Abstract
Using a sensitive differential ac conductance apparatus, we have measured transient ion movements in and the heating of bacteriorhodopsin suspensions after a light flash. The signal from the heating serves as an internal calibration of the absorbed photons and therefore the method gives the absolute quantum yield (phi) from a single measurement. At pH 4, H+ uptake precedes release, with phi = 0.4. By varying the buffer composition, we can prove that this signal is due to protons. At pH 8, however, the transient conductance increase is virtually independent of the buffer composition, showing that ions other than H+ are first released and then taken up by the purple membrane. If these ions are typical monovalent cations such as Na+ (lambda = 50 ohm-1 X cm2 X equiv-1), this process has a quantum yield of 2 or more at high salt concentrations.
Full text
PDF
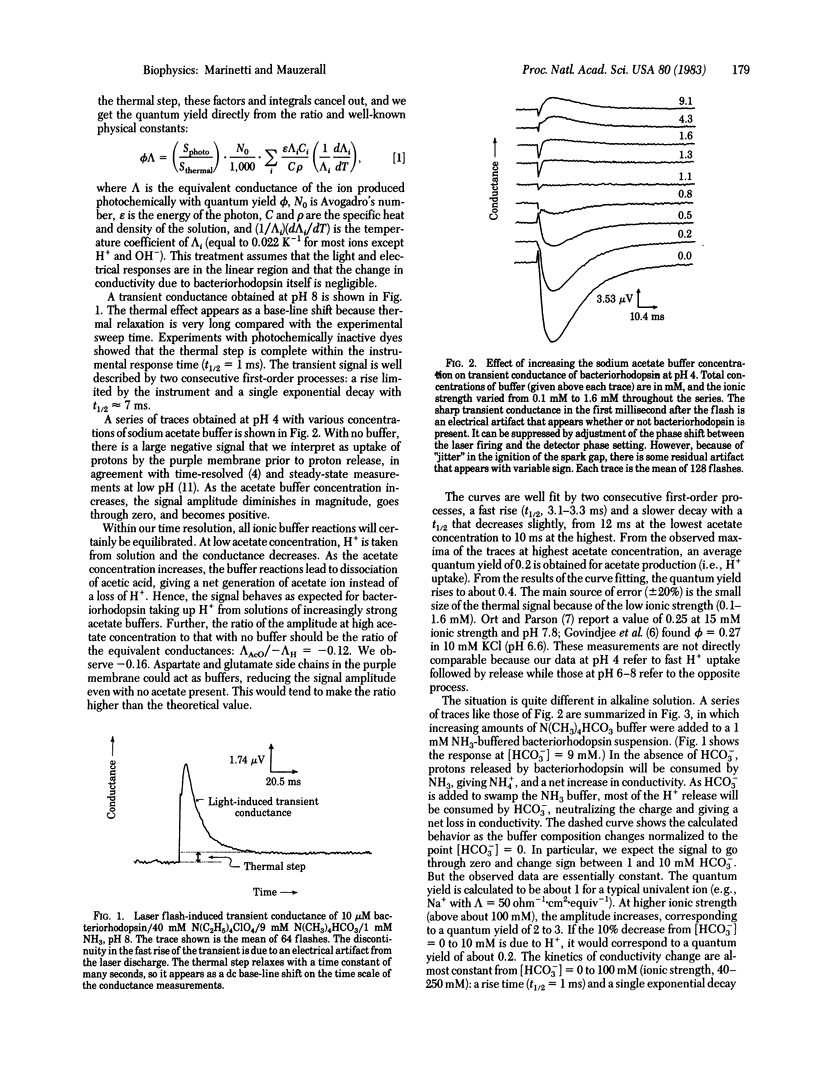
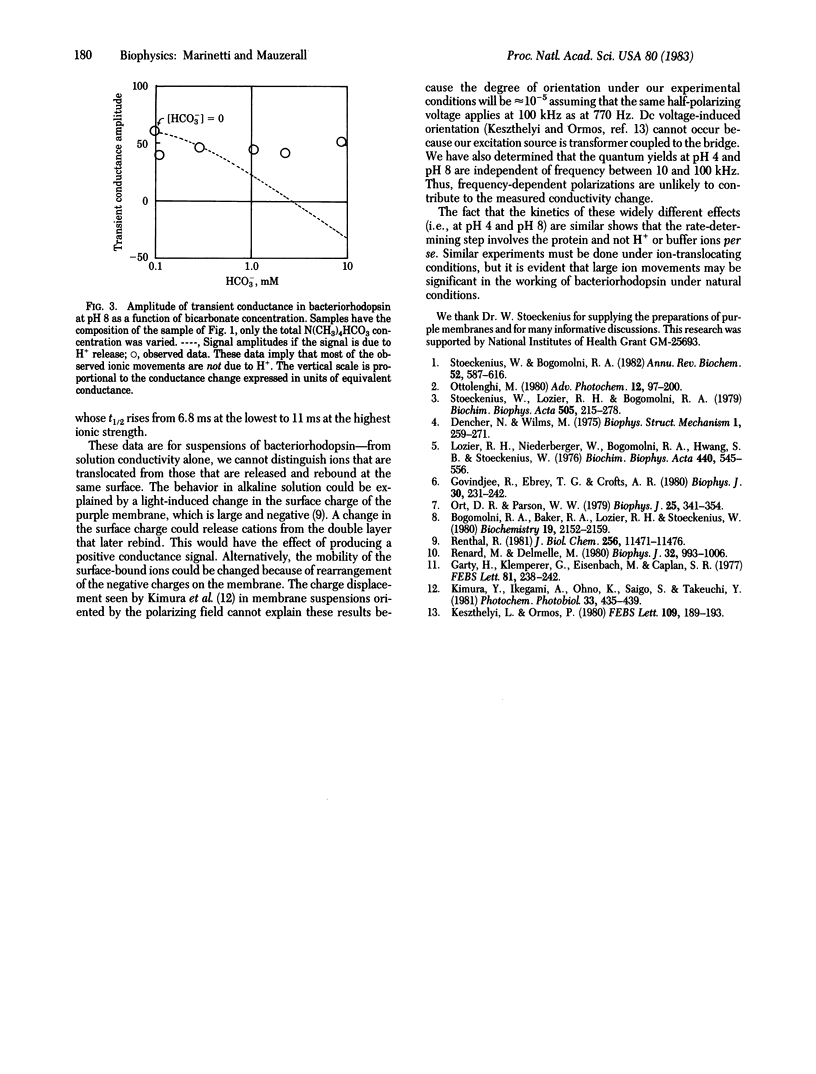
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Garty H., Klemperer G., Eisenbach M., Caplan S. R. The direction of light-induced pH changes in purple membrane suspensions. Influence of pH and temperature. FEBS Lett. 1977 Sep 15;81(2):238–242. doi: 10.1016/0014-5793(77)80526-7. [DOI] [PubMed] [Google Scholar]
- Govindjee R., Ebrey T. G., Crofts A. R. The quantum efficiency of proton pumping by the purple membrane of Halobacterium halobium. Biophys J. 1980 May;30(2):231–242. doi: 10.1016/S0006-3495(80)85091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. The quantum yield of flash-induced proton release by bacteriorhodopsin-containing membrane fragments. Biophys J. 1979 Feb;25(2 Pt 1):341–353. doi: 10.1016/s0006-3495(79)85296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M., Delmelle M. Quantum efficiency of light-driven proton extrusion in Halobacterium halobium. pH dependence. Biophys J. 1980 Dec;32(3):993–1006. doi: 10.1016/S0006-3495(80)85031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal R. Light-induced changes in H+ binding to the purple membrane. Effect of pH, light, temperature, and ionic strength. J Biol Chem. 1981 Nov 25;256(22):11471–11476. [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]



