Abstract
Reactive oxygen species (ROS) are important in regulating normal cellular processes, but deregulated ROS contribute to the development of various human diseases including cancers. Cancer cells have increased ROS levels compared to normal cells, because of their accelerated metabolism. The high ROS levels in cancer cells, which distinguish them from normal cells, could be pro-tumorigenic, but are also their Achilles’ heel. The high ROS content in cancer cells renders them more susceptible to oxidative stress induced cell death, and can be exploited for selective cancer therapy. In this review, we describe several potential therapeutic strategies that take advantage of ROS imbalance in cancer cells by further increasing oxidative stress, either alone or in combination with drugs that modulate certain signaling pathways.
BACKGROUND
Redox Homeostasis
Oxidative stress is defined as an imbalance between production of oxidants or reactive oxygen species (ROS), and their elimination by protective mechanisms or antioxidants. Disturbance in this redox balance can lead to damage of important components of the cell, including proteins, lipids, and DNA, with potential impact on the whole organism, and with an increase risk in mutagenesis (1, 2). These effects of ROS are thought to contribute to the aging process (3), but could also play a role in the genesis of cancer.
ROS are by-products of a normal cellular metabolism and play vital roles in the stimulation of signaling pathways, such as intracellular signal transduction, metabolism, proliferation and apoptosis (4, 5). Under a sustained environmental stress, ROS are produced over a long time, and thus significant damage may occur to cell structure and functions and may induce somatic mutations and neoplastic transformation (6, 7). ROS could facilitate cancer development by direct oxidative damage to DNA, induction of lipid peroxidation or oxidative protein damage leading to structural alterations in the DNA (1–2). There are several free radicals ROS and non-free radicals ROS inside cells. The major site of ROS generation inside the cells is the mitochondrial electron transport chain where electrons can escape their route and react with oxygen (8). This biological reduction of molecular oxygen is the source ROS such as superoxide anion (O2•−) which is the major free radical and hydrogen peroxide (H2O2) which is the major non-free radical ROS (8, 9). ROS can also be generated by the activation of growth factor receptors, which in turn activate NADPH oxidase. NADPH oxidase oxidizes NADPH to generate superoxide (Fig. 1).
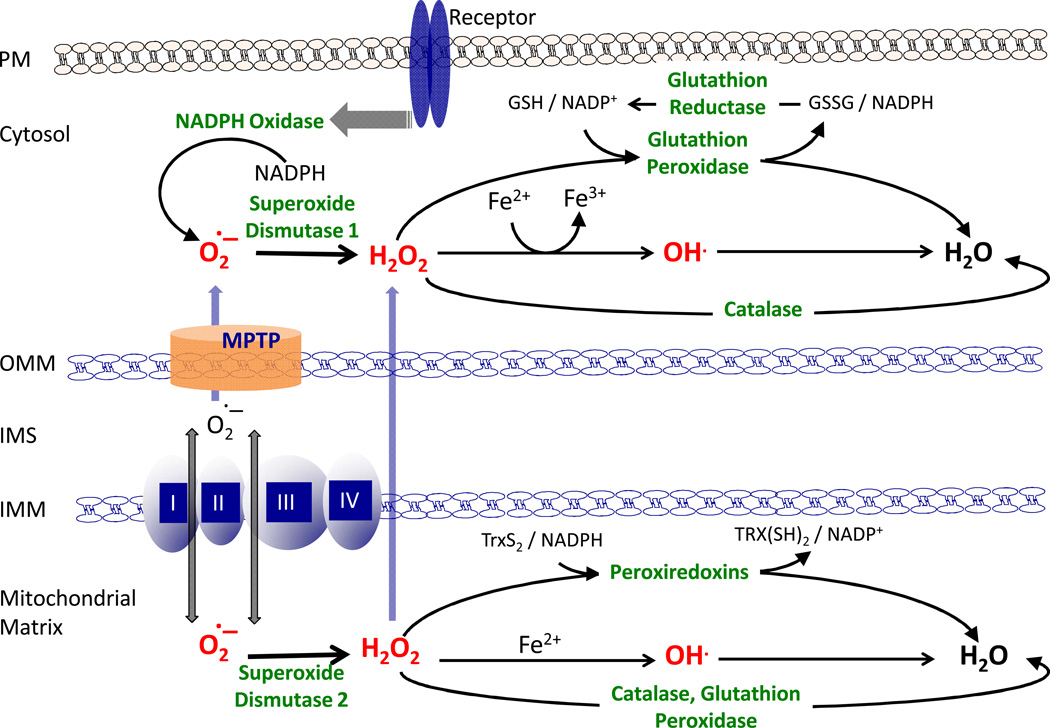
Figure 1. Cellular homeostasis of reactive oxygen species (ROS).
The major site of ROS generation inside the cells is the mitochondria. During respiration there is a leak of electrons to oxygen from complex I and complex III, which generates superoxide (O2•−). The majority of superoxide is transferred to the mitochondrial matrix, where it is dismutated to hydrogen peroxide (H2O2) by the superoxide dismutase (SOD2). Some of the superoxide is transferred to the cytosol, where it is dismutated to H2O2 by the cytoslolic SOD1. ROS can be also generated by the activation of growth factor receptors, which in turn activate NADPH oxidase that oxidizes NADPH to generate superoxide. Hydrogen peroxide can then be detoxified by 3 major mechanisms: catalase, glutathione peroxidase (GPX) and peroxiredoxins (PRX). GPX and PRX detoxifying enzymes respectively utilize glutathione (GSH) and thioredoxins (Trx), generated by NADPH. IMM/OMM, Inner/Outer Mitochondrial Membrane; IMS, Inter Mitochondrial Space; PM, Plasma Membrane; MPTP, Mitochondrial Permeability Transition Pore; GR, Glutathione Reductase
Cellular redox balance is maintained by a powerful antioxidant system that scavenges ROS. The majority of superoxide is transferred to the mitochondrial matrix, where it is dismutated to H2O2 by the superoxide dismutase (MnSOD or SOD2). Some of the superoxide is transferred to the cytosol and is dismutated to H2O2 by the cytosolic SOD (SOD1). The antioxidant system also consists of catalase, the glutathione system (reduced glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPX) and glutathione transferase), the thioredoxin system (thioredoxins (Trx), thioredoxin peroxidase and peroxiredoxins (PRX)), and vitamin E and C (9) (Fig. 1). Glutathione is the major non-enzymatic component of intracellular antioxidant defenses and plays a central role in maintaining redox balance (NADP/NADPH and GSSG/GSH ratios), via its tight regulation of NADPH intracellular levels. The intracellular NADPH level is determined by the difference between its production and its consumption. The major sources of intracellular NADPH are the pentose phosphate pathway (PPP) and mitochondrial metabolism. The major consumers of intracellular NADPH are H2O2 detoxification by GPX and PRX and fatty-acid synthesis (10). Thus, the production and the consumption of NADPH inside cells should be coordinated in order to maintain NADPH homeostasis. This is particularly important during the formation of solid tumor and during metastasis when cancer cells undergo energetic stress.
ROS and Cancer
In the past two decades, cancer promotion and progression have been linked to oxidative stress by increasing DNA mutations or inducing DNA damage, genome instability, and cell proliferation (11–13). Indeed, one of the main features of cancer cells, when compared to the normal ones, is a persistent pro-oxidative state that can lead to intrinsic oxidative stress (14, 15). The enhanced oxidative stress observed in cancer cells can result not only from ROS overproduction, but also from low levels or inactivation of antioxidant mechanisms. Survival of tumor cells is highly dependent on their capacity to control expression of endogenous antioxidants to maintain a steady state level of ROS below the threshold that will induce tumor cell death. Therefore, cancer cells have evolved mechanisms to protect themselves from intrinsic oxidative stress and have developed a sophisticated adaptation system that essentially involves the rearrangement of the antioxidant functions and the upregulation of pro-survival molecules (16, 17). In addition to the increase in mutations rate, ROS could contribute to the initiation of cancer through accelerating pro-tumorigenic signaling pathways. By oxidizing disulfide bonds of cysteine residues, ROS could change the activity of certain proteins, and particularly the tyrosine phosphatases superfamily (18). Perhaps the best example related to cancer is the documented inactivation of the tumor suppressor PTEN by oxidation (19, 20). Another example is the inhibition of the MAP kinase phosphatase by ROS, which in turn induces activation of ERKs. The inhibition of PTEN by ROS hyperactivates the PI3K/Akt signaling pathway, which is perhaps the most frequently activated signaling pathway in cancer cells [reviewed in (21–23)]. The activation of this pathway would further increase intracellular ROS, at least in part, through the inhibition of the transcription factors FOXOs, which elevate the expression of anti-oxidants such as SOD2, catalase, and sestrin3 (24).
ROS as a double-edged sword in cancer cells
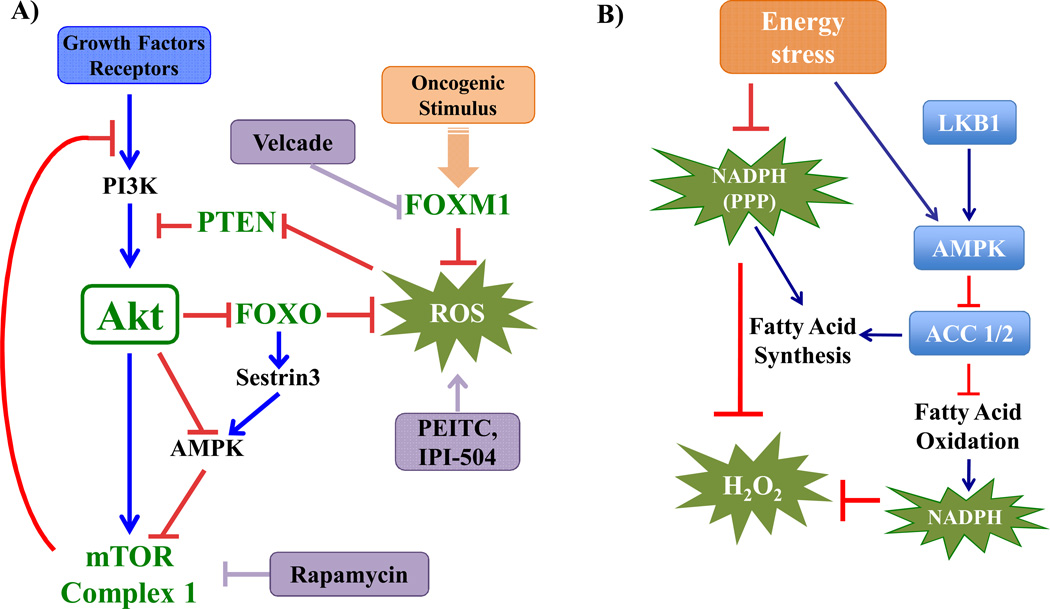
The high intracellular ROS levels in cancer cells are largely the by-products of the highly metabolic nature of these cells. These ROS levels could be pro-tumorigenic, but also increase the susceptibility of cancer cells to cell death. Activated signaling pathways that increase intracellular ROS level in cancer cells render these cells more vulnerable than normal cells to oxidative stress-induced cell death. This vulnerability was documented in cells expressing oncogenic Ras (25) and in cells that display hyperactive PI3K/Akt signaling (24). The high levels of ROS in cancer cells that display hyperactive Akt is generated by increased mitochondrial metabolism and by the suppression of anti-oxidants gene expression, through the inhibition of FOXO transcription factor (24). In addition to the elevation of SOD2 and catalase, FOXO was shown to induce the expression of Sestrin3 (24, 26). Sestrin3 is a member of a family of proteins that includes Sestrin1 and Sestrin2, which were originally identified as antioxidants induced by the tumor suppressor p53 (27–30). The FOXO-Sestrin axis is conserved in flies, and has an additional function in both mammalian and flies cells as an activator of AMPK and the inhibitor of mTORC1 (26, 31). Thus, the suppression of Sestrins expression in cancer cells could increase intracellular ROS and activate mTORC1. Because ROS inhibits PTEN activity, this would further activate the PI3K/Akt signaling in Pten-proficient cells and further activate mTORC1 (Fig. 2A). To maintain the intracellular level of ROS below a toxic threshold level, cancer cells adopted alternative mechanisms of anti-oxidation. For instance, relatively high levels of FOXM1 expression in cancer cells could compensate for the loss of FOXO activity. FOXM1 is expressed at low levels in normal cells, but its expression is markedly elevated in cancer cells (32). FOXM1 exerts multiple pro-tumorigenic activities, but was also shown to reduce ROS levels through the transcriptional induction of SOD2, catalase, and mitochondrial thioredoxin-dependent peroxide reductase (PRDX3) (33). In addition, the expression of detoxifying enzymes such as glutathione S-transferases (GSTs), NAD(P)H:quinone oxidoreductase 1 (NQO1) was reported to be elevated in cancer cells (34, 35).
Figure 2. Signaling pathways regulating intracellular ROS in cancer cells.
A) Akt is activated by extracellular signals that activate PI3K. The tumor suppressor, PTEN is a phospho-lipid phosphatase that negates the activity of PI3K. Akt activation inhibits the FOXO transcription factors and activates mTOR Complex 1 (mTORC1). By increasing cellular metabolism Akt inhibits AMPK activity and increases the level of the by-products of energy metabolism, ROS. The inhibition of FOXOs by Akt inhibits the expression of anti-oxidants, which in turn further increase ROS levels. There is also interplay between FOXOs and mTORC1, whereby Sestrin3 induced by FOXOs activates AMPK and inhibits mTORC1. The activation of mTORC1 by Akt and by AMPK inhibition promotes several anabolic functions, but also elicits negative feedback loops that inhibit Akt. The high levels of FOXM1 in cancer cells could compensate for the suppression of FOXOs through induced expression of anti-oxidants genes.
B) Energy stress during certain stages of tumor development increases oxidative stress by decreasing NADPH production through the pentose phosphate pathway (PPP). AMPK activation under energy stress conditions is required to maintain NADPH homeostasis and prevent oxidative stress induced cell death. AMPK activation inhibits the consumption of NADPH by fatty acid synthesis through the phosphorylation and inhibition of acetyl-coA carboxylase 1 (ACC1), and increases NADPH production via fatty acid oxidation, through phosphorylation and inhibition of ACC2.
Energetic stress conditions could lead to oxidative stress. Cancer cells that consume high levels of glucose undergo energetic stress during the formation of solid tumors when cells detach from the matrix and translocate to the lumen (36), or during metastasis when they leave the primary tumor site to relocate to another site. The decrease in glucose uptake during these processes suppresses ATP production and activates AMPK (36, 37), but also inhibits the generation of NADPH via the PPP. The reduced level of NADPH results in increase of intracellular ROS, which could eventually cause cell death (36, 37). However, the concomitant activation of AMPK elicits alternative mechanisms that maintain intracellular NADPH levels. The activated AMPK phosphorylates and inhibits acetyl-CoA carboxylase 1 (ACC1) and ACC2. Both enzymes may have some overlapping activities, but ACC1 is largely responsible for the generation of malonyl-CoA for fatty acid synthesis (FAS), whereas ACC2 inhibits fatty acid oxidation (FAO) (38). By inhibiting ACC1, AMPK inhibits FAS and therefore decreases NADPH consumption in FAS. By inhibiting ACC2, AMPK increases FAO and therefore NADPH production via the mitochondria (37) (Fig. 2B). Thus, AMPK activation is critical for the adaptation of cancer cells to energetic stress, which subsequently induces oxidative stress.
CLINICAL-TRANSLATIONAL ADVANCES
The high levels of ROS in cancer cells in comparison to normal cells could be exploited for cancer therapy. In fact, many of the commonly used chemotherapeutic regimens induce high level of ROS that kill cancer cells (39, 40). However most of these therapeutic regimens were not strictly developed to exploit the high level of ROS in cancer cells for cancer therapy.
The persistent pro-oxidative state characterizing cancer cells, as well as their multiple adaptation mechanisms, can be exploited to develop new therapeutic strategies that will specifically target cancer cells, in order to ensure a good therapeutic selectivity. Because the overall redox homeostasis is maintained by the balance between ROS generation and scavenging, exogenous compounds that specifically increase ROS production, or inhibit ROS elimination, can favor the accumulation of ROS in cancer cells, and hence induce cell damage or even cell death once their threshold of “tolerance” is reached (41).
ROS and Chemotherapy
Developing cancer therapies based on escalating further the high ROS level in cancer cells to a toxic level by triggering ROS accumulation directly and/or inhibition of ROS scavenging systems represent powerful avenues for selectively killing cancer cells. Several drugs have been identified as promoting ROS generation. These reagents include: (i) mitochondrial electron transport chain modulators (e.g., arsenic trioxide, doxorubicin, topotecan); (ii) redox-cycling compounds (e.g., motexafin gadolinium); (iii) agents that disrupt the antioxidant defenses mechanism, such as GSH depleting agents (e.g., buthionine sulphoximine, β-phenylethyl isothiocyanates (PEITC)) and inhibitors of SOD (e.g., 2-methoxyestradiol), and catalase (e.g., 3-amino-1,2,4-triazole) (25, 41–44). Through promoting oxidative stress or targeting the endogenous antioxidant system of cancer cells, these reagents could be used as a therapeutic weapon against cancer cells.
Rapamycin and oxidative stress combination as cancer therapy
The PI3K/AKT signaling pathway is thought to play a prominent role in the initiation and maintenance of human cancer, as many components of this pathway have been found to be mutated or amplified in a broad range of human cancers (21–23) and thereby promoting resistance to therapeutic agents that induce apoptosis.
Akt is activated by extracellular signals that activate phosphatidylinositol 3-kinase (PI3K), and is negatively regulated by phospholipid phosphatases that negate the activity of PI3K, such as the tumor suppressor PTEN. Akt activity is also downregulated by the activation of its downstream effector, the mammalian target of rapamycin complex 1 (mTORC1), which in turn induces a negative feedback mechanism that inhibits Akt activity (21, 22, 45). By virtue of its role in energy metabolism Akt can regulate the mitochondrial production of byproducts of energy metabolism, ROS. Akt can also regulate ROS, via its negative effects on FoxO transcription factor leading to downregulation of SOD2, catalase, and Sestrin3 (24) (Fig. 2A). Thus, the high levels of ROS as a consequence of Akt activation is due to an enhancement of mitochondrial activity as well as the downregulation of antioxidant defense mechanisms. In other words, Akt sensitizes cells to oxidative-stress induced apoptosis, by lowering the threshold of oxidative stress needed to induce cell death, and this could be exploited to selectively eradicate and to overcome chemoresistance of cancer cells with hyperactivated Akt.
Rapamycin analogs are currently being used in clinical trials and have been already approved for certain types of cancer (46, 47). Rapamycin alone attenuates cell proliferation and rarely elicits cell death. Furthermore, it could also increase cell survival and chemoresistance via the inhibition of mTORC1, and consequently activating Akt through the inhibition of a negative feedback loop (21, 22, 45). However, by activating Akt, rapamycin further sensitizes cells to ROS-induced cell death, and thus, the combination of rapamycin and oxidative stress could be an attractive strategy to selectively eradicate cancer cells (24).
Isothiocyanates such as the PEITC are thiol modifiers that have been shown to inhibit the GSH antioxidant system by extruding GSH from the cell and by inhibiting glutathione peroxidase (25, 44). This leads to ROS overproduction, oxidative damage of mitochondria, and apoptosis preferentially in cancer cells, presumably due to their increased constitutive ROS levels (24, 25, 44). Clinical studies with PEITC are currently being initiated (48). A combination therapy of rapamycin and PEITC was proven efficient to selectively eradicate tumors with hyperactivated Akt in pre-clinical studies (24). This strategy evades the chemoresistance induced by the hyperactivation of Akt in cancer cells.
Agents that enhance proteotoxic stress, including the HSP90 inhibitor IPI-504, are also known ROS inducers. It has been shown that IPI-504 and rapamycin synergize in Ras-driven tumors by promoting irresolvable ER stress, resulting in catastrophic ER and mitochondrial damage, and tumor regression (49). The mechanism by which these agents cooperate reveals a therapeutic paradigm that can be expanded to develop additional combinations.
FOXM1 inhibition and oxidative stress combination as cancer therapy
The transcription factor FOXM1 is overexpressed in a majority of human tumors and is implicated in tumor angiogenesis, invasion and metastasis (32). Increased FOXM1 expression in tumors is associated with advanced tumor stage and poor prognosis. FOXM1 could reduce intracellular ROS levels in cancer cells by inducing the expression of detoxifying enzymes, including catalase, SOD2, and PRDX3 (33) (Fig. 2A).
FOXM1 transcriptional activity and expression can be inhibited by proteasome inhibitors such as bortezomib (velcade) and MG132, or the thiazole antibiotics, Siomycin A and thiostrepton (50–52). It was shown that the suppression of FOXM1 by proteasome inhibitors sensitizes human cancer cells to cell death induced by DNA-damaging agents including doxorubicin and γ-irradiation (53). Therefore, targeting FOXM1, in combination with oxidative stress is a sound strategy to eliminate tumor cells.
Targeting AMPK for cancer therapy
AMPK is a master switch of metabolic adaptation (37, 54, 55), and its activation appears to be critical for cell survival during energetic stress (37) (Fig. 2B). The activation of AMPK could inhibit the proliferation of cancer cells and therefore is a considered strategy for cancer therapy (55). However, the recent studies discussed above, which show that AMPK activation is required to combat oxidative stress and promote cancer cell survival during energetic stress, suggest that the inhibition of AMPK could be also a therapeutic strategy for cancer therapy. This could be an effective therapy at certain stages of cancer progression such as during solid tumor formation and metastasis when cancer cells undergo energetic stress. Since the absence of AMPK activation during energetic stress of cancer cells elicits ROS-mediated cell death, the combination of exogenous ROS inducers and inhibitors of AMPK could be another attractive strategy for cancer therapy.
Sestrin proteins activate AMPK and also reduce ROS. As shown recently, under energetic stress conditions, Sestrin 2 expression is elevated and further potentiates AMPK activity (56, 57). Therefore, targeting Sestrins could not only elevate intracellular ROS but also attenuate AMPK activation during energetic stress, and may constitute another strategy for cancer therapy.
The use of AMPK activation for cancer therapy may stop cancer cell proliferation, but may also increase survival of cancer cells during solid tumor formation and metastasis. To circumvent this possibility and render AMPK activation a more potent therapeutic approach it would be useful to employ AMPK activation in combination with pharmacological activators of ACCs to counteract the pro-survival activity of AMPK activation (37).
In summary, we described elevated ROS level as one hallmark of cancer cells that could be exploited for selective cancer therapy. ROS are pro-tumorigenic but also toxic above a certain threshold level. This threshold level could be easily achieved in cancer cells by a relatively subtle increase in ROS level that does not increase ROS level to a toxic level in normal cells. We described several potential therapeutic strategies using ROS inducers alone or in combination with other therapeutic regimens. However, the same as with many therapeutic regimens, ROS inducers could be pro-tumorigenic for normal cells. Therefore, ROS inducers should be kept at restricted doses in order not to affect normal cells.
Footnotes
Conflicts of Interest:
Authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 2.Schraufstatter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988;82:1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 5.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 6.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 11.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240–245. [PubMed] [Google Scholar]
- 13.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 15.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 16.Farber E, Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991;51:2751–2761. [PubMed] [Google Scholar]
- 17.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 18.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. Embo J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2009;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 30.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. Embo J. 2009;28:2908–2918. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12:103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- 35.Di Pietro G, Magno LA, Rios-Santos F. Glutathione S-transferases: an overview in cancer research. Expert Opin Drug Metab Toxicol. 2010;6:153–170. doi: 10.1517/17425250903427980. [DOI] [PubMed] [Google Scholar]
- 36.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 39.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 40.Lamson DW, Brignall MS. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–329. [PubMed] [Google Scholar]
- 41.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 42.Barbieri D, Grassilli E, Monti D, Salvioli S, Franceschini MG, Franchini A, et al. D-ribose and deoxy-D-ribose induce apoptosis in human quiescent peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1994;201:1109–1116. doi: 10.1006/bbrc.1994.1820. [DOI] [PubMed] [Google Scholar]
- 43.Ceruti S, Barbieri D, Veronese E, Cattabeni F, Cossarizza A, Giammarioli AM, et al. Different pathways of apoptosis revealed by 2-chloro-adenosine and deoxy-D-ribose in mammalian astroglial cells. J Neurosci Res. 1997;47:372–383. doi: 10.1002/(sici)1097-4547(19970215)47:4<372::aid-jnr2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Xiao D, Lew KL, Zeng Y, Xiao H, Marynowski SW, Dhir R, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 45.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–1073. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 47.Populo H, Lopes JM, Soares P. The mTOR Signalling Pathway in Human Cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Accessed at: http://clinicaltrials.gov/show/NCT00691132.
- 49.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4:e6593. doi: 10.1371/journal.pone.0006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731–9735. doi: 10.1158/0008-5472.CAN-06-1576. [DOI] [PubMed] [Google Scholar]
- 53.Halasi M, Gartel AL. Suppression of FOXM1 sensitizes human cancer cells to cell death induced by DNA-damage. PLoS One. 2012;7:e31761. doi: 10.1371/journal.pone.0031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, et al. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS One. 2012;7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]