Abstract
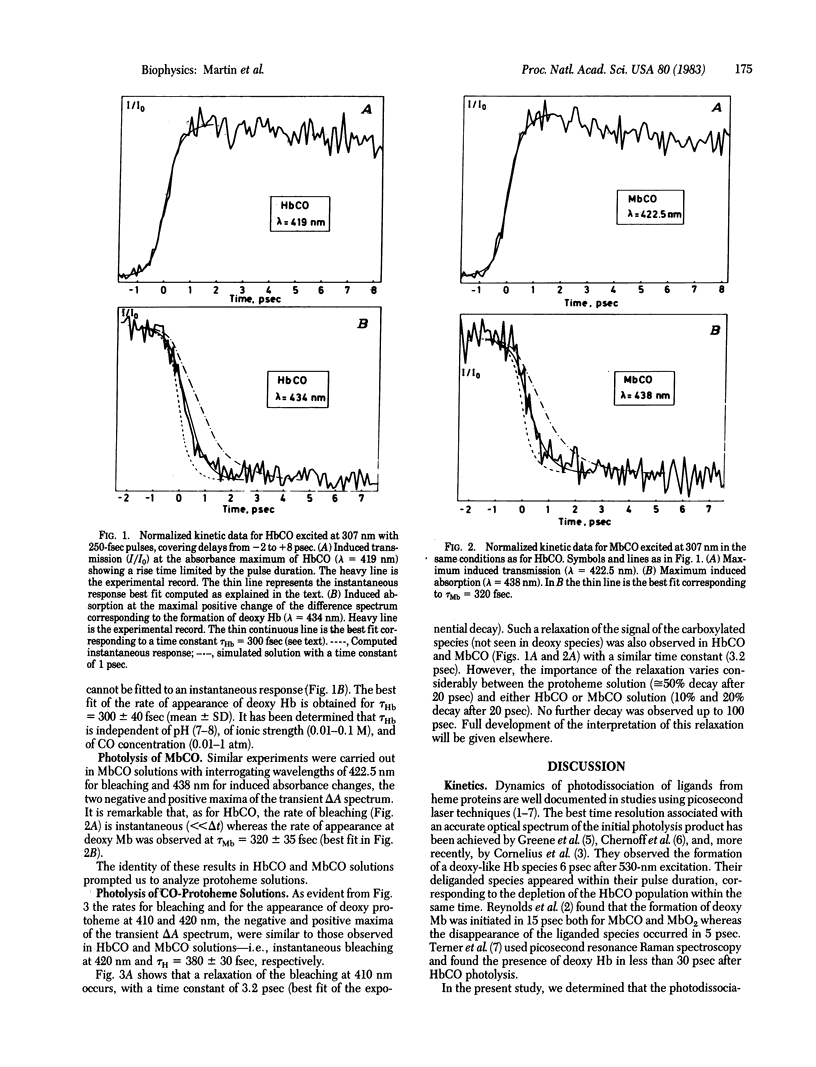
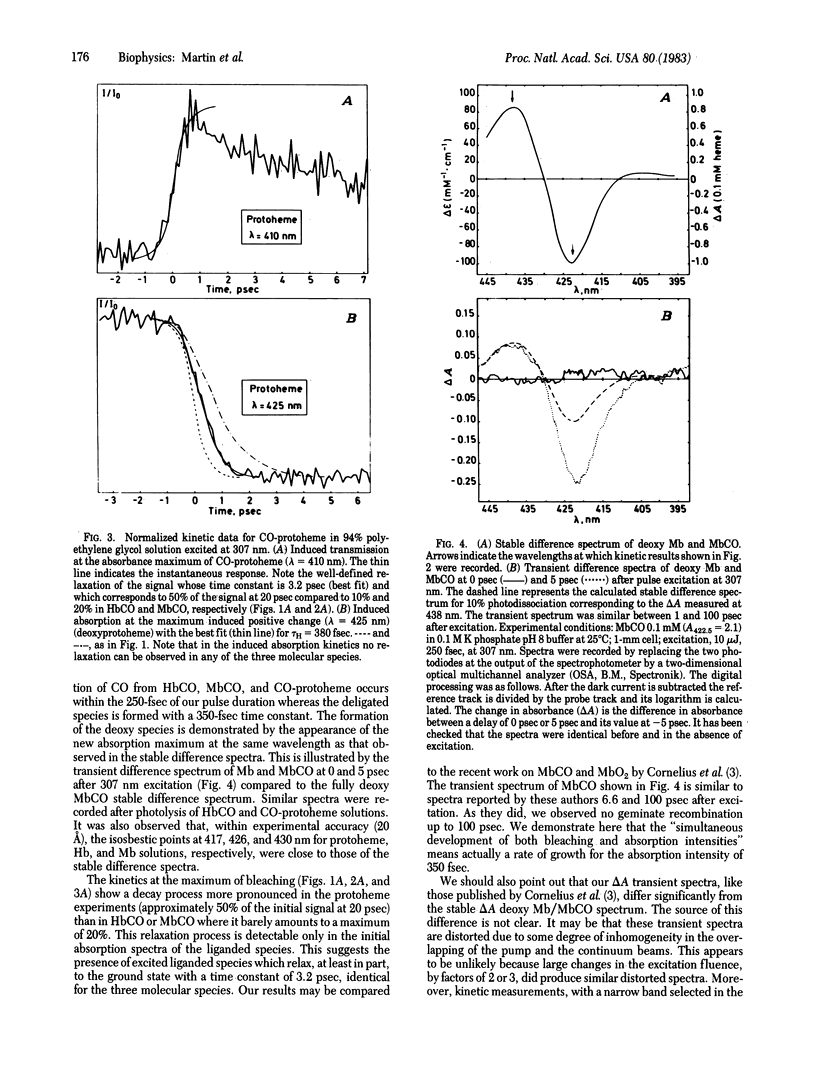
Photolysis of HbCO, MbCO, and CO-protoheme has been investigated by measuring transient differential spectra and kinetics of induced absorption after excitation with a 250-fsec laser pulse at 307 nm. Probing was performed by a part of a continuum pulse between 395 and 445 nm. Photodissociation of the three liganded species occurred within the pulse duration. By contrast, the formation of deoxy species appeared with a mean (+/- SD) response time of 350 +/- 50 fsec. This time constant was identical for the three species and independent of the presence or absence of the protein structure. Our results suggest the formation of a transient high-spin in plane iron (II) species which relaxes in 350 fsec to a high-spin stable state with concerted kinetics of CO departure and iron displacement. The spin transition is suspected to occur via liganded excited states which relax in part to non-reactive states with a 3.2-psec time constant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979 Aug 15;132(3):343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius P. A., Steele A. W., Chernoff D. A., Hochstrasser R. M. Different dissociation pathways and observation of an excited deoxy state in picosecond photolysis of oxy- and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7526–7529. doi: 10.1073/pnas.78.12.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Rates of reaction of native human globin with some hemes. J Biol Chem. 1963 Apr;238:1384–1388. [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Noe L. J., Eisert W. G., Rentzepis P. M. Picosecond photodissociation and subsequent recombination processes in carbon monoxide hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):573–577. doi: 10.1073/pnas.75.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe L. J., Eisert W. G., Rentzepis P. M. Picosecond photodissociation and subsequent recombination processes in carbon monoxide hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):573–577. doi: 10.1073/pnas.75.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Reynolds A. H., Rand S. D., Rentzepis P. M. Mechanisms for excited state relaxation and dissociation of oxymyoglobin and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2292–2296. doi: 10.1073/pnas.78.4.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. H., Rentzepis P. M. Kinetics and temperature dependence of carboxymyoglobin ligand photodissociation. Biophys J. 1982 Apr;38(1):15–18. doi: 10.1016/S0006-3495(82)84525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank C. V., Ippen E. P., Bersohn R. Time-resolved spectroscopy of hemoglobin and its complexes with subpicosecond optical pulses. Science. 1976 Jul 2;193(4247):50–51. doi: 10.1126/science.935853. [DOI] [PubMed] [Google Scholar]
- Terner J., Stong J. D., Spiro T. G., Nagumo M., Nicol M., El-Sayed M. A. Picosecond resonance Raman spectroscopic evidence for excited-state spin conversion in carbonmonoxy-hemoglobin photolysis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1313–1317. doi: 10.1073/pnas.78.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]



