Just a few years ago, the term pluripotence referred to a characteristic feature of just one type of human cells: human embryonic stem cells (hESCs). Things changed in 2007 when human induced pluripotent stem cells (hiPSCs) burst upon the scene. Derived by introducing transcription factors to reprogram somatic cells to an embryo-like state, hiPSCs are capable of differentiating into any tissue type in the body. (Takahashi et al 2007; Yu et al 2007) Induced pluripotent cells have been hailed as groundbreaking because they offer a much clearer path to disease-based modeling and individualized therapies than older, more genetically homogeneous hESC lines. (Mosher et al 2009) Moreover, some suggest the discovery will “democratize” the field by bringing pluripotent cells within the reach of many labs that have the wherewithal to make use of relatively straightforward reprogramming techniques, but have shied away from the use of more controversial and restricted hESCs. (Matlock 2009; Science 2010)
But what is not clear is how the emergence of new pluripotent technologies and U.S. federal policy impacts the adoption of new lines, the dissemination of existing lines, and the extent to which hiPSCs are supplanting or augmenting established hESC research. Complicating matters, some investigators now suspect that hiPSCs and hESCs are significantly different, issuing calls for research that uses both types of cells, most especially comparative studies. New findings have underscored these distinctions, provoking debate about the possible utility of hiPSCs for disease models and therapies. (Pera, 2011; Pasi et al 2011; Lister et al 2011; Feng et al 2010; Hu et al 2010)
How would we know if reprogramming technologies are beginning to democratize the field by lowering barriers to entry? If hiPSCs are broadening access to pluripotent cell lines, we would expect: (1) Their uptake in published literature to be significantly faster than was the case for more restricted hES lines; (2) A growing amount of research to use hiPSCs without also relying on hESC lines, indicating that the new technology obviates the need for scientists preferring less controversial methods to use embryonic cell lines; (3) Scientists working with hiPSCs would report less apprehension about or difficulty with access to research methods and funding than investigators whose research depends wholly on more restricted hESCs; and (4) Increasing use of hiPSCs would be driven by work done in labs that had not worked with hESCs extensively in the past.
This paper examines assertions 1–4 by mapping the trajectories of hESC and hiPSC technologies. We mobilize data collected from 2,104 hESC and hiPSC publications and brief face-to-face surveys with 118 active researchers (30.9% of 381 poster presenters at the 2010 ISSCR meetings in San Francisco) to examine how hiPSCs have been used in the years immediately following their discovery. These data allow us to evaluate the democratizing effects hiPSCs might exert on the more mature field of hESC research. In prior work, we have shown that the primary research literature can be a rich repository of data on patterns of use of research materials, but the underlying reasons why researchers chose some cell lines over others are not always obvious in publications. (Scott et al 2010, Scott et al 2009, Owen-Smith and McCormick 2006, Aldhous 2010)
We begin to address the related questions of who is using hiPSC and hESC lines and why by combining complementary data on material use and collaboration from an exhaustive census of publications with more suggestive, qualitative data on scientific decision-making. Because hiPSCs are a young technology our conclusions must be treated as preliminary. The field may yet grow in unexpected directions as it matures. Nevertheless, systematic information about how different types of cell lines are being used in publications offers important insights for contemporary legal and policy debates about the legality and scope of federal funding for hESC research. Our findings in this paper strongly suggest that judicial or legislative decisions that bear upon support for human embryonic stem cell research are likely to strongly impact the character and direction of human induced pluripotent stem cell research. In particular a decision that removes or significant curtails federal funding for the former will also have disastrous consequences for the latter because research using the two different types of cell lines is deeply, perhaps inextricably intertwined.
The rise of reprogramming technologies
We begin by tracking the appearance of hiPSCs and hESCs in the published literature in order to consider the speed with which new technologies penetrate the field. If hiPSC are increasing access to pluripotent cell types, we expect to see relatively quick uptake of the new method. Figures 1a and b, which track the number of human pluripotent stem cell publications yearly since 1998, suggests this is the case. From 2007, when the two initial hiPSC papers were published through 2010 we see a rapid rise of both hESC and hiPSC publications. The fast emergence of iPSCs may be attributable to several factors including ease of use and access, therapeutic potential, the entrance of new labs and investigators, and the application of previously optimized hESC culture conditions. The jump could also be due to established hESC scientists’ reactions to 8 years of funding restrictions and the funding uncertainties for hESC lines. The rise in hESC line use may result from the adoption of new lines made eligible for funding under the Obama administration, anticipation of increased federal funding under the new administration, or access to increased sources of state support for hESC research across this time period. (Kamali 2010)
Figure 1.
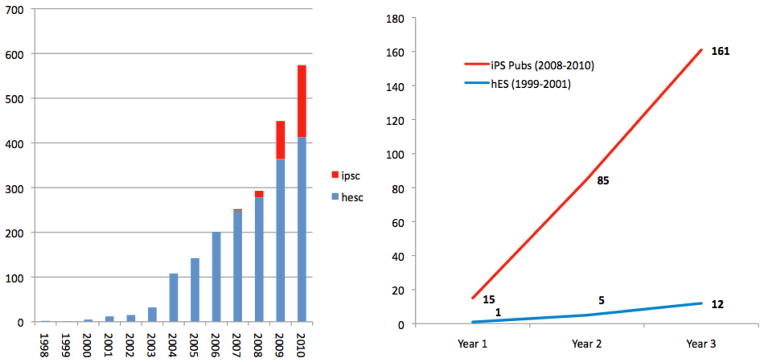
Figure 1a: hESC and hiPSC publication trends, 1998–2010
Figure 1b: Uptake of hESC and hiPSC in publications immediately following initial discovery
While hESC and hiPSC publications are both growing at a rapid pace in recent years, the uptake of induced pluripotent cell methods is much faster than was the case with hESC lines in the years immediately following their discovery. Figure 1b compares yearly rates of hESC and hiPSC publications in the three years immediately following their discovery. We believe these dramatic differences may be due in part to the very different policy environments surrounding these technologies, though we acknowledge the groundwork developed by labs working with hESC lines in the preceding decade. Embryonic stem cell researchers in the late 1990s and early 2000s faced public controversy surrounding the use of frozen embryos to derive new lines, a restrictive political and regulatory environment, and a complicated and expensive process for accessing existing cell lines protected by patent rights. This period marked a shift from the Clinton administration to the second Bush administration and it was not until August 9, 2001 that it was clear that federal funding for hESC research would be forthcoming. The chilling effect of these policies is also clear in Figure 1a, which suggests that it took hESC publications a full six years (until 2003) to surpass the number of publications using hiPSCs after just two years. In broad strokes, it appears that hiPSC technologies are being adopted much more rapidly than human embryonic stem cells were.
hiPSCs and hESCs together and separately
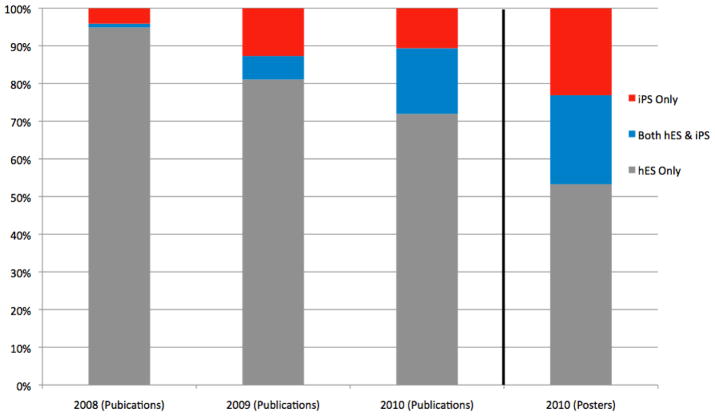
But are hiPSCs complements or substitutes for hESCs? Figure 2 shows the types of pluripotent stem cell lines used in publications from 2008–2010, identifying papers using hiPSCs alone or in concert with hESC lines and papers using hESC lines alone. Our coding scheme used information from the body of the paper as well as supplemental materials to identify the cell lines used in a particular article. We also coded the cell lines used in 381 research posters presented at the June 2010 International Society of Stem Cell Research (ISSCR) meeting held in San Francisco, CA. If hiPSCs are offering an alternative means for investigators to enter the field without having to work with embryonic cell lines, we expect to see a growing proportion of papers using induced pluripotent stem cells alone. However, there are a number of arguments suggesting that an emerging scientific consensus may lead to investigators pairing new hiPSC cells with gold standard hESCs. (Vogel 2010) Figure 2 divides publications in each year into three categories.
Figure 2.
Proportion of research by type of cell lines used, 2008–2010
The proportion of papers using hiPSC and hESC together is growing faster than those using hiPSC alone. In 2008 just 5.1% (15) of all the papers we observed used any induced pluripotent cell lines and only 20% (3) of those combined hESC and hiPSC in the same piece of research. By 2010, 26.5% (158 of 595) of all pluripotent cell papers used hiPSC technologies, but 62.0% (98 of 158) of those paired induced and embryonic cell lines. While induced pluripotent stem cells are quickly becoming an important part of the field, they do not appear to be replacing work using embryonic cell lines. Instead the two types of cells are increasingly used together. Figure 2 suggests that hiPSCs may provide a limited avenue for new investigators to enter the field if established hESC researchers come to dominate in publications using the new technique. On the other hand, we could be witnessing a shakeout period for hiPSCs as experienced researchers probe and contrast the true utility of a possible eclipsing technology.
Access and utility in materials choice
In order to probe the real reasons investigators choose to use essential materials, we surveyed 118 researchers who presented pluripotent stem cell posters at the ISSCR meeting. We scored and categorized the surveys based on major themes that characterize the field. Utility of materials scored comments about whether the lines were used because they were convenient, because they were facile in the lab, or were the best choice to derive a differentiated type of cell. In this category we included whether lines were used because they are well characterized, important for comparative studies, or useful for potential therapies. Access to specific materials scored comments on whether the researcher was instructed to use a certain line, whether it was already in use, came from a collaborator and if cost, intellectual property, or funding eligibility played a role in obtaining the line. We also asked researchers whether private, federal, national/federal, or state agencies underwrote their projects, and how policy had impacted their choice of careers, collaborators, workplace, and materials.
Researchers using hESC alone, iPSC alone, or both cell types in combination talked about access to research materials and the scientific utility of different materials about the same amount. (Table S1 Supplemental) However, qualitative coding of their actual comments revealed significant differences in how investigators on each type of paper discussed the challenges of getting the research materials they needed and the usefulness of those materials for specific scientific and technical purposes.
Access
Investigators using only hESCs manifested the most complicated thinking about access to research materials. Perhaps because embryonic lines are the core of their research programs they 1) evinced much more concern about access to federal funding and worries about the eventual disposition of particular lines under the Obama policy, 2) expressed greater reliance on state funding as an alternative to federal funding, and 3) described a much more diverse set of routes to access specific lines.
Scientists described how regulatory uncertainty and funding dilemmas could impede their research. A California researcher who relied on a specific hESC line said, “Bottom line is the ability to work with them. CIRM [California Institute for Regenerative Medicine] gives me confidence because I know I’m funded. I’m glad I’m in California.” The vagaries of federal regulation caused one New Jersey researcher to derive a new line for NIH approval when state funding was dropped for a project using H9. After a nine-month delay, H9 was eventually approved under new NIH guidelines. Another scientist remarked that federal policy has “a huge impact on the study, as our investigation had to give up NIH funding to work on the cell lines.”
Geography also played a role. One US investigator moved to Belgium in order to derive new lines “I work on methods for improved derivation of hESC lines. We created 15 new lines in the process of [our] investigation.” Researchers working in other countries remarked that either their ability to derive and use new lines was not encumbered by restrictions (Sweden) or was handicapped in some fashion (Germany).
Researchers accessed hESC lines in many ways. Some purchased them from companies while others obtained them from collaborators. Others continued to use specific lines because they were repeating previous work or because they were assigned to an ongoing project. Banking, core facilities and other repositories also played a role. One researcher remarked, “I needed a line and I learned I could get them across the street at the New York Stem Cell Foundation. They gave a vial to me the same day.”
In contrast, scientists using only iPSCs talked about access in response to our direct questions but their remarks suggested a largely unproblematic decision: in other words, they could easily derive new lines. Most concerns were whether to derive lines specific to the needs of the lab, or to obtain them from collaborators. When investigators volunteered information about the source of somatic cells used for reprogramming, they generally reported using easily accessible tissue banks, with attendant perceptions of a lower regulatory threshold. There were very few mentions of funding worries and none of policy restrictions. Investigators were split about evenly across those who derived their own lines and those who requested materials from other labs.
Researchers who used both types of pluripotent cell lines declared less concern with federal funding and access than those using hESCs only, but more than those who focused only on iPSC. Uncertainty introduced by changes in federal funding rules under the Obama administration and state alternatives to NIH research support were of little concern. We attribute this to the fact that many of the combinatorial experiments featured oft-used federally approved lines, in particular, H1, H7, and H9. In some cases, laboratories in this category were experienced hESC users. Comparative studies were thus a logical next step: “H1, H7, and H9 were already in the [lab],” one researcher said. “…so we used the three lines as controls.”
While these survey respondents invoked multiple means to get access to hESC lines, they focused more on informal routes and the legacies of past research. Notably, when asked about access, researchers who used both types of lines nearly unanimously replied by describing how they obtained their hESC lines.
Utility
We were struck by how often discussions of the usefulness and versatility of particular cell lines centered on differences among small number of well used lines. In the 38 interviews with researchers using only hESC lines where utility was an important theme, 25 (65.8%) mentioned the H1, H7, or H9 lines explicitly. Other mentions of well-characterized lines included those derived at Harvard University, HUES1, 2, 7, and 9, and the Singaporean lines HES 2 and 4. Discussions about the utility of particular research materials thus hinged not on identifying the best lines for a particular problem, but on determining which among a small set of available lines was the most satisfactory. Investigators using only hESCs evinced different conceptions of utility. Particular lines were considered valuable because they were known quantities (in scientific and regulatory terms), and thus were valuable as references in experiments that used or derived new lines. “We wanted to use H1 because they’re less likely to spontaneously differentiate,” a researcher noted. Interestingly, when investigators talk about the scientific power of particular hESC lines their discussions revolve around their ability to differentiate easily and reliably into downstream cell types (e.g. cardiac, blood, neuronal). In only three cases were specific diseases mentioned (heart, cancer, pancreas). For example, one investigator stated, “Our project was federally funded so we chose H9, and it has neurogenic ability.” A second remarked, “We chose HUES7 because of its high endogenous level of HMG A/AMGB.”
Overwhelmingly, investigators using only iPSCs picked the cells because of the scientific excitement surrounding the technology, for their ease of derivation, and most importantly, for their therapeutic usefulness. This researcher summed it up: “[We use iPSC] because it is an exciting new technique and it’s fascinating to figure out potential therapeutic implications. And, it’s a powerful comparison to what we know about hESCs.” Descriptions were framed in reference to particular diseases or markers for specific patient populations, or because the disease-based tissue banks were readily available. Somatic cells were sourced in the US, Africa, Italy, Australia, Finland, and Germany, and diseases and disorders included autism, cardiac disease, lysosomal storage disease, HIV, TB, and liver failure, suggesting a broad genetic diversity of lines.
For researchers using both types of lines, utility was framed in terms of comparing iPSCs to the best-known qualities of hESCs. Only one researcher noted disease as a study aim, and then only in the context of obtaining patient-specific lines. Because many of these labs had already studied hESCs, it was only natural that comparative work would follow. “It’s always good to use at least H9 as confirmation,” noted one researcher. Another said, “H9 is the standard. It’s needed for a positive control.” One scientist noted the conspicuous presence of hESC controls in the literature: “…we haven’t seen papers on iPSCs without comparison to ES lines.”
Co-authorship and collaboration
Content coding of materials used in hESC and hiPSC publications and interviews with stem cell researchers offer mixed support for the idea that hiPSC technologies are democratizing in the sense that they lower barriers to entry into the field by broadening access to pluripotent human stem cell lines. In this final section, we turn to analysis of co-authorship networks to determine how deeply embedded senior researchers who use hiPSC technologies are in the established field of hESC research. We treat the last authors on publications as senior representatives of labs.i Co-authorship is a key relationship defining scientific disciplines and fields (Moody 2004; Newman 2001), and often encompasses sharing of materials, expertise, goals, and sometimes students and fellows. In the aggregate, repeated patterns of co-authorship create a network structure that includes the great majority (90.77%) of scientists who have published using hESC and hiPSC lines.
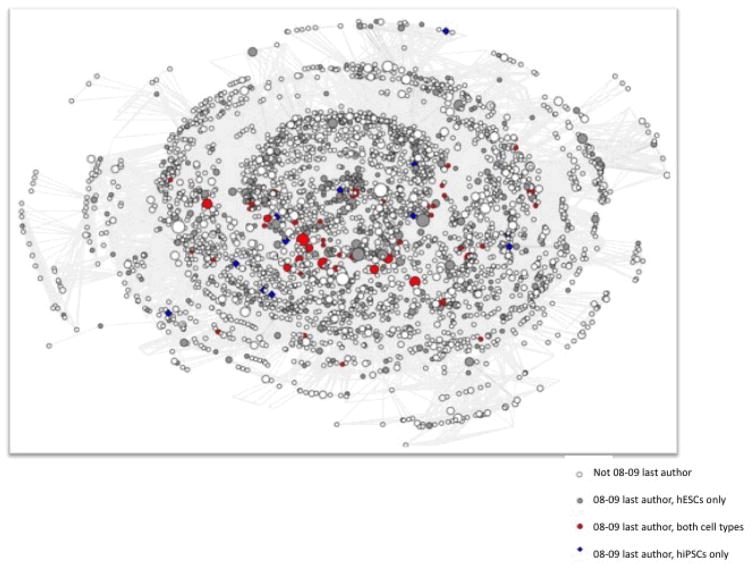
We present two network figures that examine the penetration of hiPSC research into the field of hESC science focusing particularly on the work done by more senior scientists who are listed as last author on pluripotent stem cell publications. Figure 3 examines senior scientists’ use of hiPSC techniques in recent publications by locating investigators who were last authors on 2008 and 2009 publications in the context of the aggregate (1998–2010) collaboration network. In this image, nodes represent authors (in any position) on any stem cell paper published between 1998–2010. Ties represent co-authorship on one or more papers. We color code nodes to highlight the activities of last (senior) authors on papers published in 2008 and 2009. All nodes are sized to reflect the number of papers that an author published in 2008–2009. Large white nodes represent first and middle authors from 2008–2009 publications. Small white nodes overwhelmingly represent authors on pre-2008 stem cell publications who did not publish new articles between 2008–2009. The dark gray nodes in this figure thus represent senior authors on papers that used hESC lines only. Red nodes represent senior authors on papers that used both types of lines, and blue nodes represent senior authors on papers that used only hiPSC lines.
Figure 3. Human Pluripotent Stem Cell Co-Authorship Network, 1998–2009.
This network image represents co-authorship connections among 5004 authors on publications using hES or hIPS cells from 1998–2009. 509 (9.23%, N=S513) authors are not connected to the primary network component and are not represented. Node size is proportional to the total number of hES and hiPS articles each author published in 2008–2009.
To concretize matters, consider Harvard University’s George Daley, who published 12 human pluripotent stem cell papers during this time. Six of those listed him as last author. Daley is a rare senior author in our database in that during 2008–2009 he published papers using hESC alone, hiPSC alone, and both cell types. We thus code him as a last author who has used both hESC and hiPSC. Daley appears in our network images as a large, red ellipse. Finally, the relative position of nodes in these images is meaningful. The network drawings are optimized using a pair of ‘spring embedder’ algorithms that use the overall connectivity of a network system to establish the Euclidean distance among nodes. (Fruchterman & Reingold 1991; Kamada & Kawai 1989) Thus, a position in the outer ring of the image represents a collaboration profile that has few ties into the most connected portions of the field. Likewise, scientists who are positioned close together in this figure are proximate because they are direct collaborators or they share co-authors in common, creating relatively short indirect network paths between them.
Figure 3 represents the core of the hESC field, the relative position of blue and red scientists and the relative size of their nodes, and offers useful insights into the ways in which the rise of hiPSC research has emerged from an established field constrained by significant legal uncertainty, regulatory restrictions, and ethical concerns. If hiPSCs are a widely disseminated technology that allow newcomers to enter the field without recourse to prior working relationships with established hESC investigators, we would expect to see: (1) more blue nodes than red nodes, indicating that a large portion of senior authors using hiPSCs instead of hESCs; (2) red and blue nodes smaller than gray nodes, indicating that those researchers using hiPSCs alone or in concert with hESCs are newer, less prolific investigators; and (3) red and blue nodes nearer the periphery of the image than its center, indicating that senior investigators using hiPSCs are relatively less well connected to the researchers at the established core of the hESC network.
Examining Figure 3 suggests that none of these expectations are born out. Red nodes outnumber blue nodes (55 senior investigators published papers using both hESC and hiPSC in 2008–2009 as compared with 14 senior investigators who published papers using hiPSC alone). Many red nodes are large, representing relatively prolific authors. Blue nodes in contrast tend to be small, suggesting that the last authors on hiPSC only papers are relatively junior in the larger pluripotent field or are senior investigators in allied stem cell disciplines who are experimenting with reprogramming. Finally, while red and blue nodes appear spread across the network image in Figure 3, the majority of senior authors using hiPSCs are clustered relatively tightly in a band of large red nodes that crosses the lower left quadrant of the figure. In other words, many of the investigators using hiPSC are established, prolific hESC scientists who are connected to each other directly or through relatively short indirect paths defined by shared co-authors. Such close indirect connections happen with relative frequency as senior investigators hire post-doctoral fellows from pools of newly minted Ph.D.s trained in the labs of other established PIs, or as departing post-docs carry past collaborations with them to new faculty positions.
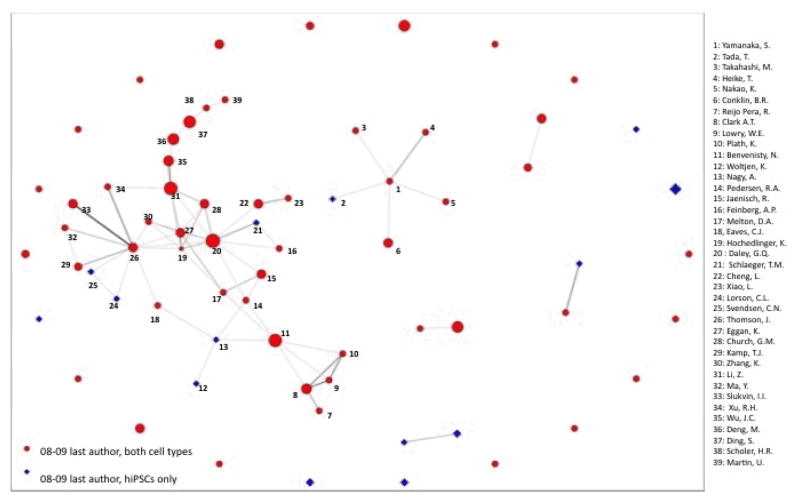
Figure 4 delves deeper into the relationships between senior hiPSC authors by extracting all 69 red and blue nodes from Figure 3 and re-optimizing them so the constellations of co-authorship among senior authors become clear. Figure 4 suggests that the clustering of red nodes in the full network is largely a result of the large set of 33 (47.83%) senior stem cell investigators connected by past and current collaborations. This cluster contains some of the most prominent scientists working with hiPSC and hESC and centers on Harvard and the University of Wisconsin. It also contains 5 of the 14 (35.7%) senior PIs who have published as last authors using hiPSC alone. In other words, the most significant cluster of established hESC researchers using hiPSC is also the source of more than 1/3 of the senior authors who are deploying hiPSC lines alone. Indeed, Figure 4 suggests an even split between hiPSC only senior authors (blue diamonds) unconnected to red nodes representing senior authors who have used hESC and hiPSC together and those who have such connections.
Figure 4. Last Authors using hIPSC, 2008–2009.
This network image represents co-authorship connections among the 69 last authors working with hiPSCs in 2008–09. 33 (47.33%) last authors are indirectly connected to each other by prior co-authorship ties in the network component to the left of the figure. That component is dominated by researchers at Harvard and the University of Wisconsin. Six other last authors are connected in the “star” centered on Shinya Yamanaka near the center of the figure. 22 hiPSC last authors (31.88%, arrayed in a circle) are part of the larger co-authorship network in Fig3, but have nut co-authored with another hiPSC last author in our data. Members of the two largest network components are labeled.
Conclusions & Implications
In conclusion, the assertion that new reprogramming technologies are engaging new investigators and reorienting the field presents a mixed picture. It is clear that iPSCs are not eclipsing hESCs but have emerged as a complimentary technology. Though use of iPSCs is increasing at a rate greater than the first years after the Thomson discovery, we believe this is due to lower regulatory thresholds, less ethical worry, increased access, and scientific excitement. In terms of who uses lines, iPSCs may not be as democratizing as recent portrayals suggest. A large proportion of early hiPSC adopters are members of the hESC establishment. This suggests three things. First, that the technology may be facile, but not so facile that a flood of new investigators is entering the pluripotent field. Experience with embryonic stem cells appears to transfer to work using induced pluripotent lines. Second, the incentives to use both types of cells in comparative studies are high. Finally, the furious activity we observe on the part of senior hESC researchers may be motivated by scientific curiosity and a dedication to pragmatic choices in uncertain funding environments. Stem cell researchers see themselves entering a new era with pluripotence, not cell type, at the center and it is only natural that experience in establishing primary cultures and deriving downstream cell types gives these labs an advantage no matter what cell enters the scene. That reprogrammed cells may hold the answers to personalized medicine or unlock the mysteries of human disease is important, but comprise a different set of objectives.
On August 23, 2010, a Washington D.C. district judge, Royce Lamberth, issued a preliminary injunction blocking Barak Obama’s 2009 executive order expanding funding for hESC research. The plaintiffs, including two adult stem cell researchers, The Christian Medical Association, and the Nightlight Christian Adoptions (an embryo “adoption” agency) sued on the basis that the two scientist’s chances for federal funding were reduced when monies were spent on hESC research. The merits of the case, in Lamberth’s view, turned on the Dickey-Wicker amendment, which has been added to the yearly HHS appropriations bill since 1996. Dickey-Wicker prohibits HHS from funding research that destroys human embryos. During that time, congress and three administrations agreed that funds could be used for research projects that did not destroy embryos. In a stunning reversal of this longstanding agreement, Lamberth held that federal funding violates Dickey-Wicker. His injunction stopped new federal funding hESC research and threw over a decade of work on human pluripotent stem cells into doubt.
A month later, a three-judge federal appeals panel stayed the injunction while it considered an appeal by the Obama administration. On April 29th it reversed Lamberth’s ruling, concluding that “the plaintiffs are unlikely to prevail because Dickey-Wicker is ambiguous and the NIH seems reasonably to have concluded that, although Dickey-Wicker bars funding for the destructive act of deriving an [h]ESC from an embryo, it does not prohibit funding a research project in which an [h]ESC will be used.” (Sherely v Sebelius 2011) Lamberth now has cross-motions for summary judgment in front of him, one by the plaintiffs, and one by the defendants, the US government. He granted the injunction based on the likelihood that the plaintiffs would prevail. Three judges appointed by conservative Republican presidents decided, 2 to 1, to allow this research. However, Lamberth might again buck legal wisdom and maintain his original position. Or, he could rule on one of the judgments and either allow federal funding for hESC research or ban it outright.
The deeper implications of a federal ban or restrictions on hESC research are largely missing from the policy discussions surrounding the Lamberth decision. We now have new evidence showing the real possibility of collateral damage that could be caused by ill conceived and politically motivated policy prescriptions. Restrictions, regulatory uncertainty, and spurious court decisions have undoubtedly retarded progress in the pluripotent stem cell field. Now, an entirely new technology, forged out the crucible of political controversy, is at risk. A major finding from this study is that iPSC and hESC are deeply intertwined and interdependent technologies. We see a decade of research using human embryonic cell types carrying the new wave of reprogramming technologies. And, while human embryonic stem cell research has made great strides over this time, our lack of understanding of early human development cannot be overestimated. Unraveling the properties of the human embryo has broad consequences for both regenerative medicine and assisted reproductive technologies. The growing and significant number of comparative studies and experiments using hESCs, combined with the heavy use of iPSCs by senior hESC investigators suggest that any federal policy that would deny funding for embryonic stem cell research would torpedo a nascent and exciting discovery that is propelling new directions in the biological sciences. Indeed, just as political debate draws artificial boundaries between adult and embryonic cell types, it is dangerous to assume the same divisions can be made for pluripotent cell types. The secrets of cells know no boundaries.
Supplementary Material
Acknowledgments
We thank the scientists who spoke with us, and J. Ostergren, R.J. Vann, L. Ooi, and M. Feret for help with data collection and research assistance. C.T.S. was supported by a US National Science Foundation (NSF) grant (SBE-0949708) and The Stanford Institute for Stem Cell Biology and Regenerative Medicine. J.B.M was supported by an NSF grant (SBE 0949708) and NIH National Center for Research Resources (UL1 RR024150-4). J.O.-S. was supported by NSF grants (SBE-0949708 and SES-0545634).
Footnotes
We do not account for the possibility that joint senior authorship driven by multi-lab collaborations may be represented by shared corresponding authorship in these images.
References
- Aldhous P. New Scientist. 2010;16:24. [Google Scholar]
- Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim KS, Lanza R. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Fruchterman TMJ, Reingold EM. Software-Practice & Experience. 1991;21:1129–1164. [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Proceedings of the National Academy of Sciences. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada T, Kawai S. Information Processing Letters. 1989;31:7–15. [Google Scholar]
- Karmali RN, Jones NM, Levine AD. Nature Biotechnology. 2010;28:1246–1248. doi: 10.1038/nbt1210-1246. [DOI] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, et al. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlock T. The league of extraordinary biologists. Boston Magazine. 09 Jun 23; [Google Scholar]
- Moody J. American Sociological Review. 2004;68:213–238. [Google Scholar]
- Newman MEJ. Proceedings of the National Academy of Sciences. 2010;98:404–409. [Google Scholar]
- Owen-Smith J, McCormick JB. Nature Biotechnology. 2006;24:391–392. doi: 10.1038/nbt0406-391. [DOI] [PubMed] [Google Scholar]
- Pasi CE, Dereli-Oz A, Negrini S, Friedli M, Fragola G, Lombardo A, Van Houwe G, Naldini L, Casola S, Testa G, Trono D, Pelicci PG, Halazonetis TD. Cell Death and Differentiation. 2011:1–9. doi: 10.1038/cdd.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- Science. Science. 2010;328:21. [Google Scholar]
- Scott CT, McCormick JB, DeRouen MC, Owen-Smith J. Nature Methods. 2010;7:866–867. doi: 10.1038/nmeth1110-866. [DOI] [PubMed] [Google Scholar]
- Scott CT, McCormick JB, Owen-Smith J. Nature Biotechology. 2009;27:696–697. doi: 10.1038/nbt0809-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley v. Sibelius, No. 10-5287. United States Court of Appeals dec. April 29, 2011.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Vogel G. Science. 2010;327:1191. doi: 10.1126/science.327.5970.1191. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.