Abstract
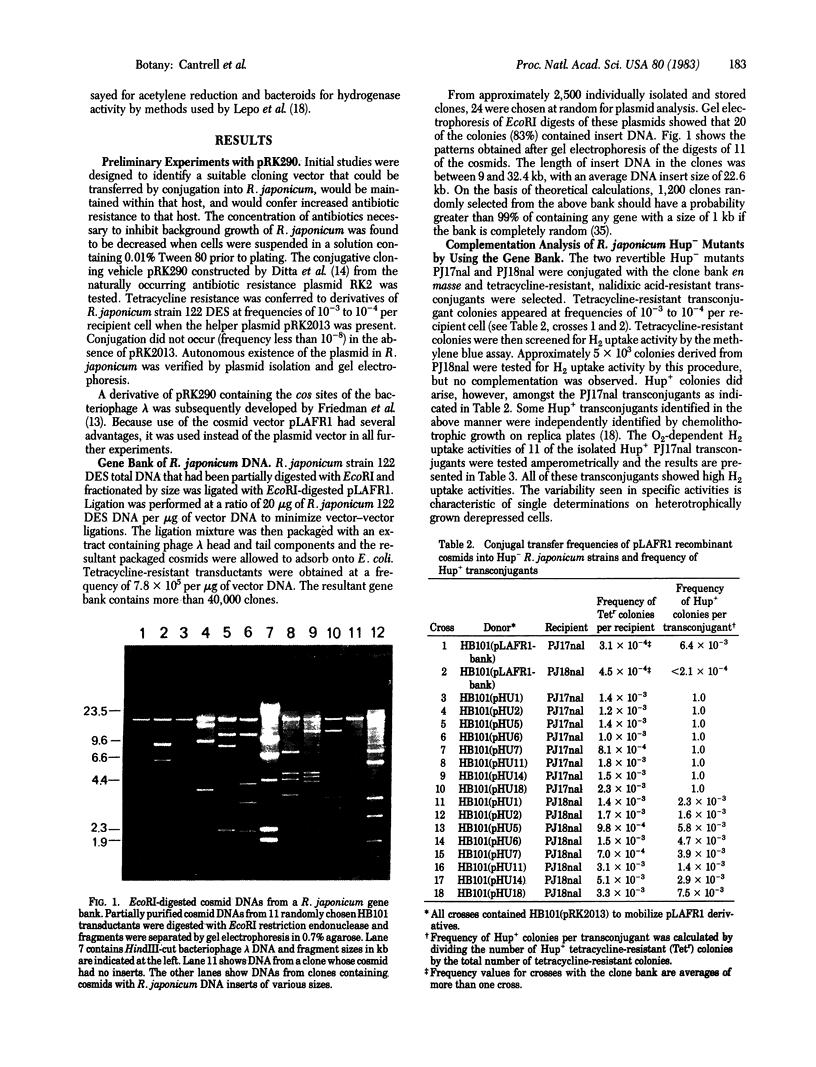
A gene bank of Rhizobium japonicum DNA was constructed by using the broad host range conjugative cosmid pLAFR1. Eighty-three percent of the clones in the bank contained cosmids with insert DNA averaging 22.6 kilobase pairs in length. A series of cosmids containing a hydrogen uptake (hup) gene was identified by transferring the gene bank into a H2 uptake-negative (Hup-) R. japonicum point mutant (PJ17nal) and screening tetracycline-resistant colonies for the ability to grow chemolithotrophically and to reduce methylene blue in a recently devised colony assay. Hup+ transconjugants arose at a frequency of approximately 6 × 10-3. Plasmid DNAs from II of the Hup+ transconjugants were isolated and used to transform Escherichia coli. EcoRI digests of all plasmids isolated from Hup+ transconjugants had three DNA fragments in common. Eight of the E. coli transformants containing hup gene cosmids were conjugated with PJ17nal and another Hup- point mutant, PJ18nal. All PJ17nal transconjugants were Hup+. The frequency of Hup+ transconjugants with PJ18nal was approximately 10-3. The results indicate that the hup gene cosmids may contain one gene and a portion of another.
Keywords: nitrogen fixation, hydrogenase, cloning, cosmid pLAFR1, soybean
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Collins J. Escherichia coli plasmids packageable in vitro in lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Nitrogenase--hydrogenase interrelationships in Rhizobia. Biochimie. 1978;60(3):233–236. doi: 10.1016/s0300-9084(78)80819-0. [DOI] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Carriers in electron transport from molecular hydrogen to oxygen in Rhizobium japonicum bacteroids. J Bacteriol. 1982 Mar;149(3):1005–1012. doi: 10.1128/jb.149.3.1005-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Lepo J. E., Hickok R. E., Cantrell M. A., Russell S. A., Evans H. J. Revertible hydrogen uptake-deficient mutants of Rhizobium japonicum. J Bacteriol. 1981 May;146(2):614–620. doi: 10.1128/jb.146.2.614-620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Mutaftschiev S. Reconstitution of H2 oxidation activity from H2 uptake-negative mutants of Rhizobium japonicum bacteroids. J Biol Chem. 1982 Feb 25;257(4):2092–2096. [PubMed] [Google Scholar]
- Maier R. J. Rhizobium japonicum mutant strains unable to grow chemoautotrophically with H2. J Bacteriol. 1981 Jan;145(1):533–540. doi: 10.1128/jb.145.1.533-540.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Salminen S. O. Uptake hydrogenase activity and ATP formation in Rhizobium leguminosarum bacteroids. J Bacteriol. 1982 Aug;151(2):989–995. doi: 10.1128/jb.151.2.989-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Mayer R. Dense autotrophic cultures of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):592–597. doi: 10.1128/aem.32.4.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]