Abstract
Purpose
Based on the preclinical evidence of topoisomerase I (Topo-1) upregulation by mitomycin C(MMC) and decreased NF-κB activation by celecoxib, we evaluated combinations of irinotecan/MMC and irinotecan/MMC/celecoxib in patients with advanced solid malignancies.
Patients–methods
Initially, patients received MMC on day 1 and irinotecan on days 2, 8, 15 and 22, every 6 weeks. MMC dose was fixed at 6 mg/m2 and cumulative doses of >36 mg/m2 were not permitted. Irinotecan was escalated in 25 mg/m2 increments. Due to late-onset diarrhea, the schedule was subsequently shortened to 4 weeks, omitting irinotecan on days 15 and 22. In the second part of the study, celecoxib 400 mg orally twice daily was added to irinotecan/MMC regimen. Potential pharmacokinetic interactions and Topo-1 and DT-diaphorase (NQ01) gene expressions in peripheral-mononuclear cells were evaluated.
Results
Forty-five patients were enrolled. Irinotecan 125 mg/m2 on days 2 and 8 in combination with MMC 6 mg/m2 on day 1 every 4 weeks is recommended for future studies; myelosuppression and diarrhea are dose-limiting. The addition of celecoxib resulted in unacceptable toxicities despite reductions on irinotecan’s dose. No relevant pharmacokinetic interactions occurred between irinotecan and MMC, and mean increases in Topo-1, were observed. Sixteen of 36 patients evaluable for response-assessment had discernable anti-tumor activity, including 1 complete, 4 partial, 10 minor and 1 tumor marker response. Four patients had prolonged (>4 months) disease-stability (stable disease, not included in CR or PR). Patients experiencing complete and partial responses had higher increments in Topo-1 expression.
Conclusions
Modulation of irinotecan by MMC is feasible, devoid of pharmacological interactions and active in solid malignancies. The lack of improvement in therapeutic index does not support the addition of celecoxib.
Keywords: Phase I, Pharmacokinetic, Mitomycin C, Irinotecan, Celecoxib, Modulation
Introduction
Irinotecan (CPT-11, Camptosar®) is a semisynthetic analog of camptothecin, a compound originally isolated from the Chinese/Tibetan ornamental tree Camptotheca acuminata. Cellular carboxylesterases (CE) cleave the ester bond of irinotecan in vivo, thereby producing the active compound 7-ethyl-10-hydroxycamptothecin (SN-38). Irinotecan/SN-38 interacts with cellular topoisomerase I (Topo-1) and single-stranded DNA breaks forming reversible Topo-1/SN-38/DNA cleavable complexes. Collision of these complexes with the advancing replication forks produce irreversible double-stranded DNA breaks and cell death [1]. G2 arrest/delay also occurs by signaling the presence of DNA damage to an S-phase checkpoint mechanism [2].
Irinotecan has demonstrated anti-tumor activity in a wide spectrum of malignancies [3]. However, several mechanisms of tumor resistance to this agent have been described [4]. Because Topo-1 is the cellular target of irinotecan, it is conceivable that its cellular level and activity would be proportional to irinotecan cytotoxic effects [5, 6]. In fact, synergism of cytotoxic effects was observed when irinotecan was combined with mitomycin C(MMC) in a human leukemia cell line in culture [7]. The mechanism responsible for this interaction was an increase in Topo-1 activity, as measured by relaxation of supercoiled DNA, following exposure to MMC [8].
Another mechanism of irinotecan resistance is activation of the transcription factor NF-κB (nuclear factor κB) by DNA damage after irinotecan treatment [9]. NF-κB upregulates the expression of a number of pro-proliferative and anti-apoptotic genes in the cell, thus theoretically dampens the cytotoxic activity of irinotecan [10]. Inhibition of NF-κB activation by intracellular introduction of the super-repressor [IκB α] significantly increased SN-38-induced cytotoxicity in colon and breast tumor cell lines, as well as in xenograft models [9, 11]. Cyclooxygenase (Cox) inhibitors have been shown to inhibit NF-κB activation by specific binding and inactivation of the enzyme that leads to the nuclear translocation of NF-κB [12, 13].
Based on the preclinical resistance data and the synergistic anti-tumor activity of the combination, we performed a phase I pharmacologic feasibility trial of the combination of irinotecan and MMC, and combination of irinotecan, MMC, and celecoxib in patients with advanced solid malignancies.
Patients and methods
Eligibility
Patients with histologically confirmed advanced solid malignancies were candidates for this study. Eligibility criteria also included (1) age ≥ 18 years; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2 (ambulatory and capable of self-care); (3) a life expectancy ≥ 12 weeks; (4) no major surgery, radiation therapy, or chemotherapy within 28 days; (5) adequate hematopoietic (absolute neutrophil count [ANC] ≥ 1,500/μL, platelet count ≥ 100,000/μL, and hemoglobin [Hgb] ≥ 9.0 g/dL), hepatic (total bilirubin level <1.5 mg/dL; transaminases [AST, ALT], and alkaline phosphatase ≤3 times the upper limit of normal), and renal (serum creatinine ≤1.5 mg/dL) functions; (6) no active neoplastic involvement of the central nervous system; (7) no prior treatment with MMC, irinotecan, or nitrosurea, and no more than six courses of chemotherapy containing an alkylating agent (four courses for carboplatin); and (8) no prior irradiation to more than 20% of bone marrow reserve. Patients receiving P450 activating or inhibiting agents were excluded, and concurrent administration of other Cox inhibitors was not allowed. All patients gave informed written consent before treatment.
Study design and dose administration
The study was conducted in two parts. The first part evaluated maximum tolerated doses (MTD) of MMC and irinotecan; the addition of celecoxib to the combination was evaluated in the second part. Initially, MMC was administered on day 1 and irinotecan on days 2 (24 h after MMC), 8, 15, and 22, with cycles repeated every 6 weeks. Due to the occurrence of late-onset diarrhea in some patients, which caused delays in day 15, 22 doses of irinotecan, the cycle duration was eventually decreased to 4 weeks, with irinotecan administered on days 2 and 8 after MMC on day 1. Once the MTD for the doublet was determined, celecoxib 400 mg orally twice daily was added in subsequent patients. Loperamide was used as per irinotecan package insert recommendations.
Dosage escalation, modifications and dose-limiting toxicities
The dose of MMC was fixed at 6 mg/m2 and limited to a maximal total dose of 36 mg/m2 to avoid potential cumulative toxicities. Irinotecan’s starting dose was 50 mg/m2 administered IV over 90 min and escalated in 25 mg/m2 increments in cohorts of at least three new patients. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) original version (https://webapps.ctep.nci.nih.gov). The recommended dose was defined as the highest dose at which no more than one of six new patients developed dose-limiting toxicities (DLT) during the first cycle of treatment. DLT was defined as: (1) ANC<500/μL lasting at least 5 days, or associated with fever and requiring parenteral antibiotics; (2) a platelet count <25,000/mL); (3) severe diarrhea, defined as >10 episodes despite optimal loperamide administration or requiring parenteral support, (4) vomiting requiring parenteral support despite optimal anti-emetics and; (5) other ≥ grade 3 non-hematologic toxicities.
Dose reductions for patients continuing on study were as follows. For patients experiencing grade 3 toxicity during a cycle of therapy, further doses were omitted until resolution of the toxicity to ≤ grade 1. The dose was reduced by one dose level in subsequent cycles for those patients experiencing any DLT. Evaluable patients were defined as patients completing one cycle (6 or 4 weeks) of treatment, unless discontinuation was due to drug-related toxicity.
Pretreatment and follow-up assessments
Histories, physical examinations, and routine laboratory studies were performed pretreatment and weekly. Routine laboratory studies included serum chemistries and complete blood cell measurement with differential counts, blood clotting times and urinalysis. Although measurable disease was not required in this phase I trial, the extent of malignant disease was evaluated prior to treatment and after every two courses. Patients continued on treatment in the absence of progressive disease or drug-limiting toxicity. A complete response (CR) was defined as disappearance of all disease on two measurements separated by a minimum of 4 weeks. A partial response (PR) required ≥50% reduction in the sum of the products of the bidimensional measurements of all measurable lesions documented by two assessments separated by at least 4 weeks; minor responses (MR) corresponded to decreases >25% but <50%. Progressive disease (PD) was defined as the presence of new lesions or a > 25% increase in pre-existing tumor lesions.
Pharmacokinetic sampling and analysis
During the first cycle of treatment, venous blood specimens were collected from a site contralateral to the drug infusion just prior to infusion and at 2, 4, and 24 h after the start of the infusion to evaluate plasma concentrations of irinotecan and its metabolites (SN-38, SN-38 glucuronide and APC). The plasma specimens were stored at ≤−20°C until assay. The assays for plasma concentration of the drugs were carried out by AvTech Laboratories, Inc (Kalamazoo, MI) using validated, sensitive and specific isocratic high-performance liquid chromatographic methods with fluorescence detection, as previously described [14, 15]. The analytical method was shown to be specific in the presence of the co-administered medication, celecoxib [16]. Irinotecan, SN-38, SN-38G, and APC plasma concentration data were analyzed by non-compartmental methods [17] via WinNonlin (Version 1.5). Irinotecan plasma concentrations and dose were expressed in free-base units. Peak plasma concentrations (Cmax) and the time at which they occurred (tmax) were determined by inspection of individual patient irinotecan, SN-38, SN-38G, and APC concentration–time curves. The area under the plasma concentration–time curve (AUC) was estimated using the linear trapezoidal rule. In those patients who had measurable plasma concentrations for 24 h following the end of the infusion, apparent terminal elimination rate constants (λz) for irinotecan and metabolites were determined by linear least-squares regression of plasma-concentration time points that were determined to lie in the terminal log-linear region of the plasma concentration–time profiles. The apparent elimination half-life (t½) was calculated as 0.693/λz and the clearance (CL) as dose/AUC0−∞.
Molecular correlates sampling and analysis
Topo-1 is the interactive target of irinotecan. DT-diaphorase (NQO1) is a reductase that catalyzes two electron transfer reaction in MMC, metabolizes quinones to hydroquinones, and is important in the activation of MMC [18]. The gene expression of Topo-1, and NQO1 were analyzed in peripheral blood mononuclear cells. RNA was extracted from blood samples collected at the following times: baseline; 5 min into the MMC infusion; at the end of MMC infusion; 3 h after the end of MMC infusion; 24 h after the end of MMC; at the end of irinotecan infusion; 2 h after the end of irinotecan; and 24 h after the end of irinotecan infusion. Gene expression samples were analyzed by reverse transcription polymerase chain reaction (RT-PCR) with a reaction-specific internal standard. Detection was accomplished by capillary electrophoresis with laser-induced fluorescence.
Results
General
Forty-five patients from two institutions were enrolled between August 1998 and May 2003. Pertinent demographic characteristics for patients in both parts of the study are displayed in Table 1. Thirty-eight patients were enrolled to the first part of the study, among them, 36 were evaluable, completing 118 cycles of the irinotecan/MMC combination. Fourteen additional cycles of irinotecan alone were administered to five of these patients after the maximum allowed number of courses with MMC (six) was exceeded. Table 2 depicts the dose escalation schema, the number of cycles administered, and the number of patients with DLT in cycle 1 at each dose level, and Tables 3 and 4 detail the hematologic and non-hematologic toxicities observed. The first 15 patients were treated on a 6-week schedule (MMC on day 1, irinotecan on days 2, 8, 15, and 22). No DLT occurred at irinotecan 50 mg/m2/week, but two of six evaluable patients at irinotecan 75 mg/m2/week developed grade 3 diarrhea during the first cycle of treatment. Since the diarrhea was of late-onset (occurred after patients received the third or fourth dose of irinotecan), the study was amended to shorten the course duration to 4 weeks (MMC on day 1, irinotecan on days 2 and 8).
Table 1.
Patient characteristics
| Patients treated | 45 |
| Sex (male/female) | 26/19 |
| Median age, years (range) | 55 (23–80) |
| ECOG performance status | |
| 0 | 21 |
| 1 | 22 |
| 2 | 2 |
| Previous radiotherapy | 25 |
| Previous chemotherapy | |
| None | 7 |
| 1 prior | 18 |
| 2 prior | 11 |
| >3 prior | 9 |
| Disease sites | |
| Lung (non-small cell) | 12 |
| Stomach | 8 |
| Esophageal | 6 |
| Breast | 7 |
| Pancreatic | 2 |
| Lung (small cell) | 2 |
| Hepatocellular | 2 |
| Unknown primary | 2 |
| Cholangiocarcinoma, bladder, thymoma, colorectal | 1 each |
Table 2.
Dose escalation scheme
| Dose level irinotecan/MMC (mg/m2) | Celecoxib (mg) | Cycle duration (weeks) | No. of patients |
Total cycles | Patients with DLT 1st (any) cycle | ||
|---|---|---|---|---|---|---|---|
| New | Reduced to this dose | Total | |||||
| 50/6 | n/a | 6 | 7 | 0 | 7 | 11 | 0 |
| 50/4 | n/a | 6 | 0 | 1 | 1 | 4 | 0 |
| 75/6 | n/a | 6 | 9 | 0 | 9 | 20 | 2 (4) |
| 75/6 | n/a | 4 | 3 | 1 | 4 | 12 | 0 |
| 50/6 | n/a | 4 | 0 | 2 | 2 | 4 | 0 |
| 100/6 | n/a | 4 | 6 | 2 | 8 | 19 | 1 (3) |
| 100/4 | n/a | 4 | 0 | 1 | 1 | 3 | 0 |
| 125/6 | n/a | 4 | 8 | 1 | 9 | 32 | 0 (2) |
| 125/4 | n/a | 4 | 0 | 1 | 1 | 1 | 0 (1) |
| 150/6 | n/a | 4 | 3 | 0 | 3 | 4 | 2 (3) |
| 125/6 | 400 BID | 4 | 2 | 0 | 2 | 2 | 2 (2) |
| 75/6 | 400 BID | 4 | 5 | 0 | 5 | 7 | 2 (2) |
| 50/4 | 400 BID | 4 | 0 | 2 | 2 | 3 | 1 |
Values in bold indicates starting dose, Values in italic indicates protocol amendment resulting in changes of cycle duration
Table 3.
Hematological toxicities
| Dose level irinotecan/MMC (mg/m2) | No. of patients (cycles) | Number of cycles with
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anemia |
Neutropenia |
Thrombocytopenia |
||||||
| G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | |||
| 1. | 50/6, 6 weeks | 7 (11) | 1 | 1 | 0 | 2 | 0 | 0 |
| 50/4, 6 weeks | 1 (4) | 0 | ||||||
| 2. | 75/6, 6 weeks | 9 (20) | 8 | 2 | 3 | 0 | 6 | 0 |
| 3. | 75/6, 4 weeks | 4 (14) | 3 | 0 | 2 | 0 | 2 | 0 |
| 50/6, 4 weeks | 2 (4) | 0 | ||||||
| 4. | 100/6, 4 weeks | 8 (23) | 11 | 0 | 7 | 0 | 2 | 2 |
| 100/4, 4 weeks | 1 (3) | 3 | 3 | 3 | ||||
| 5. | 125/6, 4 weeks | 9 (34) | 19 | 1 | 12 | 3 | 12 | 0 |
| 125/4, 4 weeks | 1 (1) | 1 | 1 | |||||
| 6. | 150/6, 4 weeks | 3 (4) | 2 | 0 | 0 | 2a | 2 | 0 |
| 7. | 125/6/celecoxib | 2 (2) | 1 | 0 | 0 | 2 (1a) | 0 | 0 |
| 8. | 75/6/celecoxib | 5 (7) | 4 | 3 | 2 | 2 | 3 | 1 |
| 50/4/celecoxib | 2 (3) | 1 | 1 | 1 | 1b | 1 | ||
For patients who had G1 anemia at base line, and no change throughout the course, the G1 anemia was not recorded. Worsening anemia to G2 or above would be recorded. One patient had G2 anemia (Hb 9.7) at the base line, progressed to Hb 8.6 at cycle 1, this was recorded
Italic indicates protocol amendment resulting in changes of cycle duration
Febrile
Sepsis
Table 4.
Non-hematological toxicities
| Dose level irinotecan/MMC (mg/m2) | No. of patients (cycles) | Number of cycles with
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nausea/vomiting |
Diarrhea |
Asthenia |
a Liver function abnormalities |
|||||||
| G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | b G3–4 | |||
| 1. | 50/6, 6 weeks | 7 (11) | 0 | 1 | 3 | 1 | 1 | 0 | 14 | 1 |
| 50/4, 6 weeks | 1 (4) | |||||||||
| 2. | 75/6, 6 weeks | 9 (20) | 1 | 1 | 2 | 3 | 0 | 0 | 9 | 4 |
| 3. | 75/6, 4 weeks | 4 (14) | 0 | 0 | 5 | 0 | 3 | 1 | 0 | 0 |
| 50/6, 4 weeks | 2 (4) | |||||||||
| 4. | 100/6, 4 weeks | 8 (23) | 0 | 0 | 4 | 2 | 2 | 0 | 8 | 0 |
| 100/4, 4 weeks | 1 (3) | |||||||||
| 5. | 125/6, 4 weeks | 9 (34) | 0 | 0 | 7 | 1 | 1 | 1 | 0 | 0 |
| 125/4, 4 weeks | 1 (1) | 1 | 1 | 2 | 0 | |||||
| 6. | 150/6, 4 weeks | 3 (4) | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 |
| 7. | 125/6/celecoxib | 2 (2) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 8. | 75/6/celecoxib | 5 (7) | 3 | 1 | 1 | 1 | 2 | 1 | 0 | 0 |
| 50/4/celecoxib | 2 (3) | 2 | 1 | 2 | ||||||
Italic indicates protocol amendment resulting in changes of cycle duration
Numbers in liver function abnormality indicate the sum of patients with elevations in alkaline phosphatase, total bilirubin, AST or ALT, one patient may be counted twice for two abnormalities
All patients had grade 3 toxicity, no one had grade 4 toxicity
This schedule was better tolerated with none of three patients developing grade 3 diarrhea at irinotecan 75 mg/m2 and only one of 14 patients at irinotecan doses of 100–125 mg/m2/week developing moderate to severe toxicity (grade 3 diarrhea) during their first cycle of treatment. Febrile neutropenia and grade 3/4 diarrhea were observed in two of three patients treated with irinotecan 150 mg/m2/week. Therefore, irinotecan 125 mg/m2 given on day 2 and day 8 every 4 weeks in combination with MMC 6 mg/m2 on day 1 is the recommended dose for phase II studies.
Celecoxib 400 mg PO twice daily was added to the above recommended doses of irinotecan and MMC in subsequent patients. Unfortunately, one of two patients treated at this dose of the triple-drug combination developed grade 3 diarrhea and neutropenia associated with sepsis during the first cycle of treatment and died. A second patient (breast primary) developed small bowel obstruction. Two of five subsequent patients treated at a reduced dose level of irinotecan (75 mg/m2/week), in combination with MMC 6 mg/m2 and celecoxib 400 mg PO twice daily developed DLT. This included grade 3 diarrhea in one patient and grade 4 neutropenia associated with small bowel obstruction, perforation, and septic peritonitis resulting in death in a colon cancer patient during his second cycle of treatment. In view of these toxicities, the triple-drug combination was considered devoid of a significant therapeutic index and not offered to subsequent patients.
In addition to the two treatment related deaths described above, two other patients died while participating in the study at the 50 and 75 mg/m2 irinotecan dose level, respectively, on the 6-week irinotecan/MMC schedule. Necropsies performed in these patients revealed congestive heart failure in one heavily pretreated and chest irradiated individual with inflammatory breast carcinoma, and saddle pulmonary embolism in the other, as the causative etiologies for their demise.
Anti-tumor activity
Sixteen patients experienced discernable anti-tumor activity, including one complete, four partial and 10 MR (25–49% decrease in the products of the two largest diameters). One additional patient had a tumor marker response. The tumor characteristics and prior treatment of these patients is described on Table 5. The most impressive response was observed in a patient with esophageal adenocarcinoma metastatic to the liver. This patient has been initially treated with neoadjuvant cisplatin and 5-fluorouracil in combination with radiation (4,500 cGy), but due to liver metastasis at the time of exploratory surgery, additional radiation and four more cycles of 5-FU and cisplatin chemotherapy were given. After demonstration of new (biopsy proven) metastatic liver nodules, intravenous paclitaxel (175 mg/m2 every 3 weeks) was administered for four cycles until disease progression, which included growth in the liver metastasis and elevations in serum levels of CA-19-9 from 310 to 7,785*/mL. Complete radiographic disappearance of tumor lesions by CT and normalization of CA-19-9 was documented after six cycles of irinotecan/MMC treatment at the 125/6 mg/m2 (4-week cycles) irinotecan/MMC dose level. The time from initiation of treatment to tumor progression (loco-regional) was 44 weeks.
Table 5.
Characteristics of patients responding to the treatment
| Sex | Age | Diagnosis | Previous treatment | Dose level | No. of cycles | Response | |
|---|---|---|---|---|---|---|---|
| 1 | M | 49 | Adenocarcinoma of the esophagus, liver mets | 5-FU/cisplatin with XRT, Taxol | 6/125 0/125 |
6 6 |
CR after 6 cycles, CA 19-9: 7,785 → 12.8 after 4 cycles, continued to be normal for 11 months |
| 2 | F | 73 | Inflammatory breast Ca, mets to chest wall | 5-FU, AC, taxotere, navelbine, gemcitabine, xeloda, XRT | 6/50 | 1 | cPR of the chest wall lesions |
| 3 | F | 53 | Inflammatory breast Ca, mets to the chest wall | Neoadjuvant AC, adjuvant Taxotere | 6/75 | 4 | cPR of the chest wall lesions after 1 cycle |
| 4 | M | 53 | Adenocarcinoma of the esophagus, liver, retroperitoneal LN mets | 5-FU/cisplatin with XRT | 6/125 | 2 | PR (55% reduction after 2 cycles) |
| 5 | M | 71 | Squamous ca of the esophagus, retroperitoneal LN mets | 5-FU/cisplatin with XRT, taxotere | 6/125 0/125 |
6 2 |
MR (39% reduction after 2 cycles), PR (58% reduction after 4 cycles), SD after 6 cycles |
| 6 | M | 28 | Gastric Ca, liver and lung mets | 5FU/Leu, doxirubicin, Taxol, LY295501, Alimta, XRT | 6/50 6/40 |
1 4 |
MR (25% reduction after 2 cycles) |
| 7 | M | 52 | Adenocarcinoma of the esophagus, lung and LN mets | 5-FU/cisplatin, Taxol | 6/75 | 6 | MR (28% reduction of metastatic LNs) |
| 0/75 | 2 | TTP = 32 weeks | |||||
| 8 | F | 56 | Bronchioloalveolar Ca, lung mets | Taxotere/xeloda | 6/75 | 4 | MR (42% after 6 cycles) |
| 6/50 | 2 | ||||||
| 0/50 | 2 | TTP = 37 weeks | |||||
| 0/75 | 1 | ||||||
| 9 | M | 80 | Non-operable NSCLC, progression after XRT and 1st line chemo | XRT, Taxol | 6/75 6/50 |
2 2 |
MR (27% reduction after 2 cycles) |
| 10 | M | 43 | NSCLC, liver mets | Taxol/carboplatin, gemcitabine/carboplatin | 6/100 | 2 | MR (Reduction in the lung mets) |
| Taxotere/xeloda, XRT | 6/75 | 2 | |||||
| 11 | M | 49 | Squamous Ca of the esophagus, recurrent bulky mediastinal LN | Neoadjuvant 5-FU/cisplatin with XRT | 6/100 | 6 | MR (28% reduction after 4 cycles) TTP 24 weeks |
| 12 | F | 75 | Gall bladder Ca, liver and LN mets | No | 6/100 | 2 | MR (36% reduction after 2 cycles) |
| 13 | F | 67 | Adenocarcinoma of unknown primary, favoring pancreas Ca, liver and lung mets | Cisplatin/gemcitabine | 6/150 | 1 | MR (Liver mets slightly decreased in size) |
| 14 | M | 53 | Gastric Ca liver mets | No | 6/125 | 4 | MR (27% reduction after 4 cycles) |
| 15 | F | 51 | Inflammatory breast Ca, recurrent chest wall lesion | CMF, Taxol | 6/125/C | 1 | Clinical MR of the chest wall lesion |
| 16 | F | 73 | Pancreatic Ca, recurrence | Adjuvant 5-FU and XRT | 6/100 | 1 | CA 19-9: 865 → 172 after 1 cycle |
AC doxorubicin, cytophosphamide, 5-FU 5-fluorouracil, Leu leucovorin, Taxol paclitaxel, LN lymph nodes, NSCLC non-small cell lung cancer, Met metastasis, CMF cytophosphamide, methotrexate, 5-fluorouracil, XRT external bean radiation treatment, Ca cancer, cPR clinical partial response, TTP total time to progression, MR minor response, C celecoxib
Two heavily pretreated patients with inflammatory breast cancer demonstrated clinical measurable >50% decrease of tumor masses in the chest wall. It is of interest, that one of the patients that died due to toxicity (bowel perforation and peritonitis after the second cycle of treatment) was noted to have a significant decrease in his metastatic abdominal tumor masses (colon primary) in the CT scan.
Pharmacokinetics and correlative studies
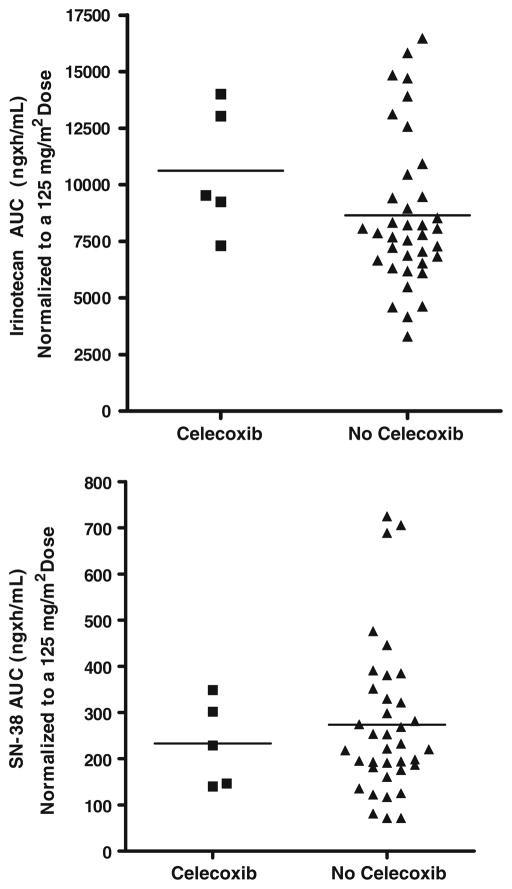
Pharmacokinetic specimens were available for 37 patients who received irinotecan in combination with MMC, and for five additional patients who received irinotecan, MMC and celecoxib. Mean (±SD) pharmacokinetic parameters for irinotecan and its metabolites are summarized in Table 6. The overall clearance, volume of distribution and half-life of irinotecan in all patients receiving the two-drug combination were 12.9 ± 5.14 L/h/m2, 105 ± 41.7 L/m2, and 5.7 ± 0.67 h, respectively. The half-life for SN-38 was 11.1 ± 3.9 and the SN-38/CPT-11 AUC0–24 ratio was 0.031 ± 0.012. The pharmacokinetic parameters for irinotecan and its metabolites determined in this study are very similar to those reported previously in single-agent irinotecan trials that utilized a similar specimen sampling schedule. Thus, no clinically significant pharmacokinetic interactions occurred between MMC and irinotecan on the schedule tested in this study. Similarly, irinotecan and SN-38 concentrations and pharmacokinetic parameters in the small number of patients receiving the three-drug combination were similar to those observed for the two-drug combination. These results indicated that there is a lack of pharmacokinetic interaction with celecoxib (Table 7 and Fig. 1).
Table 6.
Mean (±SD) CPT-11, SN-38, SN-38G, and APC pharmacokinetic parameters following a 90-min continuous infusion of irinotecan at doses ranging from 50 to 150 mg/m2
| Dose level (mg/m2) |
CPT-11 |
SN-38 |
SN-38G |
APC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cmax (ng/mL) |
AUC (ng h/mL) |
CL (L/h/m2) |
Vz (L/m2) |
Half-life (h) |
Cmax
(ng/mL) |
AUC (ng h/mL) |
Half-life (h) |
Cmax (ng/mL) | AUC (ng h/mL) |
Half-life (h) |
Cmax
(ng/mL) |
AUC (ng h/mL) |
Half-life (h) |
|
| No celecoxib | ||||||||||||||
| 50 (N = 7) | 536 ± 63.9 | 4779 ± 1451 | 12.3 ± 9.31 | 98.9 ± 68.9 | 5.7 ± 0.42 | 16.9 ± 5.15 | 180 ± 76.6 | 9.2 ± 1.94 | 50.1 ± 15.8 | 640 ± 234 | 12.1 ± 3.57 | 76.3 ± 26.7 | 1081 ± 461 | 9.2 ± 2.59 |
| 75 (N = 13) | 656 ± 138 | 4760 ± 2127 | 13.3 ± 3.94 | 108 ± 32.6 | 5.6 ± 0.40 | 15.9 ± 7.68 | 142 ± 93.6 | 10.5 ± 3.25 | 61.6 ± 37.7 | 723 ± 498 | 11.3 ± 4.59 | 86.9 ± 59.8 | 1072 ± 886 | 7.7 ± 1.39 |
| 100 (N = 6) | 825 ± 119 | 6234 ± 2265 | 14.1 ± 5.44 | 127 ± 42.8 | 6.4 ± 0.96 | 13.6 ± 3.39 | 157 ± 41.5 | 15.4 ± 3.69 | 56.1 ± 33.6 | 812 ± 532 | 15.7 ± 3.61 | 128 ± 80.4 | 1748 ± 967 | 9.2 ± 1.67 |
| 125 (N = 8) | 1109 ± 215 | 8076 ± 2033 | 12.0 ± 1.81 | 91.5 ± 24.9 | 5.2 ± 0.62 | 26.4 ± 9.52 | 251 ± 129 | 9.4 ± 3.96 | 75.8 ± 23.9 | 828 ± 314 | 11.6 ± 4.62 | 194 ± 110 | 1957 ± 759 | 7.1 ± 1.55 |
| 150 (N = 3) | 1240 ± 413 | 8844 ± 1343 | 13.7 ± 3.09 | 111 ± 33.7 | 5.5 ± 0.49 | 23.2 ± 15.7 | 279 ± 182 | 12.9 ± 5.23 | 46.9 ± 8.92 | 628 ± 71.9 | 11.8 ± 2.38 | 127 ± 46.4 | 1913 ± 821 | 8.2 ± 2.03 |
| Celecoxib | ||||||||||||||
| 75 (N = 3) | 794 ± 130 | 7258 ± 1509 | 8.77 ± 2.30 | 78.4 ± 8.03 | 6.4 ± 1.0 | 15.7 ± 3.39 | 196 ± 91.8 | 8.3 ± 0.36 | 77.3 ± 56.8 | 1197 ± 750 | 16.6 ± 11.7 | 76.3 ± 26.7 | 1081 ± 461 | 8.03 ± 1.68 |
| 125 (N = 2) | 1280 ± 70.7 | 8421 ± 1578 | 12.7 ± 2.53 | 93.7 ± 8.48 | 5.2 ± 0.56 | 23.6 ± 7.50 | 289 ± 84.7 | 9.2 ± 1.94 | 46.7 ± 15.0 | 662 ± 276 | 11.2 ± 2.17 | 86.9 ± 59.8 | 1072 ± 886 | 9.2 ± 2.59 |
Cmax peak plasma concentration, AUC area under the plasma concentration–time curve from time zero to 24 h post infusion, CL systemic clearance, Vz volume of distribution
Table 7.
Mean (±SD) CPT-11 and SN-38 Pharmacokinetic parameters following administration of irinotecan with mitomycin (no celecoxib) or with mitomycin ? celecoxib (celecoxib)
| Parameter | No celecoxib (N = 37) | Celecoxib (N = 5) | P value* |
|---|---|---|---|
| CPT-11 | |||
| Cmax (ng/mL)a | 1129 ± 228 | 1306 ± 159 | 0.1070 |
| AUC 0–24 (ng h/mL)* | 8655 ± 3316 | 10626 ± 2799 | 0.1204 |
| CL (L/h/m2) | 13.0 ± 5.2 | 10.3 ± 3.0 | 0.2481 |
| Vz (L/m2) | 106 ± 42.2 | 84.5 ± 11.0 | 0.3446 |
| t½ (h) | 5.7 ± 0.7 | 5.9 ± 1.0 | 0.8173 |
| SN-38 | |||
| Cmax (ng/mL)a | 27.3 ± 13.4 | 25.1 ± 5.7 | 0.9535 |
| AUC 0–24 (ng h/mL)* | 274 ± 164 | 233 ± 92.7 | 0.8460 |
| t½ (h) | 11.1 ± 3.9 | 10.2 ± 2.3 | 0.8831 |
| SN-38/CPT-11 AUC0–24 ratio | 0.031 ± 0.012 | 0.030 ± 0.008 | 0.9381 |
Non-parametric Mann–Whitney test
Normalized to an irinotecan dose of 125 mg/m2
Fig. 1.
Comparison of irinotecan and SN-38 AUC values when given with MMC or with MMC and celecoxib. All AUC values were normalized to an irinotecan dose of 125 mg/m2 since doses differed among the two groups. CTP-11 dose-normalized AUC, p = 0.1204; SN-38 dose-normalized AUC, p = 0.8460
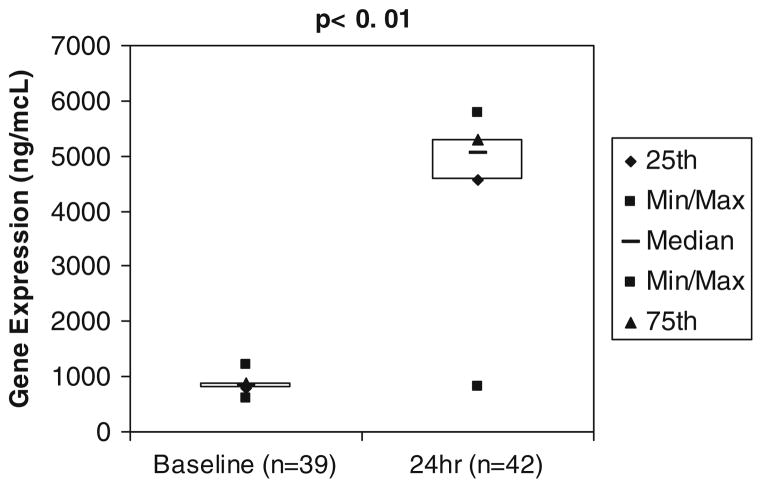
Topo-1 gene expression at baseline was 844 ng/mcL ± 108 with maximal induction (5274 ng/mcL ± 602) by 24 h after MMC infusion (Fig. 2). Patients with major responses to therapy (complete and partial responses) had the greatest Topo-1 induction, to 6,114 ng/mcL ± 1,019 compared to the rest of the patients at 151 ng/mcL ± 25.2 (p = 0.023). MMC-induced NQO1 gene expression from a baseline level of 4.4 ng/mcL ± 1.5 to maximum induction of 14.8 ng/mcL ± 1.8 by 3 h after MMC infusion.
Fig. 2.
Topo-1 gene expression before and after MMC administration. Topo-1 gene expression in peripheral blood mononuclear cells at baseline and 24 h after MMC administration was evaluated by RT-PCR, demonstrating a significant induction in topo-1 gene expression after MMC administration, p ≤ 0.01, one tail t test
Discussion
Irinotecan and MMC are both potent chemotherapy agents that have demonstrated anti-tumor activity in multiple tumor types [3]. Mutation and stable decrements in Topo-1 are potential mechanisms of tumor resistance to irinotecan [5, 6]. Preclinical studies have shown synergism in cytotoxicity with the combination of these two drugs [7]. Exposure to irinotecan produces a rapid, transient decrease in Topo-1 concentration in vitro and in vivo, which in cell cultures correlates with a decrease in cytotoxicity throughout the exposure period [19]. On the other hand, treatment of human MCF-7 breast adenocarcinoma cells with MMC resulted in increased Topo-1 activity [8], including the relaxation of supercoiled plasmid DNA and stabilization of the covalent Topo-1-DNA complex. On the basis of this preclinical rationale and the overlapping clinical anti-tumor spectra of these agents, this phase I pharmacologic study was performed to evaluate the feasibility of administering irinotecan and MMC, to study the pharmacologic profiles and potential pharmacokinetic interactions between these agents. More interestingly, this phase I study also attempts to test the hypothesis that MMC reverses tumor resistance to irinotecan by modulating the Topo-1 expression level.
Myelosuppression, predominantly neutropenia and diarrhea, were the principal DLTs observed in this study. Similar to other combination regimens using weekly irinotecan [20], elimination of irinotecan doses late in cycle resulted in a better tolerability of the irinotecan/MMC combination. The rate of moderate to severe diarrhea and neutropenia seen at the recommended doses of this combination, 22% and 44%, compares favorably to single agent irinotecan 125 mg/m2 given weekly for 4 weeks and 2 weeks off reported in a phase III study, as 36% and 29%, respectively [21].
The pharmacokinetic data obtained indicates that there are no pharmacological interaction between MMC and irinotecan when these agents are given 24 h apart, a schedule that was hypothetically designed to eliminate potential interference with activation of MMC by irinotecan and to provide for a time interval prior to the irinotecan administration to allow for Topo-1 upregulation. Previous studies had shown that MMC requires a reductase, DT-diaphorase (NQOR), for the activation of MMC. NQOR is a unique flavoenzyme coded by NADPH-quinone oxidoreductase gene (NQO1) that displays non-specific reactivity towards NADH and NADPH and shows a broad electron acceptor specificity, which catalyzes two electron transfer reactions important in the activation of MMC [18, 22]. Our group has shown that irinotecan decreases NQO1 gene expression in peripheral blood lymphocytes by approximately 50%, suggesting that infusion of irinotecan before or at the same time of MMC may interfere with the ability to activate mitomycin [23]. Therefore, in this study we chose to infuse MMC on day 1, and irinotecan on day 2 to avoid this interaction. To further this observation, our study indeed demonstrated increased NQO1 expression induced by MMC. This autocrine interaction may potentially increase the activity of MMC, and contribute to the anti-tumor activity observed in this study.
As predicted from in vitro models, MMC induces Topo-1 gene expression in human subjects, and responders (complete and partial responses) demonstrated the largest Topo-1 induction 24 h following MMC infusion. Since maximum Topo-1 upregulation in PBMC is reached at least 3 h, and at maximum 24 h after administration of MMC, the data provides pharmacodynamic evidence that a delay in the administration of irinotecan after MMC may be of utility. However, an important pitfall in any practical implications of this observation, is that it is not known if the pharmacodynamic effect at the tumor level is different from that in peripheral cells.
Although it is beyond the scope of a phase I and pharmacokinetic trial to assess clinical benefit, we observed anti-tumor effects with the combination of irinotecan and MMC in a wide variety of tumors, including refractory esophageal carcinoma and inflammatory breast cancer refractory to multiple lines of previous treatment. Breast cancer is of particular interest, since irinotecan has not demonstrated as a single agent of high level of activity in this disease. MMC has known activity in breast cancer treatment, at doses of 12–20 mg/m2 [24, 25]. However, one randomized study comparing the effects of “standard dose” MMC at 20 mg/m2 every 6 weeks versus “low dose” MMC at 5 mg/m2 showed comparable response rate, and less hematologic toxicities [24]. Thus, it is possible that the anti-tumor activity observed with the combination of irinotecan and MMC was due to MMC only and not to a potential synergistic effect of the combination.
In support of potential synergy for the drug combination, a study conducted by a separate group of investigators reported that the combination of intravenous irinotecan with intraperitoneal MMC produces a high response rate in patients with cisplatin-resistant ovarian adenocarcinoma [26]. Furthermore, the value of Topo-1 expression, as a predictor of response to irinotecan has been recently brought to attention by reported data from the FOCUS study, where Topo-1 expression was one of the most important parameters predictive of clinical benefit [27]. Moderate and high levels of Topo-1 expression are associated with statistically significant higher benefits from chemotherapy measured as failure free survival (FFS) with irinotecan and oxaliplatin-based chemotherapy [27].
In this study of biomodulation of drug resistance associated with irinotecan, we chose to combine celecoxib with the recommended doses of the MMC and irinotecan doublet. In addition to the discussed potential decrease in activation of NF-κB, previous reports had also supported amelioration or no increased side effects in irinotecan-related toxicity with the addition of celecoxib to irinotecan [28–31]. To further support the above notion, the updated report from the BICC-C study also showed no increased toxicity when oral celecoxib (400 mg twice a day) was combined with irinotecan 180 mg/m2 every 2 weeks with in addition to infusional 5-flurouracil in FOLFURI regimen, or was combined with weekly irinotecan 125 mg/m2 in addition to bolus 5-flurouracil in modified IFL regimen [32]. However, we observed a higher incidence of severe side effects, such as diarrhea and neutropenia, both at 125 and 75 mg/m2 of irinotecan. Notably, the difference between our study and all the above studies is the use of MMC instead of 5-flurouracil. Yet, our pharmacokinetic data showed no significant change in the pharmacokinetics of irinotecan or its metabolites with the addition of celecoxib (Table 7); thus, we have no plausible explanation for this surprising finding. It is possible that double modulation of irinotecan at the cellular level translates into increased toxicity.
In summary, the combination of MMC and irinotecan is feasible and preliminarily active in refractory malignancies. Phase II clinical trials in breast and esophageal/gastroesophageal junction adenocarcinomas at the doses and schedule recommended in this study are under way.
Contributor Information
Y. Xu, The Division of Hematology/Oncology, Departments of Internal Medicine, The Ohio State University College of Medicine and Public Health, Columbus, OH, USA
J. M. Kolesar, The University of Wisconsin, Madison, WI, USA
L. J. Schaaf, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210-1240, USA
R. Drengler, The Cancer Therapy and Research Center, The University of Texas Health Science Center, San Antonio, TX, USA
W. Duan, The Division of Hematology/Oncology, Departments of Internal Medicine, The Ohio State University College of Medicine and Public Health, Columbus, OH, USA
G. Otterson, The Division of Hematology/Oncology, Departments of Internal Medicine, The Ohio State University College of Medicine and Public Health, Columbus, OH, USA
C. Shapiro, The Division of Hematology/Oncology, Departments of Internal Medicine, The Ohio State University College of Medicine and Public Health, Columbus, OH, USA
J. Kuhn, The Cancer Therapy and Research Center, The University of Texas Health Science Center, San Antonio, TX, USA
M. A. Villalona-Calero, Email: miguel.villalona@osumc.edu, The Division of Hematology/Oncology, Departments of Internal Medicine and Pharmacology, The Ohio State University College of Medicine and Public Health, Columbus, OH, USA
References
- 1.Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]
- 2.Shao RG, Cao CX, Zhang H, et al. Replication-medated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA: DNA-PK complexes. EMBO J. 1999;18:1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothenberg ML. Irinotecan (CPT–11): recent developments and future directions—colorectal cancer and beyond. Oncologist. 2001;6:66–80. doi: 10.1634/theoncologist.6-1-66. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13:1841–1851. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]
- 5.Reid RJ, Benedetti P, Bjornsti MA. Yeast as a model organism for studying the actions of DNA topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:289–300. doi: 10.1016/s0167-4781(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 6.Kanzawa F, Sugimoto Y, Minato K, et al. Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res. 1990;50:5919–5924. [PubMed] [Google Scholar]
- 7.Kano Y, Suzuki K, Akutsu M, et al. Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer. 1992;50:604–610. doi: 10.1002/ijc.2910500420. [DOI] [PubMed] [Google Scholar]
- 8.Gobert C, Bracco L, Rossi F, et al. Modulation of DNA topoisomerase I activity by p53. Biochemistry. 1996;35:5778–5786. doi: 10.1021/bi952327w. [DOI] [PubMed] [Google Scholar]
- 9.Cusack JC, Liu R, Baldwin AS. Inducible chemoresistance to 7-Ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-κB activation. Cancer Res. 2000;60:2323–2330. [PubMed] [Google Scholar]
- 10.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Cusack JC, Liu R, Baldwin AS. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Yin MJ, Lin KM, et al. Sulindac inhibits activation of the NF-κB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 13.Yin M, Yamamoto T, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 14.Knuth DW. Bioanalytical method validation: determination of irinotecan (CPT-11) and its SN-38 and APC metabolites in human plasma by isocratic HPLC-FL with protein precipitation and emission wavelength switching. AvTech Laboratories, Inc; 1999. Pharmacia & Upjohn Study Report a0032518, 12 Feb 1999. [Google Scholar]
- 15.Knuth DW. Bioanalytical method validation: Determination of the SN-38 glucuronide metabolite of irinotecan (CPT-11) in human plasma by isocratic HPLC-FL following hydrolysis. AvTech Laboratories, Inc; 1999. Pharmacia & Upjohn Study Report a0032541, 25 Feb 1999. [Google Scholar]
- 16.Method Validation Report Addendum No 7: Evaluation of the Specificity of the HPLC-FL Method for the Determination of CPT-11, SN-38 and APC in Human Plasma for the Coadministered Medication Celecoxib (SC-58635, Celebrex®) and SC-62807 and SC-60613 (2 metabolites of celecoxib). Smart Number CPTAIV-0020-VR1-AD7, 5 March 2003
- 17.Gibaldi M, Perrier D. Pharmacokinetics. 2. Marcel Dekker; New York: 1982. [Google Scholar]
- 18.Dorr RT, Von Hoff DD, editors. Cancer chemotherapy handbook. Norwalk: Appleton & Lange; 1994. p. 2. [Google Scholar]
- 19.Beidler DR, Cheng YC. Camptothecin induction of a time and concentration dependent decrease of topoisomerase I and its implication into camptothecin activity. Mol Pharmacol. 1995;47:907–914. [PubMed] [Google Scholar]
- 20.Rothenberg ML, Meropol NJ, Poplin EA, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol. 2001;19(18):3801–3807. doi: 10.1200/JCO.2001.19.18.3801. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Moore MR, Harker G, et al. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21:807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinine metabolism. Biochem Pharm. 1995;49:127–130. doi: 10.1016/s0006-2952(94)00333-5. [DOI] [PubMed] [Google Scholar]
- 23.Kolesar J, Villalona-Calero M, Eckhardt G, et al. Detection of a point mutation in NQO1 (DT-diaphorase) in a patient with colon cancer. J Natl Caner Inst. 1995;87(13):1022–1024. doi: 10.1093/jnci/87.13.1022-a. [DOI] [PubMed] [Google Scholar]
- 24.Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus MMC plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 study group. J Clin Oncol. 1999;17(5):1413–1424. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- 25.Walters RS, Frye D, Au Buzdar, et al. A randomized trial of two dosage schedules of MMC C in advanced breast carcinoma. Cancer. 1992;69(2):476–481. doi: 10.1002/1097-0142(19920115)69:2<476::aid-cncr2820690234>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu Y, Umezawa S, Hasumi K. A phase II study of combined CPT-11 and mitomycin C in platinum refractory clear cell and mucinous ovarian carcinoma. Ann Acad Med Singapore. 1998;27(5):650–656. [PubMed] [Google Scholar]
- 27.Braun MS, Richman SD, Adlard JW, et al. Association of topoisomerase-1 (Topo1) with the efficacy of chemotherapy in a randomized trial for advanced colorectal cancer patients (FOCUS) J Clin Oncol. 2006;24(18S):10009. [Google Scholar]
- 28.Kohne F, de Greve J, Bokemeyer I, et al. Capecitabine plus irinotecan versus 5-FU/FA/irinotecan ?/1 celecoxib in first line treatment of metastatic colorectal cancer. Safety results of the prospective multicenter EORTC phase III study 40015, ASCO 2005 GI Cancers Symposium; 2005. p. Abstract 3525. [Google Scholar]
- 29.El-Rayes BF, Zalupski MM, Shields AF, et al. A phase II trial of celecoxib, irinotecan and capecitabine in metastatic colorectal cancer. ASCO GI Cancers Symposium; 2005; 2005. p. Abstract 3677. [Google Scholar]
- 30.Lee F, Roach G, Parasher G, et al. Irinotecan, capecitabine and celecoxib (ICC) is an effective palliative regimen for unreectable/metastatic cholangiocarcinoma. ASCO GI Cancers Symposium; 2005. p. Abstract 14830. [Google Scholar]
- 31.Trifan OC, Durham WF, Salazar VS, et al. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62(20):5778–5784. [PubMed] [Google Scholar]
- 32.Fuchs CS, Marshall J, Mitchell EP, et al. Updated results of BICC-C study comparing first-line irinotecan/fluoropymidine combinations ± celecoxib in mCRC: clinical data cut-off September 1, 2006. J Clin Oncol. 2007;25(18S):4027. [Google Scholar]