Abstract
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by airflow limitation that is not fully reversible. In a significant proportion of patients with COPD, reduced lung elastic recoil combined with expiratory flow limitation leads to lung hyperinflation during the course of the disease. Development of hyperinflation during the course of COPD is insidious. Dynamic hyperinflation is highly prevalent in the advanced stages of COPD, and new evidence suggests that it also occurs in many patients with mild disease, independently of the presence of resting hyperinflation. Hyperinflation is clinically relevant for patients with COPD mainly because it contributes to dyspnea, exercise intolerance, skeletal muscle limitations, morbidity, and reduced physical activity levels associated with the disease. Various pharmacological and nonpharmacological interventions have been shown to reduce hyperinflation and delay the onset of ventilatory limitation in patients with COPD. The aim of this review is to address the more recent literature regarding the pathogenesis, assessment, and management of both static and dynamic lung hyperinflation in patients with COPD. We also address the influence of biological sex and obesity and new developments in our understanding of hyperinflation in patients with mild COPD and its evolution during progression of the disease.
Keywords: chronic obstructive pulmonary disease, hyperinflation, expiratory flow limitation, operational lung volumes
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable lung disease characterized by airflow limitation that is not fully reversible.1 COPD is a leading cause of mortality and morbidity worldwide, even if it remains largely underdiagnosed.2,3 Currently, the prevalence of the disease is estimated to be around 10% in the population aged >40 years4 and could reach around 20%–30%5,6 when including milder patients (Global initiative for chronic Obstructive Lung Disease [GOLD] stage 1).1
In a significant proportion of patients with COPD, reduced lung elastic recoil combined with expiratory flow limitation eventually leads to lung hyperinflation during the course of the disease.7 In patients with COPD, the lung can be hyperinflated at rest (static hyperinflation) and/or during exercise (dynamic hyperinflation) when ventilatory requirements are increased and expiratory time is shortened. Hyperinflation is clinically relevant for patients with COPD mainly because it contributes to the dyspnea8 and morbidity associated with the disease.9 In fact, although measurement of expiratory flows is a prerequisite for the diagnosis and staging of COPD, the effects of the disease on static and dynamic lung volumes correlate better with patient symptoms and impairment in functional capacity than spirometric indices of the disease.10 Moreover, dynamic lung hyperinflation is related to reduced daily physical activity in COPD,11 which is an important component of quality of life.12
Despite the difficulties in establishing a cause-effect relationship, exercise intolerance and lung hyperinflation are closely interrelated in COPD.13,14 While exercise intolerance in patients with COPD is complex and multifactorial,15–17 dynamic hyperinflation remains a major contributor to exercise limitation that is consistently observed in this disease.18 During exercise, hyperinflation may impede cardiac19,20 and respiratory muscle function and increase the work of breathing.21 Finally, this phenomenon can also occur in patients with mild disease,22–24 a category of individuals likely representing a great portion of patients diagnosed with COPD.5
This review addresses the more recent literature regarding the pathogenesis of both static and dynamic lung hyperinflation. The pathophysiology and physiological consequences of lung hyperinflation are summarized, as well as management, pharmacological treatment, and the impact of pulmonary rehabilitation on hyperinflation. We also address the influence of biological sex and obesity and new developments in our understanding of hyperinflation in mild COPD patients and its evolution during progression of the disease. The review is based on literature available on the PubMed database, irrespective of the year of publication.
Pathophysiology of hyperinflation
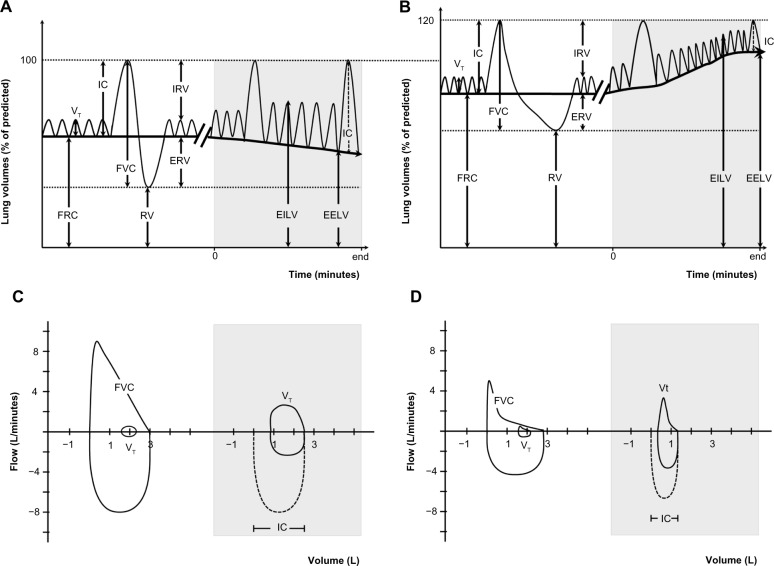
Lung volumes can be divided into several compartments defined by the normal cycle of tidal breathing and the maximum capacity to inhale and exhale (Figure 1A). In health, during relaxed tidal breathing, the lungs tend to return to a basal level of inflation, which is termed functional residual capacity (FRC) or end-expiratory lung volume (EELV). During the hyperpnea of exercise, both tidal volume (VT) and respiratory rate increase to meet the increased ventilatory requirements. Therefore, maintenance of stable lung volumes requires that expiratory muscles must be recruited to elevate pleural and alveolar pressure, increase expiratory flow, and force the increased VT to be completely exhaled before the next inhalation.25
Figure 1.
Lung volumes and capacities at rest and during exercise.
Notes: Lung volumes and capacities in a healthy elderly control (A and C) and in an aged-matched COPD patient (B and D). Gray parts represents lung volumes during exercise.
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; ERV, expiratory reserve volume; FRC, functional residual capacity; FVC, forced vital capacity; IC, inspiratory capacity; IRV, inspiratory reserve volume; RV, residual volume; VT, tidal volume.
Hyperinflation, defined as an increased volume of air remaining in the lung at the end of spontaneous expirations, is present when resting FRC or EELV is increased above normal.26 Two types of hyperinflation can be distinguished, ie, static and dynamic hyperinflation. A significant proportion of patients with COPD have some degree of lung hyperinflation, which often remains undetected in the absence of detailed physiological analysis (see section on assessment). Both static and dynamic effects of breathing contribute differently to lung hyperinflation in COPD.
Static and dynamic hyperinflation
Static hyperinflation
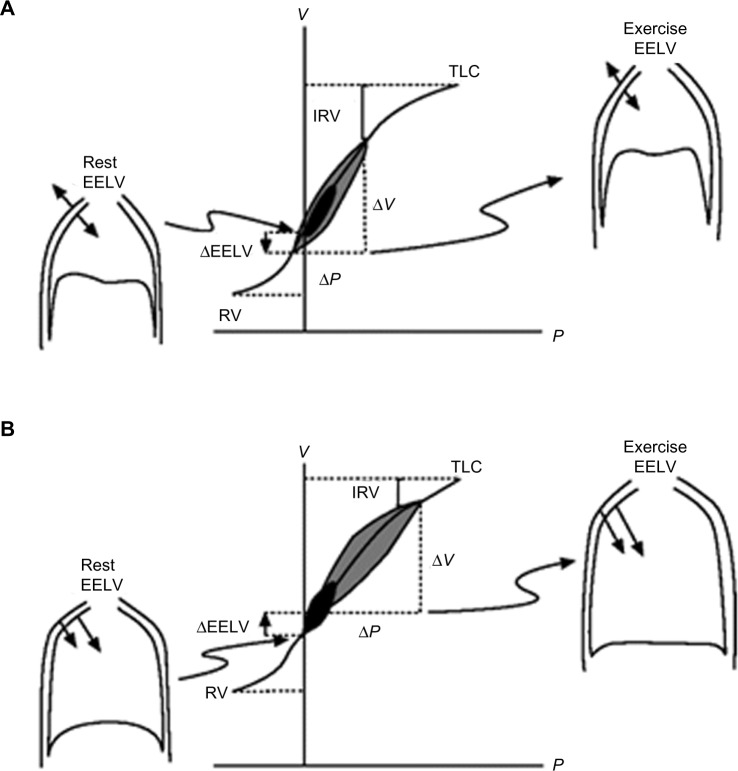
Under normal physiological conditions, for a given change in pleural pressure generated by the respiratory muscles, the attainable end-inspiratory lung volume (EILV) and EELV are determined by the passive pressure–volume relationship of the respiratory system (Figure 2).26,27 In healthy subjects, elastic recoil pressure of the respiratory system decreases progressively during exhalation, reaching zero at FRC or EELV and the elastic work of breathing is minimized by maintaining VT within 20%–80% of the vital capacity range. With advancing age, damage to the connective tissue of the lung occurs, resulting in a reduction of the lung elastic recoil pressure.28 The equilibrium point (FRC or EELV) therefore occurs at a higher lung volume than in younger subjects, with a consequence of an increased volume of air remaining in the lung at the end of spontaneous expirations. This is referred to as static hyperinflation, which exists at rest.28 In COPD with emphysema, the lung recoil pressure is further reduced by a reduced elastic load related to smoking or α1-antitrypsin deficiency.29 Therefore, the elastic recoil pressure of the respiratory system falls to zero at a larger FRC or EELV, resulting in more static hyperinflation.
Figure 2.
Pressure–volume relationships of the total respiratory system.
Notes: Pressure–volume relationships of the total respiratory system in healthy subjects (A) and in COPD (B). Tidal pressure–volume curves during rest (black) and exercise (gray) are shown. In COPD, the ability to further expand tidal volume is reduced. In contrast with health, the combined recoil pressure of the lungs and chest wall in hyperinflated patients with COPD is inwardly directed during both rest and exercise. Reproduced with permission of the European Respiratory Society. Eur Respir Rev December 2006 15:61–67; doi:10.1183/09059180.00010002.26 Copyright © remains with European Respiratory Society.
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; IRV, inspiratory reserve volume; P, pressure; RV, residual volume; TLC, total lung capacity; V, volume.
Dynamic hyperinflation
Dynamic lung hyperinflation refers to the temporary increase in EELV above the resting value during periods of increased ventilatory needs (eg, exercise). It is dependent on operational lung volumes and expiratory time, and is thus a key mechanistic consequence of expiratory flow limitation.14
During exercise, respiratory rate increases and VT expands to accommodate increased respiratory demands. The hyperpnea induces phasic activity of expiratory muscles in both healthy individuals and in those with COPD.30,31 In healthy individuals, the increased expiratory effort progressively decreases EELV and expiratory airflows are sufficient to allow complete exhalation of the inhaled VT before the next inhalation, even when breathing approaches maximal ventilation. In contrast, the combined effects of decreased lung elastic recoil pressure and increased airways resistance in patients with COPD results in an increased mechanical time constant for lung emptying in many alveolar units. Thus, as the respiratory rate and expiratory flow increases, the expiratory time available for exhalation can become insufficient and complete exhalation of VT to the relaxation volume becomes increasingly compromised, and EELV usually increases with hyperpnea.32 In addition, similar to healthy subjects, patients with COPD recruit expiratory muscles to increase their pleural and alveolar pressures, in an effort to increase expiratory flow. However, in these patients, the airways typically collapse when the pleural pressure becomes positive, thereby preventing increased expiratory flow.33 As a result, exhalation may not be completed prior to the onset of the next breath, causing an increase in operational lung volumes and progressive air retention called “air trapping”.13,26,34 This is referred to as dynamic hyperinflation, which can occur independently of static hyperinflation. Usually observed during exercise, the onset of dynamic hyperinflation will also occur at lower minute ventilations as disease severity limiting exhalation worsens, and may even occur during quiet breathing in severe patients or during an acute exacerbation.14,35
Natural history of hyperinflation
Development of hyperinflation during the course of COPD is insidious. In early COPD, the forced expiratory volume in one second (FEV1) may not be the optimal indicator of small airways obstruction.36 In fact, considering the extent of small airway inflammation reported in patients with mild COPD,37 it is conceivable that substantial structural damages could have taken place before marked expiratory flow limitation is objectively measured via FEV1.38 Early changes observed in pulmonary function of heavy smokers without COPD likely imply increased total lung capacity (TLC) and residual volume because of the loss of elastic recoil.39 These early changes reflecting lung hyperinflation are observed without any apparent reduction in FEV1.39 In mild COPD, measures of TLC, FRC, and residual volume were found to be significantly above predicted values while vital capacity and inspiratory capacity (IC) were preserved.40 Throughout the continuum of hyperinflation from mild to more severe COPD, vital capacity and IC decrease linearly with the progression of airflow obstruction (FEV1 decline). On the other hand, the progressive increase in TLC, FRC, and residual volume appears to be exponential with the worsening airflow limitation during the course of COPD.40
During exercise, some studies report that dynamic hyperinflation is already present in patients with mild disease (GOLD stage 1), even when resting hyperinflation is slightly present22,23 or absent.24,41–43 Even if patients with mild COPD usually have preserved resting IC, they still exhibit dynamic hyperinflation and abnormal ventilatory mechanics during exercise when compared with healthy controls.22,44 At peak exercise, notwithstanding the severity of the disease, patients seem to show a consistent fall of approximately 20% of their resting IC at peak exercise.22–24,45–48
In patients with moderate-to-severe COPD, the level of dynamic hyperinflation is poorly related to FEV1.49 However, when comparing two patients with similar FEV1, the one presenting with a reduced diffusion capacity, more severe small airway obstruction, and a higher ventilatory response to exercise will tend to develop more dynamic hyperinflation early during exercise.13 Moderate levels of dynamic hyperinflation can even be observed in healthy elderly individuals aged >70 years without any pulmonary disease following normal aging of the lung parenchyma.50–52 Likewise, the ventilatory response during exercise of a healthy elderly subject could be similar to that of a patient with GOLD stage 1 COPD.53
Physiological and sensory consequence of lung hyperinflation
Dyspnea
The interrelation between hyperinflation and dyspnea has been evaluated indirectly using regression analysis. O’Donnell and Webb54 evaluated 23 patients with severe COPD and found that the change in EILV from baseline was the strongest predictor of the change in Borg dyspnea ratings (r=0.63, P<0.001). In this study, EELV and VT (both components of EILV) combined with breathing frequency accounted for 61% of the variance in dyspnea intensity. A subsequent study in a larger cohort of COPD patients (n=105) demonstrated that the VT/IC ratio, an index of EILV and VT constraint, was the strongest predictor of exertional dyspnea based on multiple linear regression analysis.13 Moreover, interventions that deflate the lungs (ie, reduce EILV and EELV) and delay the onset of critical VT constraints consistently reduce dyspnea intensity in patients with COPD during exercise.45,46,55,56
Although dynamic hyperinflation is a cardinal feature of COPD with important physiological consequences, a small proportion of patients (~15%–20%) do not dynamically hyperinflate during exercise even though they still experience intolerable dyspnea.13,42,57 Guenette et al42 recently evaluated the effects of dynamic hyperinflation on dyspnea by comparing a group of well characterized COPD patients who did not acutely increase their EELV during exercise (nonhyperinflators, n=65) with those that did increase their EELV (hyperinflators, n=65). Despite being well matched for age, sex, body mass index, and baseline airflow obstruction, the authors were not able to show that the hyperinflators experienced more dyspnea than the nonhyperinflators. The authors concluded that perhaps the regulation of EILV provides a better index of critical constraints to ventilation (and therefore dyspnea) during exercise than the behavior of dynamic EELV per se. This finding does not necessarily diminish the physiological and sensory significance of dynamic hyperinflation, but rather shows that some individuals with airflow obstruction can still experience similar critical VT constraints (and thus similar dyspnea ratings), regardless of how they regulate EELV.
Respiratory and limb muscle function
Respiratory muscles
Static lung hyperinflation alters the geometry of the thorax and shortens the diaphragm,58 thereby placing the diaphragm in a suboptimal contractile position to generate pressure. This mechanical disadvantage reduces the force-generating capacity of the inspiratory muscles and is likely to become further exaggerated in patients who dynamically hyperinflate.59 Indeed, the ability of the respiratory muscles to generate pressure decreases at high lung volumes in humans.58,60 These functionally weakened respiratory muscles coupled with the increased elastic and threshold loading of the inspiratory muscles61 results in a substantial increase in the work and oxygen cost of breathing.21,62
Despite the known deleterious effects of static and dynamic hyperinflation on respiratory muscle function, some have postulated that respiratory muscle strength and function may actually be preserved in some patients with COPD.58,63,64 Chronic exposure to lung hyperinflation may result in physiological adaptations to preserve inspiratory muscle strength and perhaps obviate the development of diaphragmatic fatigue.65 Some of the documented adaptations include: an increase in the relative fraction of fatigue-resistant slow-twitch (type I) muscle fibers66 that can occur even in mild-to-moderate COPD;67 a reduction in sarcomere length which permits an increase in pressure production at higher lung volumes;68 increased mitochondrial density;68 and/or an improvement in mitochondrial respiratory chain capacity.69
Limb muscles
A direct link between dynamic hyperinflation and peripheral muscle function has not been fully established. Studies in healthy subjects suggest that high levels of respiratory muscle work may result in a sympathetically mediated metaboreflex which causes redistribution of blood flow from the locomotor muscles to the respiratory muscles.70–72 A reduction in locomotor muscle blood flow could result in an accelerated rate of development of limb muscle fatigue during exercise. This contention is supported by studies that show reduced limb muscle fatigue and corresponding improvements in perceived leg discomfort when the work of breathing is mechanically unloaded during exercise in healthy humans73 and in patients with COPD.74 In theory, dynamic hyperinflation and the associated increase in work and oxygen cost of breathing may compromise blood flow to the periphery, leading to compromised oxygen delivery and therefore causing increased leg fatigue. Indeed, studies that have unloaded the respiratory muscles of hyperinflated patients with bronchodilators or heliox resulted in an improvement in indices of limb muscle fractional oxygen extraction.75,76 For example, Louvaris et al77 recently demonstrated that improving operating lung volumes in hyperinflated COPD patients with heliox enhanced oxygen delivery to the quadriceps muscles during exercise by increasing arterial oxygen content and blood flow to the quadriceps muscles. The authors speculated that this was likely due to blood flow redistribution from the respiratory muscles since cardiac output was similar between heliox and room air.
Cardiac function
Lung hyperinflation has been shown to adversely affect cardiovascular function in patients with COPD. Lung hyperinflation reduces right ventricular preload and venous return at rest and during exercise.78–81 Left ventricular afterload may also increase due to the high intrathoracic pressure swings needed to overcome the high elastic and resistive loads encountered by patients with COPD during exercise.80 In addition, right ventricular afterload increases during exercise because there is an increase in pulmonary vascular resistance resulting from patients breathing at a high EILV.82,83 There is also indirect evidence to suggest that lung hyperinflation is associated with pulmonary hypertension. A number of mechanisms have been proposed to explain this association as recently described,84 including increased intrathoracic pressures, cardiovascular effects, increased lung volume, altered gas exchange, pulmonary vascular remodeling, and endothelial dysfunction. Collectively, these cardiovascular consequences of lung hyperinflation likely contribute, in highly variable combinations, to the reduced cardiac performance observed in some COPD patients during exercise.20,85 However, it should be acknowledged that not all studies have been able to demonstrate a direct link between dynamic hyperinflation and cardiac performance during exercise. For example, Stark-Leyva et al86 found that voluntary hyperinflation in healthy subjects did not adversely affect cardiac output during exercise. It remains to be determined if these findings in a healthy model can be extrapolated to patients with both static and dynamic hyperinflation, such as those with COPD.
Exercise tolerance
The mechanisms of exercise intolerance in COPD are complex and multifactorial and have been the subject of rigorous scientific debate.16–18 Potential mechanisms include abnormal ventilatory mechanics, limb muscle dysfunction, and impaired cardiac function, among other factors.87 All of these mechanisms are related, at least in part, to lung hyperinflation as previously described. Thus, it is difficult to directly demonstrate a cause–effect relationship between hyperinflation and exercise performance because interventions that reduce hyperinflation may also improve any one or a combination of these contributory factors to varying degrees. Nevertheless, correlative evidence indicates that there is a link between exercise performance and indices of lung hyperinflation. For example, peak VT relative to predicted vital capacity was found to be the best predictor of peak aerobic capacity (r=0.68, P<0.0005) in 105 patients with COPD.13 Work from other groups supports these results by showing a significant correlation between resting IC and peak work rate and peak oxygen uptake, particularly in patients with demonstrable expiratory flow limitation at rest.88 The notion that lung hyperinflation is inversely related to exercise tolerance is also supported, albeit indirectly, by studies showing statistically significant correlations between improvements in resting and exercise IC and improvements in peak oxygen uptake and cycle endurance time following different interventions.46,56,89,90
Influence of comorbidities and sex on hyperinflation
Obesity
Obesity is an abnormal or excessive fat accumulation that may impair health.91 Added weight on the thorax and abdomen (and also the neck), can significantly affect static and dynamic lung volumes along with respiratory mechanics,92–112 usually in a dose-response fashion. While several studies have addressed this issue, significant variability has been observed when evaluating the effects of obesity on lung volumes. These discrepancies may arise from heterogeneity in the severity of obesity and/or fat distribution, the precision of its measurement, or other confounding factors, such as underlying lung disease or sex differences. These uncertainties may well be exaggerated when the respiratory effects of obesity are studied alongside another heterogeneous disease such as COPD. As such, caution is recommended in drawing conclusions.
Total respiratory system compliance is usually reduced in obese patients. Obesity alone appears to have a “deflationary” effect. Obese patients consistently have a reduced expiratory reserve volume (or FRC) proportional to the magnitude of obesity.99,108,110,113–120 Total lung capacity is usually not affected (ie, it remains within the lower limits of normal values), although some studies report decreases in cases of very severe obesity (body mass index >45 kg/m2).110,112,121 Obesity is associated with a small decrease in FEV1 and forced vital capacity (although they remain within normal values)108,122,123 and the FEV1/forced vital capacity ratio is preserved.124 The physiological consequences of a combination of obesity and COPD are not well known and could theoretically provide advantages and disadvantages. On the one hand, both of these pathologies may have opposing effects in terms of lung volumes, COPD being primarily hyperinflating and obesity being deflating. This could provide an advantage to patients with COPD who are obese by reducing the deleterious effects of dynamic hyperinflation. On the other hand, this combination could increase mechanical loading and airway closure,125 and thus worsen trapping of air in the lung.
While very few studies have addressed the impact of the combination of obesity and COPD on lung volumes, available results suggest that compared with normal weight patients, obese patients with COPD have reduced TLC and FRC126–129 and that lower lung volumes are maintained throughout exercise.126,128,129 Obese patients with COPD still hyperinflate to a similar degree (Δ IC from rest to peak exercise capacity) than their normal weight counterparts.126,128,129 Obesity in COPD appears to have a deflating effect at rest, and as a consequence, even if patients hyperinflate at a similar rate, they remain at lower volumes during exercise. Therefore, these studies all report that obese patients with COPD have either preserved or increased exercise capacity,126–129 except when walking is the testing modality.127 Mechanistic data126 showed that the elastic properties of the lung were better preserved and that diaphragmatic function appeared not to be better in obese patients with COPD. Also, the increased metabolic load induced by obesity appeared to be compensated by an increased ventilatory efficiency (ie, lower ventilatory equivalent for CO2) in these patients. The precise mechanisms by which obesity and COPD interact to affect lung volumes are presently not well known. They are likely influenced by several factors, such as COPD phenotype42 and fat distribution.96
Sex
Respiratory volumes and flows are significantly different between the sexes, as shown by the reference equations for lung function.130,131 These differences (mainly smaller lungs and maximal flow rates in women) may also affect dynamic volumes because fit women may suffer from expiratory flow limitation that induces an increase in EELV.132–134 It is therefore possible that COPD affects women differently than men. Women with COPD appear to be more susceptible to resting hyperinflation, despite lower tobacco use and younger age.135 When restricted to emphysema, women also present a different pattern of disease compared with men, ie, smaller airway lumen and thicker airway walls.136,137 During constant work rate cycle exercise testing at the same relative intensity, women with COPD hyperinflated at a rate similar to that in men (Δ IC).138 However, considering their smaller lung volumes, they reached a critical inspiratory reserve volume sooner than men and thus stopped exercise earlier than men. Similar results were obtained in another sample of COPD patients.139 It would appear that women may be more susceptible to the deleterious effects of COPD because of their smaller respiratory systems compared with men.
Assessment of hyperinflation
Static assessments
In order to calculate lung hyperinflation at baseline, two subdivisions of the vital capacity must be measured. These are the IC and the expiratory reserve volume (Figure 1B).140 Methods used for assessment of these parameters in COPD are body plethysmography, nitrogen washout, and helium dilution techniques.141 Body plethysmography is considered the gold standard. This test is performed in a body plethysmograph allowing measurement of intrathoracic gas while airflow is occluded. Based on Boyle’s law,142 changes in thoracic volumes caused by a compression or decompression of the gas in the lungs during respiratory maneuvers can be computed. FRC is thus obtained and constitutes the key measurement of static hyperinflation. A minimum of three values must be obtained, and the difference between the lowest and the highest FRC must be within 5% to be considered reliable. The mean value is then reported. In elderly healthy subjects, residual volume and FRC represent 30% and 55% of TLC, respectively.130 In COPD, these values can be increased to 70% and 85% of the TLC for residual volume and FRC, respectively.143 Usually, lung volumes/capacities exceeding 120%–130% of the predicted value are considered to be clinically relevant in COPD, but this remains arbitrary given that no consensus about the definition or severity of lung hyperinflation is available.131,141 It seems that the FRC calculated by body plethysmography is overestimated because it includes both ventilated and nonventilated lung compartments.130,142 In contrast, nitrogen washout and helium dilution techniques underestimate FRC in the presence of severe airflow obstruction or emphysema.32,140 Complete details about these three techniques are available in the latest American Thoracic Society/European Respiratory Society task force document.141 Finally, because of a lack of standardization, radiographic techniques144–147 are not commonly used clinically to measure static hyperinflation in COPD.32 In fact, lung volumes calculated from radiographic techniques are based on the volume of gas within the outline of the thoracic cage and thus include the volume of tissue as well as the lung gas volume.140 This method is usually reserved for patients with a limited ability to correctly perform the other techniques. Nevertheless, high-resolution computed tomography might constitute a useful upcoming technique to assess hyperinflation in COPD.32
Dynamic assessments
Dynamic hyperinflation is determined from assessment of EELV (Figure 1B). This volume can be used interchangeably with FRC, although it is usually more appropriate to use it during exercise because this value is temporarily increased. EELV is commonly measured during exercise or any condition increasing minute ventilation by assessment of serial IC measurements as recently described by Guenette et al.41 As for the resting EELV, a minimum of three IC maneuvers must be performed at rest. Values within 10% or 150 mL of the largest acceptable IC are usually considered reproducible. During exercise, patients are asked to take a deep inspiration after a normal expiration at specific intervals ranging from 1 to 3 minutes as well as at symptom limitation and during recovery. Because TLC remains stable during exercise,148,149 a temporary decrease in IC reflects a temporary increase in EELV (Figure 1B and D). More than 80% of patients with moderate-to-severe COPD showed significant increases in EELV during exercise.11,13,46,150 This volume has been shown to be reliably measurable and is responsive to treatment in COPD.57,89 Moreover, inspiratory-to-total lung capacity ratio <25% has also been used as a prognostic tool in COPD.151 A recent study showed that reduction of the inspiratory reserve volume (IC – VT, Figure 1A and B) reflecting “room to breathe” was even more related to exercise dyspnea than EELV42 (Figure 1B). Finally, other methods such as optoelectronic plethysmography152 and respiratory inductance plethysmography153 are available for the assessment of dynamic hyperinflation, but they are still mainly used for research purposes in COPD.
Management and treatment of hyperinflation
Bronchodilator therapy
Pharmacological interventions that reduce operating lung volumes and delay the onset of ventilatory limitation consistently reduce the intensity of dyspnea during exercise in patients with COPD.46,55,56 It should be noted, however, that the rates of increase in EELV (dynamic hyperinflation) and dyspnea symptoms during exercise are not modified after administration of bronchodilators. Rather, pharmacotherapy delays the development of restrictive ventilatory mechanics during exercise by deflating the lungs and decreasing EELV at rest. The resulting increase in resting IC causes a parallel downward shift in operating lung volumes during exercise in comparison with exercise performed without bronchodilation (Figure 3).41,150 Thus, for any given exercise intensity or ventilation, patients breathe on the more linear portion of the respiratory system pressure–volume curve, with attendant improvements in neuromechanical coupling and, by extension, dyspnea. However, the absolute magnitude of dynamic hyperinflation does not change, and may even increase during peak exercise, reflecting the higher levels of ventilation that can be achieved following pharmacotherapy.10,45,90
Figure 3.
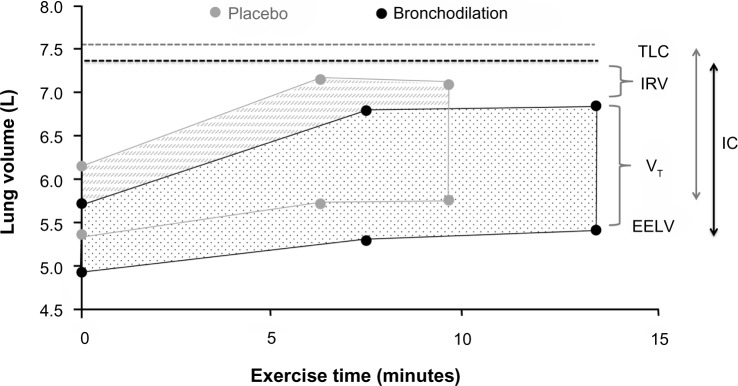
Acute effects of bronchodilation therapy on operational volume during constant work rate cycle ergometry in patients with COPD.
Notes: Example of operating lung volumes during constant work rate cycle ergometry performed at 75% maximal workload after dosing of placebo (gray symbols) or bronchodilation therapy (black symbols). Adapted with permission from the American College of Chest Physicians. Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128(3):1168–1178.150
Abbreviations: COPD, chronic obstructive pulmonary disease; EELV, end-expiratory lung volume; IC, inspiratory capacity; IRV, inspiratory reserve volume; TLC, total lung capacity; VT, tidal volume.
Nonpharmacological interventions
Ventilatory support
The use of noninvasive ventilatory support consistently increases endurance time and reduces perception of dyspnea during constant load cycling tasks in patients with COPD.154,155 However, assisting ventilation by continuous positive airway pressure or pressure support will not affect EELV at rest or the increase in EELV during exercise.156 The use of ventilatory support techniques will therefore not directly impact either static or dynamic lung hyperinflation. The effects of these interventions on dyspnea are probably mostly related to unloading of the inspiratory muscles during exercise.155,157–160 Respiratory muscle function is often impaired in patients with COPD.161 As previously described, these muscles have to overcome higher elastic and threshold loads during exercise which increases the work and oxygen cost of breathing in comparison with healthy subjects.26,162 Optimal continuous positive airway pressure reduces the elastic work of breathing throughout inspiration, counterbalances intrinsic positive end expiratory pressure, and takes away the threshold load on the inspiratory muscles while pressure support provides variable resistive and elastic unloading of the ventilatory muscles.156,163
Unloading respiratory muscles by proportional assisted ventilation improved leg blood flow and exercise performance during sustained high intensity exercise in healthy trained cyclists, indicating a competition for blood flow between respiratory and limb muscles.164,165 One study has so far investigated these mechanisms in patients with moderate-to-severe COPD.166 These authors found positive effects of respiratory muscle unloading by proportional assisted ventilation during a relatively short (average of 4–5 minutes) constant load cycling task on endurance time, leg muscle oxygenation, and dyspnea and leg fatigue symptoms.166
Oxygen/heliox administration
Supplemental oxygen during exercise consistently improves endurance and maximal exercise capacity and reduces ventilation and dyspnea at isotime during endurance exercise testing in COPD patients with and without resting hypoxemia.167 Oxygen supplementation during exercise delays the attainment of ventilatory limitation and accompanying intolerable symptoms of dyspnea during exercise by reducing ventilatory demand.168,169 Oxygen supplementation will however not affect EELV and IC at rest and will also not change EELV for a given level of ventilation during exercise.168,170 The improvements observed at a given level of exertion are therefore not caused by a direct effect on static or dynamic hyperinflation. Both improved oxygen delivery to the peripheral muscles (resulting in less reliance on anaerobic metabolism), and attenuated peripheral chemoreceptor stimulation have been proposed as possible explanations for the reduction in ventilatory demand for a given level of exertion.168,169
Heliox is a low density gas mixture (79% helium, 21% oxygen) that has been used in patients with COPD to reduce airflow resistance with increasing ventilatory requirements during exercise.171 Heliox supplementation has been shown to improve exercise intensity and endurance in patients with COPD in comparison with room air breathing.172 Effects on dyspnea are likely but less clearly documented in the current literature.172 Two papers evaluating dyspnea at isotime during an endurance cycling task however consistently showed significant reductions in perception of dyspnea.171,173 Heliox breathing increases the size of the maximal resting flow–volume envelope and seems to actually slow down the increase in EELV during exercise by decreasing airflow resistance, thereby directly altering dynamic hyperinflation.170,171 The response with regard to exercise capacity seems to be correlated with the magnitude of change in EELV during exercise.171 In three studies, the responses to hyperoxic helium (60%–70% helium, 30%–40% oxygen) and oxygen supplementation alone were compared during a constant load cycling task in patients with moderate (nonhypoxemic),173 severe,174 and very severe (on long-term oxygen therapy) symptoms.175 These studies all found significant differences in endurance time in favor of the hyperoxic helium group.173–175 They further demonstrated reductions in the resistive work of breathing,173 and reductions in exercise-induced dynamic hyperinflation (increases in EELV)174,175 in comparison with hyperoxia alone.
Lung volume reduction surgery
In selected patients, lung volume reduction surgery decreases static and dynamic hyperinflation, and improves neuromechanical coupling, respiratory muscle function, exertional dyspnea, and exercise performance.176–179 Lung volume reduction surgery increases maximal ventilatory capacity as evidenced by increases in both maximal voluntary ventilation and maximal minute ventilation at peak exercise.176–178,180–182 The positive effects of this intervention on airflow obstruction have been ascribed to increases in lung elastic recoil or to reductions in TLC and residual volume leading to an increased vital capacity and improvements in respiratory muscle function.183,184 However, the understanding of the exact mechanisms of improvement in lung function remains incomplete and needs to be improved to select the optimal patients for this procedure.183,184 Besides the effects on static hyperinflation, it seems that the intervention also exerts a direct effect on dynamic hyperinflation during exercise.176–178 While minute ventilation has been reported to be stable at comparable work rates after lung volume reduction surgery, decreases in EELV have been observed, with reductions in breathing frequency and increases in VT.183 Thus, lung volume reduction surgery improves airway conductance and lung emptying both at rest (comparable with bronchodilators) and during exercise (comparable with heliox breathing).
Exercise
The improvements in dyspnea and exercise capacity during constant load cycling tasks after properly conducted exercise training programs are larger than those observed with any of the previously described interventions.185,186 Several physiological and psychological factors, including a reduction in dynamic hyperinflation, have been proposed to explain these improvements.187–189 It is generally accepted that exercise training, unlike bronchodilators, does not have an impact on resting pulmonary mechanics.190 From the available data, it also appears that, unlike heliox breathing or lung volume reduction surgery, exercise training does not have a direct effect on the rate of increase in EELV (dynamic hyperinflation) during exercise.170 Similar to the acute effects of oxygen supplementation, exercise training reduces ventilatory needs for a given level of exertion.170,190,191 This decrease in ventilatory needs is probably related to improvements in limb muscle function after training with an accompanying reduced reliance on anaerobic metabolism during exercise.187,189 Less ventilation will allow patients to reduce their respiratory rate, increase VT, and reduce EELV for a given workload and will eventually result in reduced symptoms of dyspnea and improved exercise endurance.187,189 For a given level of ventilation, EELV seems, however, not to be altered after exercise training.187–189
Breathing techniques
Pursed lip breathing is used spontaneously by some patients with severe dyspnea, airflow obstruction, and lung hyperinflation.192 Therapeutically, it has been applied to reduce breathing frequency and increasing VT during exercise in several small studies, with mixed results in terms of dyspnea reduction and improvements in exercise capacity.192–194 Spahija et al192 observed that during constant work bicycle exercise, a reduction in dyspnea during application of pursed lip breathing was related to changes in EELV and pressure generation of the inspiratory muscles. Even though the evidence base is limited, pursed lip breathing might be used on a trial-and-error basis in individual patients. A recent study by Collins et al195 used a computerized ventilation feedback intervention aimed at slowing respiratory rate in combination with an exercise training program and showed reductions in respiratory rate, ventilation, and dynamic hyperinflation at isotime during a constant load cycling task. Feasibility of this approach on a larger scale needs to be addressed.
Inspiratory muscle training
Strengthening inspiratory muscles by specific training programs has been applied frequently in patients with COPD with the aim to alleviate dyspnea and improve exercise capacity. Reduced contractile muscle effort has been proposed as an important dyspnea relieving mechanism in studies that used ventilatory support to unload these muscles during exercise.157–160 Inspiratory muscle training aims to increase the capacity of these muscles to allow them to function at a lower fraction of their maximal capacity during exercise. Strong evidence supports effects of inspiratory muscle training to improve inspiratory muscle function (strength and endurance) and to reduce dyspnea and improve exercise capacity when applied as a standalone intervention.196 Positive effects of inspiratory muscle training on operational lung volumes and breathing patterns during exercise have so far only been demonstrated in a single study.197 More research into the mechanisms linking inspiratory muscle training to reduction of dyspnea during daily activities is warranted.
Summary
Although measurement of FEV1 is mandatory to establish a diagnosis of COPD, research in recent years has clearly demonstrated that hyperinflation, at rest and/or during exercise, is more closely associated with important clinical outcomes such as dyspnea and exercise intolerance than with expiratory flow indices. Hyperinflation has become an important endpoint in several clinical trials evaluating the efficacy of pharmacological and nonpharmacological therapeutic approaches to COPD. These trials have shown that measuring hyperinflation at rest and/or during exercise in the context of a multicenter randomized trial is feasible and valid. These trials have also confirmed that reducing hyperinflation in patients with COPD is a realistic therapeutic objective and is associated with relevant clinical benefits.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 3.Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182(7):673–678. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 5.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 6.Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 7.Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321–340. doi: 10.1164/ajrccm.159.1.ats898. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225–236. doi: 10.1080/15412550701480455. [DOI] [PubMed] [Google Scholar]
- 9.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–549. doi: 10.1164/ajrccm.160.2.9901038. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506–512. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 12.Jones PW. Activity limitation and quality of life in COPD. COPD. 2007;4(3):273–278. doi: 10.1080/15412550701480265. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 15.Pepin V, Saey D, Laviolette L, Maltais F. Exercise capacity in chronic obstructive pulmonary disease: mechanisms of limitation. COPD. 2007;4(3):195–204. doi: 10.1080/15412550701480489. [DOI] [PubMed] [Google Scholar]
- 16.Aliverti A, Macklem PT. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J Appl Physiol. 2008;105(2):749–751. doi: 10.1152/japplphysiol.90336.2008. [DOI] [PubMed] [Google Scholar]
- 17.Debigare R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol. 2008;105(2):751–753. doi: 10.1152/japplphysiol.90336.2008a. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol. 2008;105(2):753–755. doi: 10.1152/japplphysiol.90336.2008b. [DOI] [PubMed] [Google Scholar]
- 19.Watz H, Waschki B, Meyer T, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138(1):32–38. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 20.Tzani P, Aiello M, Elia D, et al. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir Res. 2011;12:150. doi: 10.1186/1465-9921-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 2009;107(1):309–314. doi: 10.1152/japplphysiol.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(6):622–629. doi: 10.1164/rccm.200707-1064OC. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell DE, Laveneziana P, Ora J, Webb KA, Lam YM, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax. 2009;64(3):216–223. doi: 10.1136/thx.2008.103598. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon P, Saey D, Provencher S, et al. Walking exercise response to bronchodilation in mild COPD: a randomized trial. Respir Med. 2012;106(12):1695–1705. doi: 10.1016/j.rmed.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1988;64(1):135–146. doi: 10.1152/jappl.1988.64.1.135. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15(100):61–67. [Google Scholar]
- 27.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22(1):95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 28.Pride NB. Ageing and changes in lung mechanics. Eur Respir J. 2005;26(4):563–565. doi: 10.1183/09031936.05.00079805. [DOI] [PubMed] [Google Scholar]
- 29.Thurlbeck WM. Overview of the pathology of pulmonary emphysema in the human. Clin Chest Med. 1983;4(3):337–350. [PubMed] [Google Scholar]
- 30.Grimby G, Goldman M, Mead J. Respiratory muscle action inferred from rib cage and abdominal V-P partitioning. J Appl Physiol. 1976;41(5 Pt 1):739–751. doi: 10.1152/jappl.1976.41.5.739. [DOI] [PubMed] [Google Scholar]
- 31.Dodd DS, Brancatisano T, Engel LA. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis. 1984;129(1):33–38. doi: 10.1164/arrd.1984.129.1.33. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–232. doi: 10.1080/15412550600977478. [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 34.Milic-Emili J. Dynamic pulmonary hyperinflation and intrinsic PEEP: consequences and management in patients with chronic obstructive pulmonary disease. Recenti Prog Med. 1990;81(11):733–737. Italian. [PubMed] [Google Scholar]
- 35.Haluszka J, Chartrand DA, Grassino AE, Milic-Emili J. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(5 Pt 1):1194–1197. doi: 10.1164/ajrccm/141.5_Pt_1.1194. [DOI] [PubMed] [Google Scholar]
- 36.Barnes N, Bush A. Howling for the moon. Thorax. 2011;66(8):645–646. doi: 10.1136/thx.2011.161323. [DOI] [PubMed] [Google Scholar]
- 37.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 38.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbin RP, Loveland M, Martin RR, Macklem PT. A four-year follow-up study of lung mechanics in smokers. Am Rev Respir Dis. 1979;120(2):293–304. doi: 10.1164/arrd.1979.120.2.293. [DOI] [PubMed] [Google Scholar]
- 40.Deesomchok A, Webb KA, Forkert L, et al. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD. 2010;7(6):428–437. doi: 10.3109/15412555.2010.528087. [DOI] [PubMed] [Google Scholar]
- 41.Guenette JA, Chin RC, Cory JM, Webb KA, O’Donnell DE. Inspiratory capacity during exercise: measurement, analysis, and interpretation. Pulm Med. 2013;2013:956081. doi: 10.1155/2013/956081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J. 2012;40(2):322–329. doi: 10.1183/09031936.00157711. [DOI] [PubMed] [Google Scholar]
- 43.Chin RC, Guenette JA, Cheng S, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187(12):1315–1323. doi: 10.1164/rccm.201211-1970OC. [DOI] [PubMed] [Google Scholar]
- 44.Babb TG, Rodarte JR. Lung volumes during low-intensity steady-state cycling. J Appl Physiol. 1991;70(2):934–937. doi: 10.1152/jappl.1991.70.2.934. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J. 2004;24(1):86–94. doi: 10.1183/09031936.04.00072703. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 47.Neder JA, Fuld JP, Overend T, et al. Effects of formoterol on exercise tolerance in severely disabled patients with COPD. Respir Med. 2007;101(10):2056–2064. doi: 10.1016/j.rmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Maltais F, Celli B, Casaburi R, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Respir Med. 2011;105(4):580–587. doi: 10.1016/j.rmed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Calverley PM, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–199. doi: 10.1183/09031936.04.00113204. [DOI] [PubMed] [Google Scholar]
- 50.Johnson BD, Dempsey JA. Demand vs capacity in the aging pulmonary system. Exerc Sport Sci Rev. 1991;19:171–210. [PubMed] [Google Scholar]
- 51.Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA. Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis. 1991;143(5 Pt 1):960–967. doi: 10.1164/ajrccm/143.5_Pt_1.960. [DOI] [PubMed] [Google Scholar]
- 52.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis. 1991;143(5 Pt 1):968–977. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 53.Laveneziana P, Parker CM, O’Donnell DE. Ventilatory constraints and dyspnea during exercise in chronic obstructive pulmonary disease. Appl Physiol Nutr Metab. 2007;32(6):1225–1238. doi: 10.1139/H07-119. [DOI] [PubMed] [Google Scholar]
- 54.O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148(5):1351–1357. doi: 10.1164/ajrccm/148.5.1351. [DOI] [PubMed] [Google Scholar]
- 55.Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):967–975. doi: 10.1164/ajrccm.153.3.8630581. [DOI] [PubMed] [Google Scholar]
- 56.O’Donnell DE, Hamilton AL, Webb KA. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol. 2006;101(4):1025–1035. doi: 10.1152/japplphysiol.01470.2005. [DOI] [PubMed] [Google Scholar]
- 57.O’Donnell DE, Travers J, Webb KA, et al. Reliability of ventilatory parameters during cycle ergometry in multicentre trials in COPD. Eur Respir J. 2009;34(4):866–874. doi: 10.1183/09031936.00168708. [DOI] [PubMed] [Google Scholar]
- 58.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325(13):917–923. doi: 10.1056/NEJM199109263251304. [DOI] [PubMed] [Google Scholar]
- 59.Sinderby C, Spahija J, Beck J, et al. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(7):1637–1641. doi: 10.1164/ajrccm.163.7.2007033. [DOI] [PubMed] [Google Scholar]
- 60.Polkey MI, Hamnegard CH, Hughes PD, Rafferty GF, Green M, Moxham J. Influence of acute lung volume change on contractile properties of human diaphragm. J Appl Physiol. 1998;85(4):1322–1328. doi: 10.1152/jappl.1998.85.4.1322. [DOI] [PubMed] [Google Scholar]
- 61.Gea J, Casadevall C, Pascual S, Orozco-Levi M, Barreiro E. Respiratory diseases and muscle dysfunction. Expert Rev Respir Med. 2012;6(1):75–90. doi: 10.1586/ers.11.81. [DOI] [PubMed] [Google Scholar]
- 62.Shindoh C, Hida W, Kikuchi Y, et al. Oxygen consumption of respiratory muscles in patients with COPD. Chest. 1994;105(3):790–797. doi: 10.1378/chest.105.3.790. [DOI] [PubMed] [Google Scholar]
- 63.Byrd RB, Hyatt RE. Maximal respiratory pressures in chronic obstructive lung disease. Am Rev Respir Dis. 1968;98(5):848–856. doi: 10.1164/arrd.1968.98.5.848. [DOI] [PubMed] [Google Scholar]
- 64.Singh B, Eastwood PR, Finucane KE. Volume displaced by diaphragm motion in emphysema. J Appl Physiol. 2001;91(5):1913–1923. doi: 10.1152/jappl.2001.91.5.1913. [DOI] [PubMed] [Google Scholar]
- 65.Mador MJ, Kufel TJ, Pineda LA, Sharma GK. Diaphragmatic fatigue and high-intensity exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(1):118–123. doi: 10.1164/ajrccm.161.1.9903010. [DOI] [PubMed] [Google Scholar]
- 66.Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337(25):1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- 67.Doucet M, Debigare R, Joanisse DR, et al. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. Eur Respir J. 2004;24(6):971–979. doi: 10.1183/09031936.04.00020204. [DOI] [PubMed] [Google Scholar]
- 68.Orozco-Levi M, Gea J, Lloreta JL, et al. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):371–378. doi: 10.1183/09031936.99.13237199. [DOI] [PubMed] [Google Scholar]
- 69.Ribera F, N’Guessan B, Zoll J, et al. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(6):873–879. doi: 10.1164/rccm.200206-519OC. [DOI] [PubMed] [Google Scholar]
- 70.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529Pt 2:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537Pt 1:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130(1):3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 73.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571Pt 2:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amann M, Regan MS, Kobitary M, et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiappa GR, Queiroga F, Jr, Meda E, et al. Heliox improves oxygen delivery and utilization during dynamic exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(11):1004–1010. doi: 10.1164/rccm.200811-1793OC. [DOI] [PubMed] [Google Scholar]
- 76.Laveneziana P, Palange P, Ora J, Martolini D, O’Donnell DE. Bronchodilator effect on ventilatory, pulmonary gas exchange, and heart rate kinetics during high-intensity exercise in COPD. Eur J Appl Physiol. 2009;107(6):633–643. doi: 10.1007/s00421-009-1169-4. [DOI] [PubMed] [Google Scholar]
- 77.Louvaris Z, Zakynthinos S, Aliverti A, et al. Heliox increases quadriceps muscle oxygen delivery during exercise in COPD patients with and without dynamic hyperinflation. J Appl Physiol. 2012;113(7):1012–1023. doi: 10.1152/japplphysiol.00481.2012. [DOI] [PubMed] [Google Scholar]
- 78.Mahler DA, Brent BN, Loke J, Zaret BL, Matthay RA. Right ventricular performance and central circulatory hemodynamics during upright exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(5):722–729. doi: 10.1164/arrd.1984.130.5.722. [DOI] [PubMed] [Google Scholar]
- 79.Light RW, Mintz HM, Linden GS, Brown SE. Hemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exercise. Am Rev Respir Dis. 1984;130(3):391–395. doi: 10.1164/arrd.1984.130.3.391. [DOI] [PubMed] [Google Scholar]
- 80.Montes de Oca M, Rassulo J, Celli BR. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med. 1996;154(5):1284–1289. doi: 10.1164/ajrccm.154.5.8912737. [DOI] [PubMed] [Google Scholar]
- 81.Vizza CD, Lynch JP, Ochoa LL, Richardson G, Trulock EP. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest. 1998;113(3):576–583. doi: 10.1378/chest.113.3.576. [DOI] [PubMed] [Google Scholar]
- 82.Ranieri VM, Dambrosio M, Brienza N. Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failure. Eur Respir J. 1996;9(6):1283–1292. doi: 10.1183/09031936.96.09061283. [DOI] [PubMed] [Google Scholar]
- 83.Oswald-Mammosser M, Apprill M, Bachez P, Ehrhart M, Weitzenblum E. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration. 1991;58(5–6):304–310. doi: 10.1159/000195950. [DOI] [PubMed] [Google Scholar]
- 84.Wrobel JP, Thompson BR, Williams TJ. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic review. J Heart Lung Transplant. 2012;31(6):557–564. doi: 10.1016/j.healun.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 85.Vassaux C, Torre-Bouscoulet L, Zeineldine S, et al. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J. 2008;32(5):1275–1282. doi: 10.1183/09031936.00151707. [DOI] [PubMed] [Google Scholar]
- 86.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96(5):1920–1927. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 87.Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic lung diseases. Compr Physiol. 2012;2(3):1779–1817. doi: 10.1002/cphy.c110015. [DOI] [PubMed] [Google Scholar]
- 88.Diaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J. 2000;16(2):269–275. doi: 10.1034/j.1399-3003.2000.16b14.x. [DOI] [PubMed] [Google Scholar]
- 89.O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557–1565. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- 90.Peters MM, Webb KA, O’Donnell DE. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPD. Thorax. 2006;61(7):559–567. doi: 10.1136/thx.2005.053470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [No authors listed] [PubMed] [Google Scholar]
- 92.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17(1):43–49. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
- 93.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108(1):206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 94.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 95.Sutherland TJ, Goulding A, Grant AM, et al. The effect of adiposity measured by dual-energy X-ray absorptiometry on lung function. Eur Respir J. 2008;32(1):85–91. doi: 10.1183/09031936.00112407. [DOI] [PubMed] [Google Scholar]
- 96.Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008;134(4):704–711. doi: 10.1378/chest.07-1728. [DOI] [PubMed] [Google Scholar]
- 97.Ofir D, Laveneziana P, Webb KA, O’Donnell DE. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol. 2007;102(6):2217–2226. doi: 10.1152/japplphysiol.00898.2006. [DOI] [PubMed] [Google Scholar]
- 98.Hamoui N, Anthone G, Crookes PF. The value of pulmonary function testing prior to bariatric surgery. Obes Surg. 2006;16(12):1570–1573. doi: 10.1381/096089206779319356. [DOI] [PubMed] [Google Scholar]
- 99.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 100.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jubber AS. Respiratory complications of obesity. Int J Clin Pract. 2004;58(6):573–580. doi: 10.1111/j.1368-5031.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 102.Canoy D, Luben R, Welch A, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159(12):1140–1149. doi: 10.1093/aje/kwh155. [DOI] [PubMed] [Google Scholar]
- 103.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321(4):249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 104.Rochester DF. Obesity and pulmonary function. In: Alpert M, Alexander J, editors. The Heart and Lung in Obesity. Armonk, NY, USA: Futura Publishing Co; 1998. [Google Scholar]
- 105.Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 1998;85(4):1236–1243. doi: 10.1152/jappl.1998.85.4.1236. [DOI] [PubMed] [Google Scholar]
- 106.Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. 1998;68(1):35–41. doi: 10.1093/ajcn/68.1.35. [DOI] [PubMed] [Google Scholar]
- 107.Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107(5):1298–1302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 108.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103(5):1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 109.Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. 1991;85(4):309–311. doi: 10.1016/s0954-6111(06)80102-2. [DOI] [PubMed] [Google Scholar]
- 110.Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112(11):828–832. doi: 10.7326/0003-4819-112-11-828. [DOI] [PubMed] [Google Scholar]
- 111.Suratt PM, Wilhoit SC, Hsiao HS, Atkinson RL, Rochester DF. Compliance of chest wall in obese subjects. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(2):403–407. doi: 10.1152/jappl.1984.57.2.403. [DOI] [PubMed] [Google Scholar]
- 112.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128(3):501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 113.Ladosky W, Botelho MA, Albuquerque JP., Jr Chest mechanics in morbidly obese non-hypoventilated patients. Respir Med. 2001;95(4):281–286. doi: 10.1053/rmed.2001.1035. [DOI] [PubMed] [Google Scholar]
- 114.Kelly TM, Jensen RL, Elliott CG, Crapo RO. Maximum respiratory pressures in morbidly obese subjects. Respiration. 1988;54(2):73–77. doi: 10.1159/000195504. [DOI] [PubMed] [Google Scholar]
- 115.Thomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989;44(5):382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Emirgil C, Sobol BJ. The effects of weight reduction on pulmonary function and the sensitivity of the respiratory center in obesity. Am Rev Respir Dis. 1973;108(4):831–842. doi: 10.1164/arrd.1973.108.4.831. [DOI] [PubMed] [Google Scholar]
- 117.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318(5):293–297. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 118.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87(3):654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 119.Collet F, Mallart A, Bervar JF, et al. Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes (Lond) 2007;31(4):700–706. doi: 10.1038/sj.ijo.0803460. [DOI] [PubMed] [Google Scholar]
- 120.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98(2):512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 121.Burki NK, Baker RW. Ventilatory regulation in eucapnic morbid obesity. Am Rev Respir Dis. 1984;129(4):538–543. [PubMed] [Google Scholar]
- 122.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56(1):4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162(13):1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 124.Babb TG, Wyrick BL, Chase PJ, et al. Weight loss via diet and exercise improves exercise breathing mechanics in obese men. Chest. 2011;140(2):454–460. doi: 10.1378/chest.10-1088. [DOI] [PubMed] [Google Scholar]
- 125.Boiselle PM, Litmanovich DE, Michaud G, et al. Dynamic expiratory tracheal collapse in morbidly obese COPD patients. COPD. 2013;10(5):604–610. doi: 10.3109/15412555.2013.781149. [DOI] [PubMed] [Google Scholar]
- 126.Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O’Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol. 2011;111(1):10–19. doi: 10.1152/japplphysiol.01131.2010. [DOI] [PubMed] [Google Scholar]
- 127.Sava F, Laviolette L, Bernard S, Breton MJ, Bourbeau J, Maltais F. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10:55. doi: 10.1186/1471-2466-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laviolette L, Sava F, O’Donnell DE, et al. Effect of obesity on constant workrate exercise in hyperinflated men with COPD. BMC Pulm Med. 2010;10:33. doi: 10.1186/1471-2466-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–971. doi: 10.1164/rccm.200904-0530OC. [DOI] [PubMed] [Google Scholar]
- 130.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 131.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 132.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985) 1998;84(6):1872–1881. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 133.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581Pt 3:1309–1322. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deruelle F, Nourry C, Mucci P, et al. Difference in breathing strategies during exercise between trained elderly men and women. Scand J Med Sci Sports. 2008;18(2):213–220. doi: 10.1111/j.1600-0838.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 135.Laviolette L, Lacasse Y, Doucet M, et al. Chronic obstructive pulmonary disease in women. Can Respir J. 2007;14(2):93–98. doi: 10.1155/2007/463435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480–1488. doi: 10.1378/chest.09-0676. [DOI] [PubMed] [Google Scholar]
- 138.Laviolette L, O’Donnell DE, Webb KA, Hamilton AL, Kesten S, Maltais F. Performance during constant workrate cycling exercise in women with COPD and hyperinflation. COPD. 2009;6(5):340–351. doi: 10.1080/15412550903140873. [DOI] [PubMed] [Google Scholar]
- 139.Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O’Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: physiological mechanisms. Respir Physiol Neurobiol. 2011;177(3):218–227. doi: 10.1016/j.resp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 140.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 141.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 142.Coates AL, Peslin R, Rodenstein D, Stocks J. Measurement of lung volumes by plethysmography. Eur Respir J. 1997;10(6):1415–1427. doi: 10.1183/09031936.97.10061415. [DOI] [PubMed] [Google Scholar]
- 143.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 144.Burki NK, Krumpelman JL. Correlation of pulmonary function with the chest roentgenogram in chronic airway obstruction. Am Rev Respir Dis. 1980;121(2):217–223. doi: 10.1164/arrd.1980.121.2.217. [DOI] [PubMed] [Google Scholar]
- 145.Simon G, Pride NB, Jones NL, Raimondi AC. Relation between abnormalities in the chest radiograph and changes in pulmonary function in chronic bronchitis and emphysema. Thorax. 1973;28(1):15–23. doi: 10.1136/thx.28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 147.de Jong PA, Muller NL, Pare PD, Coxson HO. Computed tomographic imaging of the airways: relationship to structure and function. Eur Respir J. 2005;26(1):140–152. doi: 10.1183/09031936.05.00007105. [DOI] [PubMed] [Google Scholar]
- 148.Stubbing DG, Pengelly LD, Morse JL, Jones NL. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J Appl Physiol Respir Environ Exerc Physiol. 1980;49(3):511–515. doi: 10.1152/jappl.1980.49.3.511. [DOI] [PubMed] [Google Scholar]
- 149.Vogiatzis I, Georgiadou O, Golemati S, et al. Patterns of dynamic hyperinflation during exercise and recovery in patients with severe chronic obstructive pulmonary disease. Thorax. 2005;60(9):723–729. doi: 10.1136/thx.2004.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128(3):1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 151.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 152.Aliverti A, Stevenson N, Dellaca RL, Lo Mauro A, Pedotti A, Calverley PM. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59(3):210–216. doi: 10.1136/thorax.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Clarenbach CF, Senn O, Brack T, Kohler M, Bloch KE. Monitoring of ventilation during exercise by a portable respiratory inductive plethysmograph. Chest. 2005;128(3):1282–1290. doi: 10.1378/chest.128.3.1282. [DOI] [PubMed] [Google Scholar]
- 154.van’t Hul A, Kwakkel G, Gosselink R. The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil. 2002;22(4):290–297. doi: 10.1097/00008483-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 155.van’t Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Acute effects of inspiratory pressure support during exercise in patients with COPD. Eur Respir J. 2004;23(1):34–40. doi: 10.1183/09031936.03.00016903. [DOI] [PubMed] [Google Scholar]
- 156.O’Donnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001;33(Suppl 7):S647–S655. doi: 10.1097/00005768-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 157.O’Donnell DE, Sanii R, Younes M. Improvement in exercise endurance in patients with chronic airflow limitation using continuous positive airway pressure. Am Rev Respir Dis. 1988;138(6):1510–1514. doi: 10.1164/ajrccm/138.6.1510. [DOI] [PubMed] [Google Scholar]
- 158.Petrof BJ, Calderini E, Gottfried SB. Effect of CPAP on respiratory effort and dyspnea during exercise in severe COPD. J Appl Physiol. 1990;69(1):179–188. doi: 10.1152/jappl.1990.69.1.179. [DOI] [PubMed] [Google Scholar]
- 159.Maltais F, Reissmann H, Gottfried SB. Pressure support reduces inspiratory effort and dyspnea during exercise in chronic airflow obstruction. Am J Respir Crit Care Med. 1995;151(4):1027–1033. doi: 10.1164/ajrccm/151.4.1027. [DOI] [PubMed] [Google Scholar]
- 160.Polkey MI, Kyroussis D, Mills GH, et al. Inspiratory pressure support reduces slowing of inspiratory muscle relaxation rate during exhaustive treadmill walking in severe COPD. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1146–1150. doi: 10.1164/ajrccm.154.4.8887619. [DOI] [PubMed] [Google Scholar]
- 161.Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J Suppl. 2003;46:41s–51s. doi: 10.1183/09031936.03.00004607. [DOI] [PubMed] [Google Scholar]
- 162.Rochester DF. The respiratory muscles in COPD. State of the art. Chest. 1984;85(Suppl 6):47S–50S. doi: 10.1378/chest.85.6.47s. [DOI] [PubMed] [Google Scholar]
- 163.Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J. 2004;24(2):313–322. doi: 10.1183/09031936.04.00002904. [DOI] [PubMed] [Google Scholar]
- 164.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82(5):1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 165.Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89(1):131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- 166.Borghi-Silva A, Oliveira CC, Carrascosa C, et al. Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax. 2008;63(10):910–915. doi: 10.1136/thx.2007.090167. [DOI] [PubMed] [Google Scholar]
- 167.Bradley JM, Lasserson T, Elborn S, Macmahon J, O’Neill B. A systematic review of randomized controlled trials examining the short-term benefit of ambulatory oxygen in COPD. Chest. 2007;131(1):278–285. doi: 10.1378/chest.06-0180. [DOI] [PubMed] [Google Scholar]
- 168.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(4):892–898. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 169.Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18(1):77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- 170.Dolmage TE, Evans RA, Goldstein RS. Defining hyperinflation as ‘dynamic’: moving toward the slope. Respir Med. 2013;107(7):953–958. doi: 10.1016/j.rmed.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 171.Palange P, Valli G, Onorati P, et al. Effect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patients. J Appl Physiol. 2004;97(5):1637–1642. doi: 10.1152/japplphysiol.01207.2003. [DOI] [PubMed] [Google Scholar]
- 172.Hunt T, Williams MT, Frith P, Schembri D. Heliox, dyspnoea and exercise in COPD. Eur Respir Rev. 2010;19(115):30–38. doi: 10.1183/09059180.00006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(7):763–771. doi: 10.1164/rccm.200509-1533OC. [DOI] [PubMed] [Google Scholar]
- 174.Hussain O, Collins EG, Adiguzel N, Langbein WE, Tobin MJ, Laghi F. Contrasting pressure-support ventilation and helium-oxygen during exercise in severe COPD. Respir Med. 2011;105(3):494–505. doi: 10.1016/j.rmed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 175.Queiroga F, Jr, Nunes M, Meda E, et al. Exercise tolerance with helium-hyperoxia versus hyperoxia in hypoxaemic patients with COPD. Eur Respir J. 2013;42(2):362–370. doi: 10.1183/09031936.00087812. [DOI] [PubMed] [Google Scholar]
- 176.O’Donnell DE, Webb KA, Bertley JC, Chau LK, Conlan AA. Mechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in emphysema. Chest. 1996;110(1):18–27. doi: 10.1378/chest.110.1.18. [DOI] [PubMed] [Google Scholar]
- 177.Benditt JO, Wood DE, McCool FD, Lewis S, Albert RK. Changes in breathing and ventilatory muscle recruitment patterns induced by lung volume reduction surgery. Am J Respir Crit Care Med. 1997;155(1):279–284. doi: 10.1164/ajrccm.155.1.9001325. [DOI] [PubMed] [Google Scholar]
- 178.Martinez FJ, de Oca MM, Whyte RI, Stetz J, Gay SE, Celli BR. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med. 1997;155(6):1984–1990. doi: 10.1164/ajrccm.155.6.9196106. [DOI] [PubMed] [Google Scholar]
- 179.Laghi F, Jubran A, Topeli A, et al. Effect of lung volume reduction surgery on neuromechanical coupling of the diaphragm. Am J Respir Crit Care Med. 1998;157(2):475–483. doi: 10.1164/ajrccm.157.2.9705082. [DOI] [PubMed] [Google Scholar]
- 180.Benditt JO, Lewis S, Wood DE, Klima L, Albert RK. Lung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometry. Am J Respir Crit Care Med. 1997;156(2 Pt 1):561–566. doi: 10.1164/ajrccm.156.2.9611032. [DOI] [PubMed] [Google Scholar]
- 181.Cordova F, O’Brien G, Furukawa S, Kuzma AM, Travaline J, Criner GJ. Stability of improvements in exercise performance and quality of life following bilateral lung volume reduction surgery in severe COPD. Chest. 1997;112(4):907–915. doi: 10.1378/chest.112.4.907. [DOI] [PubMed] [Google Scholar]
- 182.Keller CA, Ruppel G, Hibbett A, Osterloh J, Naunheim KS. Thoracoscopic lung volume reduction surgery reduces dyspnea and improves exercise capacity in patients with emphysema. Am J Respir Crit Care Med. 1997;156(1):60–67. doi: 10.1164/ajrccm.156.1.9609101. [DOI] [PubMed] [Google Scholar]
- 183.Marchand E, Gayan-Ramirez G, De Leyn P, Decramer M. Physiological basis of improvement after lung volume reduction surgery for severe emphysema: where are we? Eur Respir J. 1999;13(3):686–696. doi: 10.1183/09031936.99.13368699. [DOI] [PubMed] [Google Scholar]
- 184.Fessler HE, Scharf SM, Ingenito EP, McKenna RJ, Jr, Sharafkhaneh A. Physiologic basis for improved pulmonary function after lung volume reduction. Proc Am Thorac Soc. 2008;5(4):416–420. doi: 10.1513/pats.200708-117ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lacasse Y, Guyatt GH, Goldstein RS. The components of a respiratory rehabilitation program: a systematic overview. Chest. 1997;111(4):1077–1088. doi: 10.1378/chest.111.4.1077. [DOI] [PubMed] [Google Scholar]
- 186.Casaburi R, Porszasz J. Reduction of hyperinflation by pharmacologic and other interventions. Proc Am Thorac Soc. 2006;3(2):185–189. doi: 10.1513/pats.200508-095DO. [DOI] [PubMed] [Google Scholar]
- 187.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(13):1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 188.O’Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. 2009;167(1):116–132. doi: 10.1016/j.resp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 189.Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19(115):24–29. doi: 10.1183/09059180.00007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest. 2005;128(4):2025–2034. doi: 10.1378/chest.128.4.2025. [DOI] [PubMed] [Google Scholar]
- 191.Puente-Maestu L, Abad YM, Pedraza F, Sanchez G, Stringer WW. A controlled trial of the effects of leg training on breathing pattern and dynamic hyperinflation in severe COPD. Lung. 2006;184(3):159–167. doi: 10.1007/s00408-005-2576-x. [DOI] [PubMed] [Google Scholar]
- 192.Spahija J, Marchie M, Ghezzo H, Grassino A. Factors discriminating spontaneous pursed-lips breathing use in patients with COPD. COPD. 2010;7(4):254–261. doi: 10.3109/15412555.2010.496820. [DOI] [PubMed] [Google Scholar]
- 193.Casciari RJ, Fairshter RD, Harrison A, Morrison JT, Blackburn C, Wilson AF. Effects of breathing retraining in patients with chronic obstructive pulmonary disease. Chest. 1981;79(4):393–398. doi: 10.1378/chest.79.4.393. [DOI] [PubMed] [Google Scholar]
- 194.Garrod R, Dallimore K, Cook J, Davies V, Quade K. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chron Respir Dis. 2005;2(2):67–72. doi: 10.1191/1479972305cd068oa. [DOI] [PubMed] [Google Scholar]
- 195.Collins EG, Langbein WE, Fehr L, et al. Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2008;177(8):844–852. doi: 10.1164/rccm.200703-477OC. [DOI] [PubMed] [Google Scholar]
- 196.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37(2):416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 197.Petrovic M, Reiter M, Zipko H, Pohl W, Wanke T. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:797–805. doi: 10.2147/COPD.S23784. [DOI] [PMC free article] [PubMed] [Google Scholar]