Neurol Sci. 1997;150:153–159. doi: 10.1016/s0022-510x(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 34.Belec L, Meillet D, Hernvann A, Gresenguet G, Gherardi R. Differential elevation of circulating interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6 in AIDS-associated cachectic states. Clin Diagn Lab Immunol. 1994;1:117–120. doi: 10.1128/cdli.1.1.117-120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–487. [PubMed] [Google Scholar]
- 36.Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 37.Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE, et al. Age-related skeletal muscle decline is similar in HIVinfected and uninfected individuals. J Gerontol A Biol Sci Med Sci. 2011;66A:332–340. doi: 10.1093/gerona/glq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.