Abstract
Background
Previous studies suggest that the antioxidants vitamins C and E may protect against development of knee OA. We examined the association of circulating levels of vitamin C and E with incident whole knee radiographic osteoarthritis (WKROA).
Methods
We performed a nested case-control study of incident WKROA in MOST, a cohort of 3026 men and women aged 50-79 years with, or at high risk of, knee OA. Incident cases were knees without either tibiofemoral (TF) or patellofemoral (PF) OA at baseline that developed TF and/or PF OA by 30 month follow-up. Two control knees per case were selected from those eligible for WKROA that did not develop it. Vitamin C and E (alpha-tocopherol) assays were done on baseline supernatant plasma (PCA) and serum samples, respectively. We examined the association of gender-specific tertiles of vitamin C and E with incident WKROA using logistic regression with GEE, adjusting for age, gender, and obesity.
Results
Subjects without WKROA at baseline who were in the highest tertile of vitamin C had a higher incidence of WKROA [adjusted OR= 2.20 (95% CI: 1.12-4.33); p-value= 0.021], with similar results for the highest tertile of vitamin E [adjusted OR= 1.89 (1.02-3.50); p-value= 0.042]], compared to those in the lowest tertiles. P-values for the trend of vitamin C and E tertiles and incident WKROA were 0.019 and 0.030, respectively.
Conclusions
Higher levels of circulating vitamin C and E did not provide protection against incident radiographic knee OA, and may be associated with an increased risk of knee OA.
Keywords: knee osteoarthritis, antioxidants, vitamin C, vitamin E
Background
Antioxidants have been investigated for their protective effect in several chronic diseases. Vitamin C is a water soluble vitamin with antioxidant properties. Its role as an antioxidant has been investigated in association with cardiovascular disease (1), lipid profiles (1) and cancer prevention (2). The antioxidant vitamin E (present in the forms alpha-, beta- delta-, and gamma-tocopherol), has also been studied in the prevention of cardiovascular diseases, (3) and cancer(4) and for its effects on bone turnover markers in osteoporosis(5).
Both vitamin C and E have also been considered for a potential role in protecting joints from the development and progression of osteoarthritis. A cross-sectional study in patients with knee OA found that those with severe OA had a decreased quantity of reactive oxygen species scavengers in their joint fluid compared to patients with no knee OA (6), suggesting a possible role for reactive oxygen species in the development of OA and for antioxidants in preventing it. Vitamin C is also an essential nutrient for the biosynthesis of collagen matrix components (7). In studies of animal cartilage explants, vitamin C has been shown to be chondroprotective; supplementation of goat articular chondrocytes with vitamin C reduced morphological degeneration of these chondrocytes by static loading (7). Similarly, vitamin C was found to stimulate collagen synthesis in a study of guinea pig cartilage (8). However, not all animal studies suggest a protective effect of vitamin C in OA. A recent study in Hartley guinea pigs found that exposure to increased levels of vitamin C worsened the severity of incident OA(9) .
In epidemiological studies, a higher dietary intake of vitamin C, but not vitamin E, was associated with reduced structural progression of knee OA (10), while dietary intake of these micronutrients was not associated with the incidence of radiographic knee OA. A study of healthy adults found that subchondral bone marrow lesions in the knee measured by MRI, which may be involved in the pathogenesis of OA, were less common in those with higher dietary intake of vitamin C (11), but there was no association with cartilage defects or cartilage volume nor of dietary intake of vitamin E with any MRI measure. These studies estimated dietary consumption of antioxidants from food frequency questionnaires (FFQ), a method prone to misclassification of micronutrient intake (12). Dietary vitamin C intake data show great variability and plasma levels may better reflect long term vitamin C status (12). For Vitamin E, the alpha-tocopherol isoform has been shown to be reasonably estimated by the FFQ method (13, 14). Two population studies have examined the cross-sectional association of serum tocopherol (Vitamin E) levels with prevalent knee OA, with inconsistent results that varied by tocopherol isoform (15) (16) and gender (15). Finally, a randomized trial in individuals with clinical knee OA found no effect of daily vitamin E supplementation (500 mg) on cartilage loss assessed by MRI or on symptoms over two years(17).
In this study we examined the association of baseline plasma levels of vitamin C and serum levels of vitamin E with incident whole knee radiographic OA (WKROA) during 30 months of follow-up using data from the Multicenter Osteoarthritis (MOST) Study.
Methods
Study Subjects
We performed a nested case-control study of incident knee OA among subjects in MOST, a prospective cohort study of 3,026 persons aged 50 to 79 years of age who have knee OA or are at high-risk for knee OA. Individuals at high risk include were overweight, had knee pain, aching or stiffness on most of the last 30 days, or had a history of knee surgery or knee injury that made it difficult to walk for at least one week (18). Subjects were recruited from two U.S communities, Birmingham, Alabama and Iowa City, Iowa through mass mailings of letters and study brochures, supplemented by media and community outreach campaigns; study protocols were approved by Institutional Review Boards at all participating institutions. Persons who screened positive for rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis (19), renal insufficiency requiring dialysis, a history of cancer, bilateral knee replacement surgery, inability to walk without assistance, or plans to leave the study area were excluded (18).
Radiographic Methods
Weight-bearing PA fixed flexion and lateral view knee radiographs were obtained at the baseline and 30 month visit using a plexiglass positioning frame (SynaFlexer TM) (20). A musculoskeletal radiologist and a rheumatologist experienced in evaluating knee radiographs scored paired radiographs for Kellgren-Lawrence (K-L) grade and medial and lateral tibiofemoral (TF) and patellofemoral (PF) JSN (0-3) (21). If readers disagreed on the presence or incidence of TF or PF OA in a knee at any time-point, or worsening of JSN (TF or PF) between time-points, final determinations were made by an adjudication panel of three readers (22). Weighted kappas for agreement between the two readers were as follows: K-L grade= 0.79; medial TF compartment JSN= 0.81; lateral TF compartment JSN= 0.86; PF JSN = 0.75. The kappa for PF OA (yes/no) was 0.80 and for TF OA yes/no) was 0.83.
Definition of Whole Knee Radiographic OA (WKROA)
Whole knee ROA was considered present when a knee had either TF OA, defined as a K-L grade ≥ 2, and/or the presence of PF OA, defined as 1) a PF osteophyte grade ≥ 2 or 2) as a PF osteophyte grade ≥ 1 plus one or more of PF JSN ≥ 2 or sclerosis ≥ 2 or cysts ≥ 2 (23). Incident WKROA was defined as a knee that developed WKROA at the 30 month follow-up that did not have it at baseline. Incident TFROA and incident PFROA were defined as knees without prevalent TF or PF ROA, respectively, at baseline that developed it at follow-up.
Selection of Subjects for Vitamin C and E Assays
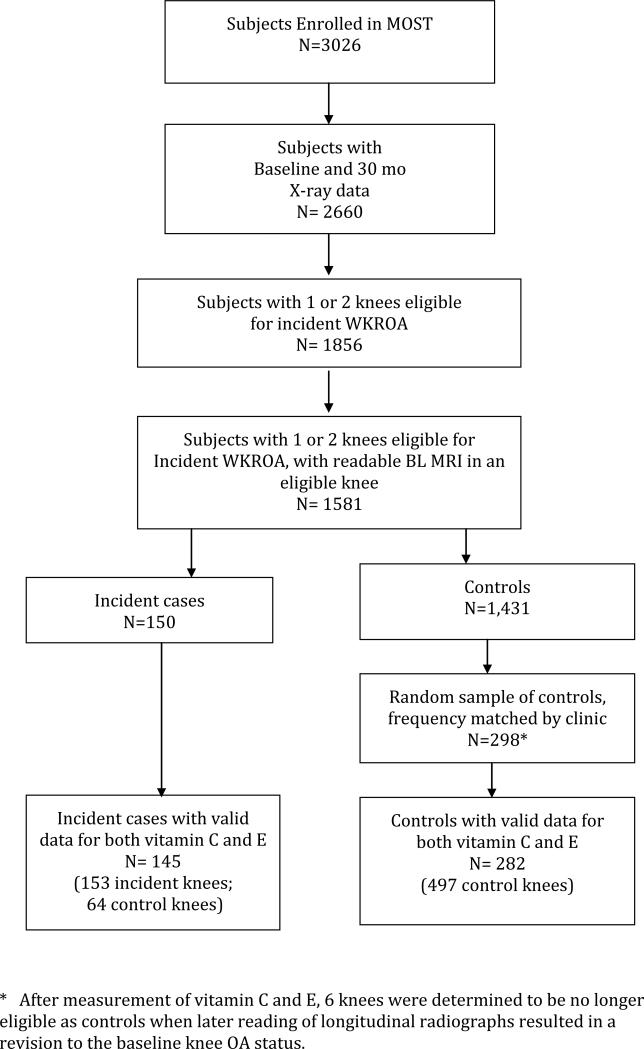
To be eligible for the nested case-control study of vitamin C and E and other nutritional assays subjects had to have 30 month follow-up radiographic outcome data, 1 or 2 knees eligible for incident WKROA and a readable baseline MRI and (n=1,581) (Figure 1). Although not the focus of this report, baseline MRIs were required for parallel studies of nutritional and MRI-derived predictors of incident WKROA. From among subjects eligible for incident WKROA in one or both knees, we included as cases all subjects who developed incident WKROA in at least one knee (n= 150) and randomly selected approximately 2 control subjects per case (n=298), frequency matched by clinic site, from subjects eligible for incident WKROA who did not develop it. Valid vitamin C and E assay results were available for 145 (97%) incident cases and 282 (95%) controls.
Figure 1.
* After measurement of vitamin C and E, 6 knees were determined to be no longer eligible as controls when later reading of longitudinal radiographs resulted in a revision to the baseline knee OA status.
Vitamin C and E Assay Methods
Blood samples for vitamin C and E assays were collected at the baseline visit after an overnight fast. Both a plasma/PCA sample for vitamin C, using light-protected processing tubes, and a serum sample for vitamin E were processed immediately and stored at −70 degrees before being shipped to Tufts-USDA Human Nutrition Research Center on Aging (Boston, MA) where the samples were again stored at −70 degrees. Sample concentrations of vitamin C and E (alpha-tocopherol only) were then measured using HPLC assays (Waters Associates, Inc.). (24) (25). Assays were run with subjects grouped in several separate batches, each sent to the laboratory at different times. The ratio of incident knee OA cases and controls in each batch was 1 to 2.
The intraassay CV for vitamin C was 6.0%., and the interasay CV was 4.5% .The normal range for vitamin C was considered to be 0.4 – 2.20 mg/dL, similar to normal ranges reported in other published studies (26). The intraassay CV for assays for serum vitamin E was 5.6%, and the interassay CV was 3.5%. The normal range for vitamin E was considered to be 500 - 1800 ug/dL, also similar to published ranges (27). There were no significant differences in mean vitamin C (p-value = 0.40) or E (p-value = 0.58) levels between different batches sent at different times. The median batch values for the four batches of Vitamin C (1.326, 1.308, 1.310,1.269) and vitamin E (1521.5, 1490.3, 1681.7, 1469.5) were all similar. All assays were run in duplicate.
Other Baseline Measures
Prescribed and over-the-counter medication and micronutrient supplement use within the previous 30 days of the baseline visit was collected by self-report and inventory of participant-supplied medication bottles. Current use and dose of supplements for vitamin C and vitamin E were also assessed by direct question. The modified self-report Charlson comorbidity questionnaire was collected at baseline and the Charlson comorbidity index was calculated (28). Standing height (measured by Harpenden Stadiometer) and weight were measured at baseline and used to calculate the BMI (29). Serum levels of 25-hydroxy vitamin D were measured using commercially available radioimmunoassay kits (DiaSorin, Stillwater, Minn), serum vitamin A using HPLC assays (Waters Associates, Inc.) and plasma vitamin K using HPLC assays (40).
Statistical Analysis
Ranges and sex-specific tertile cutpoints for baseline vitamin C and E levels were determined for men and women separately as previous studies indicate that these levels may vary by sex (30), (14).
We performed knee-level analyses of incident WKROA using all knees eligible for incident WKROA in both cases and controls. Among the subjects with vitamin C and E data, there were 153 incident WKROA knees in 145 subjects and 561 knees without WKROA at both baseline and follow-up in all 427 subjects. We performed analyses separately in subjects who had both knees eligible for incident WKROA (n= 287) and in those who already had unilateral WKROA at baseline (n=140).
Logistic regression with GEE to adjust for a subject's potential inter-knee correlation (Statistical Analysis System PROC GENMOD, Version 9.2) was used to determine the association of sex-specific tertiles of vitamin C and E with incident OA, adjusting for sex, age, BMI, nonwhite race, and clinic site and in a separate analysis mutually adjusting for vitamin C and E tertiles in a single model. Tests for trend used the sex-specific median vitamin C and E values for each tertile. We tested for gender by vitamin C and E level interactions and performed stratified analyses when there were significant interactions.
We performed several sensitivity analyses. We evaluated the effects of adding additional individual covariates to the logistic models, including use at baseline (yes/no) of vitamin C, vitamin E, vitamin D and multivitamin supplements, the dose of vitamin C, dietary intake of vitamin E supplements, glucosamine/chondroitin supplement use, the season in which blood samples were obtained, Vitamin C and E batch number, the subject's fasting status at the time of phlebotomy, education level, Charlson co-morbidity score, smoking status and the presence of baseline frequent knee pain. We also analyzed our results adjusting for circulating blood levels of vitamin A, vitamin K and vitamin D. We conducted a separate analysis excluding all vitamin C and E supplement users. Finally, we performed key analyses using incident TF OA (K-L grade ≥2) as the outcome.
We evaluated the possible effects of selection bias in the samples of incident cases and controls from each clinical site that might result from the requirement to have readable baseline MRIs. For this we re-estimated the key results using a weighted logistic regression implemented by invoking the “weight” option in PROC GENMOD, with the weights based on the clinic-specific probabilities of inclusion of incident WKROA cases and controls as a proportion of the total number of cases and controls at the clinic. Results were similar to the unweighted regression analysis and are not reported here.
Results
Subject Characteristics
Baseline characteristics of subjects with incident WKROA and controls were similar, except that the incident cases had a higher average BMI and a greater percentage took glucosamine supplements, were more likely to have unilateral WKROA at baseline and to have frequent knee pain in one or both knees (Table 1). Among knees that developed incident WKROA, 82% of these knees developed incident TF ROA only, and 14% developed incident PF ROA only, and 4% developed ROA in both joints.
Table 1.
Baseline characteristics of participants by incident whole knee radiographic OA (ROA) status at follow-up
| Incident Whole Knee ROA Sample | ||
|---|---|---|
| Subjects with Incident Case knees (n= 145) | Subjects without Incident Case knees (n= 282) | |
| Age, mean (SD) | 61.7 (7.7) | 61.5 (7.7) |
| Sex (%Female) | 88 (61%) | 166 (59%) |
| BMI, mean (SD) | 30.9 (5.3) | 28.7 (4.4)* |
| Caucasian (%) | 117 (81%) | 244 (87%) |
| Clinic (% UIowa) | 59 (41%) | 121 (43%) |
| Vitamin C level (mg/dL) (SD) | 1.37 (0.62) | 1.31(0.54) |
| Vitamin E level (ug/dL) (SD) | 1671.9 (762.6) | 1691.7(735.0) |
| Chondroitin Use(%) | 28 (19%) | 41 (15%) |
| Glucosamine Use(%) | 42 (29%) | 54(19%)* |
| Vitamin C supplementation (%) | 31 (21%) | 67 (24%) |
| Vitamin E supplementation (%) | 49 (34%) | 87 (31%) |
| Multivitamin supplementation (%) | 78 (54%) | 153 (54%) |
| Any vitamin supplements reported (%) | 104 (72%) | 205 (73%) |
| PASE activity score (SD) | 182.9 (88.2) | 185.6 (94.9) |
| Baseline Charlson co- morbidity score | 0.36 (0.78) | 0.55 (1.07) |
| Any comorbidity (%) | 34 (23%) | 84 (30%) |
| Subjects with unilateral knee ROA at baseline (%) | 73 (50%) | 67 (23%)* |
| Subjects with frequent knee pain in either or both knees | 72 (50%) | 96 (34%)* |
| Subjects with knee ROA or frequent knee pain in either or both knees (%) | 100 (69%) | 138 (49%)* |
P-value <0.05.
Vitamin C and E Ranges and Sex-Specific Tertiles
There were significant age-adjusted differences (p value <.0001) in Vitamin C levels between women (mean value 1.43 mg/dL (SD=0.55)) and men (mean value 1.18 mg/dL (SD=0.56)) as well as for vitamin E (women: mean value 1828 ug/dL (SD=789); men 1562 ug/dL (SD= 738). Sex-specific ranges for the tertiles of the baseline vitamin C and E are shown in Table 2. Vitamin C and vitamin E levels were moderately correlated in both men (Pearson's r= 0.25) and women (Pearson's r= 0.29).
Table 2.
Association of baseline plasma vitamin C and E level and incident whole knee radiographic osteoarthritis (ROA) in subjects with no ROA in either knee at baseline
| Incident ROA in subjects with no ROA in either knee at baseline (n = 574 knees, 287 subjects/80 events) | ||||
|---|---|---|---|---|
| Baseline vitamin C level | Incident Knee Events/Observations (%) | Model 1: Adjusted OR (95%CI); P-value | Model 2: Adjusted OR (95%CI); P-value | Model 3: Adjusted OR (95%CI); P-value |
| Lowest Tertile Women: 0.12 - 1.21 Men: 0.096 - 0.89 |
18 / 166 (10.8%) | Reference 1.0 | Reference 1.0 | Reference 1.0 |
| Middle Tertile Women: 1.22 - 1.58 Men: 0.90 - 1.33 |
27 / 202 (13.4%) | 1.33 (0.68-2.62) 0.403 |
1.53 (0.76-3.10) 0.232 |
1.43 (0.71-2.89) 0.323 |
| Highest Tertile Women: 1.584 - 3.69 Men: 1.34 - 2.66 |
35 / 206 (17.0%) | 1.76 (0.93-3.32) 0.083 |
2.20 (1.12-4.33) 0.021 |
1.97 (1.00-3.88) 0.048 |
| Test for trend | 0.075 | 0.019 | 0.041 | |
| Baseline vitamin E level | Incident Knee Events/Observations (%) | Model 1: Adjusted OR (95%CI); P-value | Model 2: Adjusted OR (95%CI); P-value | Model 3: Adjusted OR (95%CI); P-value |
|---|---|---|---|---|
| Lowest Tertile Women: 582 - 1390 Men: 333 - 1172 |
23 / 198 (11.6%) | Reference 1.0 | Reference 1.0 | Reference 1.0 |
| Middle Tertile Women: 1391 -1940 Men: 1173 - 1660 |
21 / 164 (12.8%) | 1.16 (0.59-2.25) 0.668 |
1.33 (0.68-2.63) 0.406 |
1.30 (0.66-2.56) 0.455 |
| Highest Tertile Women: 1941 - 9222 Men: 1661 - 5156 |
36 / 212 (17.0%) | 1.70 (0.92-3.14) 0.088 |
1.89 (1.02-3.50) 0.042 |
1.66 (0.91-3.04) 0.101 |
| Test for trend | 0.061 | 0.030 | 0.081 |
Model 1 - Adjusted for age, sex, nonwhite race, clinic site
Model 2 - Adjusted for age, sex, nonwhite race, clinic site and BMI
Model 3 - Adjusted for age, sex, nonwhite race, clinic site, BMI and vitamin E/C tertiles.
Vitamin C and E and Incident WKROA
Among subjects with no WKROA in either knee ((n = 287 subjects, 574 knees) at baseline, there was a trend (p=0.075) for an association between sex-specific tertiles of vitamin C and incident WKROA in a model adjusted for age, sex, nonwhite race and clinic site (Table 2). With additional adjustment for BMI there was a significant test for trend across vitamin C tertiles for the association with incident WKROA, with the estimated odds ratios consistent with a dose-response relationship. The highest tertile of Vitamin C had twice the odds of incident WKROA compared to the lowest. This association was attenuated but still significant when additionally adjusted for Vitamin E (Table 2). The interaction terms for Vitamin C tertiles and gender were not significant (p = 0.579).
Subjects without baseline WKROA in the highest tertile of plasma vitamin E also had a significant (p-value= 0.042) twice the odds increased risk of incident WKROA after adjusting for age, race, gender, clinic site and BMI, which was attenuated with additional adjustment for plasma vitamin C levels (Table 2). An interaction term for vitamin E and gender was significant (p= 0.039). In further analyses stratifying for gender (Table 3), women in the highest tertile of vitamin E had an increased risk of incident WKROA [OR= 3.43 (1.36-8.66); p-value=0.009; p-value for trend=0.014], but there was no association in men [OR= 0.99 (0.41-2.41); p-value=0.989; p-value for trend=0.786] . Results were unchanged when mutually adjusting for vitamin C and E levels.
Table 3.
Association of baseline plasma vitamin E level and incident whole knee radiographic OA (WK ROA) in subjects with no ROA at baseline: A. Women and B. Men
| A. Incident WK ROA-Women only (n=336 knees, 168 ppts/50 events) | B. Incident WK ROA-Men only (n=238 knees, 119 ppts/30 events) | |||||
|---|---|---|---|---|---|---|
| Baseline vitamin E level | Incident Knee Events/Observations (%) | Model 2: Adjusted OR (95%CI); P-value | Model 3: Adjusted OR (95%CI); P-value | Incident Knee Events/Observations (%) | Model 2: Adjusted OR (95%CI); P-value | Model 3: Adjusted OR (95%CI); P-value |
| The Lowest Tertile | 10 / 112 (8.9%) | Reference 1.0 | Reference 1.0 | 13 / 86 (15.1%) | Reference 1.0 | Reference 1.0 |
| Middle Tertile | 16 / 96 (16.7%) | 2.86 (1.09-7.52) 0.033 |
2.72 (1.04-7.09) 0.041 |
5 / 68 (7.4%) | 0.48 (0.17-1.34) 0.160 |
0.49 (0.17-1.39) 0.177 |
| The Highest Tertile | 24 / 128 (18.8%) | 3.43 (1.36-8.66) 0.009 |
3.07 (1.24-7.60) 0.016 |
12 / 84 (14.3%) | 0.99 (0.41-2.41) 0.990 |
0.85 (0.35-2.10) 0.729 |
| Test for trend | 0.014 | 0.026 | 0.786 | 0.934 | ||
Model 2 - Adjusted for age, nonwhite race, clinic site, and BMI
Model 3 - Adjusted for age, nonwhite race, clinic site, BMI and vitamin E tertiles and vitamin C tertiles.
Results for vitamin C levels were similar, although nonsignificant, in the much smaller group of subjects (n = 140 subjects, 140 knees) who had unilateral WKROA at baseline (data not shown). The risk of incident WKROA in the contralateral knee was increased for the highest tertile of vitamin C [adjusted OR= 1.97 (95% CI: 0.83-4.70); p=0.125; p for trend=0.231]. For the subjects in the highest tertile of vitamin E, however, the risk of developing contralateral WKROA was nonsignificantly decreased compared to the lowest tertile [adjusted OR=0.57 (0.24-1.39); p=0.218; (p for trend: 0.174)]. Including levels of both antioxidants in the same model did not substantially change these results.
Additional Analyses
Individual adjustment for the additional covariates described in the Methods, including glucosamine/chondroitin and vitamin supplement use, and circulating levels of vitamins A, D and K did not materially change any of the results. Subjects using vitamin C or E supplements had significantly higher blood levels of vitamin C or E; excluding them did not change any of the results. Our results were largely unchanged when incident TF ROA alone was analyzed as the outcome (data not shown). There were two few cases of incident PF OA for valid analysis.
Discussion
We evaluated the relationship of blood levels of the antioxidant vitamins C and E with the risk of developing incident radiographic knee OA and found higher plasma levels of vitamin C and E (specifically alpha-tocopherol) did not protect against incident radiographic whole knee OA. Rather, subjects with vitamin C levels and vitamin E levels in the highest tertile had a significantly increased odds of developing WKROA. In addition, there was a significant dose-response trend for increasing levels of vitamin C and E and odds of developing incident WKROA.
Our finding that higher plasma vitamin C levels increased incident WKROA is consistent with a recent animal study showing that exposure to increased levels of vitamin C supplements worsened the severity of incident OA(9). Kraus et al. evaluated the dose response of ascorbic acid with incident OA lesions in Hartley guinea pigs. Guinea pigs fed a high concentration of ascorbic acid (150 mg/day) over 8 months had a greater degree of proteoglycan loss, cartilage fibrillation and osteophyte formation than the group fed low levels of ascorbic acid (2.5 – 3 mg/day). The investigators postulated that the degenerative joint changes observed in the guinea pig model may be mediated in part through the local production of TGF-beta, which was found to be actively expressed in the osteophytes of guinea pigs given higher doses of vitamin C.
Our results differ from our expectation that antioxidants may protect against the development of knee OA and from some previous studies. In the longitudinal Framingham OA study, McAlindon and colleagues(31) found that higher self-reported dietary intake of vitamin C was associated with reduced joint space narrowing progression in knees with existing TF ROA, but dietary vitamin E intake was not related to progression and neither micronutrient was associated with incident TF ROA. Wang et al (11) assessed dietary intake of vitamin C and E in healthy middle-aged subjects without knee pain and found that 10 years later the prevalence of tibiofemoral bone marrow lesions assessed by MRI was lower in those with a greater vitamin C intake, but there was no association with the later prevalence of cartilage defects. Vitamin E intake was not associated with either MRI finding. Both studies used food frequency questionnaires to assess dietary vitamin C and E intake, a method limited by weak correlations with objective micronutrient biomarkers and misclassification of nutrient intake for Vitamin C especially (32) (33). A more recent study suggested that users of vitamin C supplements (34) had a modest decreased risk of incident TF ROA. However, this study did not distinguish past supplement intake from use at the study baseline, and excluded over half of all enrolled subjects because they had used multivitamins of any kind in the past.
Two previous cross-sectional population studies have examined the association of blood levels of vitamin E with radiographic knee OA , with results for alpha-tocopherol that are consistent with ours in not finding a protective effect of higher levels. In the Johnson County Osteoarthritis study, Jordan et al. (15) measured serum levels of tocopherols in subjects with and without radiographic TF knee OA, and found that a higher ratio of alpha to gamma tocopherol level was associated with a significantly decreased prevelance of OA, while higher levels of delta-tocopherol and gamma-tocopherol were associated with a significant increase in the prevalence of OA. There was no significant association seen between levels of alpha tocopherol and radiographic OA. A cross-sectional study done in Japan found no association of alpha-tocopherol levels with prevalent TF OA, but subjects in the highest tertile of undifferentiated beta/gamma tocopherol had a significantly reduced prevalence of OA (16). Finally, in the only randomized, placebo controlled trial of antioxidant supplementation and knee OA progression, subjects receiving 500 mg vitamin E supplementation (which contains mainly alpha-tocopherol) daily for two years showed no difference compared to placebo in cartilage changes assessed over the two year period by MRI (17).
Several strengths and limitations of our study deserve comment. Ours is the first study to our knowledge to evaluate the risk of incident radiographic knee OA with blood levels of both vitamin C and E, which better reflect long-term circulating levels of vitamin C than assessments of dietary intake using food frequency questionnaires (32). In addition,the MOST longitudinal cohort study had a high rate of follow-up (89%) for radiographic outcomes.
We examined antioxidant levels and the risk of new radiographic knee OA, a design valuable for establishing that the antioxidant exposure occurred prior to the radiographic endpoint. We do not present results for baseline antioxidant levels and radiographic progression of existing knee OA because restricting our analysis to knees with existing OA can generate substantial bias (attenuating and even reversing true causal relationships) in the apparent association of disease worsening with a chronic risk factor, such as blood antioxidant levels, when that risk factor is also associated with disease onset (35) (36). Thus, associations of blood antioxidant levels with disease progression would be largely uninterpretable.
Our study also has several potential limitations. Incident OA outcomes are based on radiographs, which are relatively insensitive to very early knee OA (37). However, this would tend to bias any associations of vitamin C and E levels toward null findings. Analyses of vitamin C and E levels and knee MRI findings are warranted. Even in a prospective study of incident OA such as ours, it is possible that subjects with early signs or symptoms of knee OA may have been more likely to take vitamin C and E and other nutritional supplements in order to prevent disease onset, resulting in confounding by indication. To try to control for this possibility, we did sensitivity analyses excluding vitamin C and E supplement users, and adjusting for glucosamine/chondroitin and other supplement use, and this did not change our results. We studied radiographic OA of the whole knee, which is appropriate since circulating levels of vitamin C and E are likely to affect both TF and PF compartments of the knee. However, although we were able to analyze incident TF OA separately and found similar associations with vitamin C and E levels, there were too few incident PFROA cases for separate analyses. Therefore, our findings may reflect primarily associations with TFROA.
Vitamin C and E levels were measured from a single plasma and serum sample at baseline only and may not reflect long-term exposure or diurnal variation, while blood levels may not correlate strongly with antioxidant levels in joint fluid. We assessed only alpha-tocopherol levels. Alpha tocopherol is found in higher plasma and tissue concentrations than other forms, is the main form of vitamin E in supplements and is one of the most potent antioxidant tocopherols (27). Nevertheless, our results are not directly comparable with studies that have analyzed other tocopherol isoforms and isoform ratios (16, 17). Gamma-tocopherol may have anti-inflammatory in addition to antioxidant properties and is a major source of vitamin E in the diet (38). We did not adjust alpha-tocopherol levels for blood cholesterol levels, which may be a source of confounding for fat soluble vitamin E. Further investigation of the role of various isoforms of tocopherol in OA is warranted.
The association of vitamin C and E levels with incident WKROA was only significant in models adjusted for BMI, a potent risk factor for incident knee OA. Obese individuals have lower circulating levels of ascorbic acid and other micronutrients antioxidants (38) (39), possibly due to low dietary intake or depletion of antioxidants from scavenging of reactive oxygen species, which are increased in obesity. In our study adjustment for BMI strengthened the association of vitamin C and and E with incident disease. Consistent with one other study (15), our results suggested possible gender differences in the relationship of alpha-tocopherol and the risk of knee OA; men in our study in the higher tertiles of vitamin E had a small and nonsignificantly decreased risk and women had a large and significant increased risk of knee OA. These results should be treated with caution given the relatively small number of events in the men and wide confidence intervals.
In conclusion, higher plasma level of the antioxidants vitamin C and serum level of vitamin E at baseline were associated with an increased risk of incident whole knee radiographic OA and therefore we would argue against the routine use of vitamin C or E supplementation to prevent knee osteoarthritis. A possible causal role of high vitamin C and E levels in knee OA development and the full spectrum of antioxidant effects in knee OA warrant further investigation.
Acknowledgements
The MOST Study is supported by National Institutes of Health (NIH) grants from the National Institute on Aging to Drs. Torner (U01-AG-18832), Nevitt (U01-AG-19069) and Felson (U01-AG-18820) and Dr. Beth Lewis (UAB) (U01-AG18947). Dr. Neogi is supported by an NIH grant K23AR055127.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Study Conception and Design: Chaganti, Tolstykh, Lane, Felson, Nevitt
Acquisition of Data: Chaganti, Tolstykh, Lane, Nevitt, Neogi, Javaid, Torner, Jacques, Curtis
Analysis and Interpretation of Data: Chaganti, Tolstykh, Lane, Nevitt, Neogi, Javaid, Torner, Jacques, Curtis, Felson
Author Disclosures: None
References
- 1.Abdollahzad H, Eghtesadi S, Nourmohammadi I, Khadem-Ansari M, Nejad-Gashti H, Esmaillzadeh A. Effect of vitamin C supplementation on oxidative stress and lipid profiles in hemodialysis patients. Int J Vitam Nutr Res. 2009 Sep;79(5-6):281–7. doi: 10.1024/0300-9831.79.56.281. [Randomized Controlled Trial] [DOI] [PubMed] [Google Scholar]
- 2.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of internal medicine. 2004 Nov 22;164(21):2335–42. doi: 10.1001/archinte.164.21.2335. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't]
- 3.Ohrvall M, Sundlof G, Vessby B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J Intern Med. 1996 Feb;239(2):111–7. doi: 10.1046/j.1365-2796.1996.410753000.x. [Research Support, Non-U.S. Gov't]
- 4.Slattery ML, Edwards SL, Anderson K, Caan B. Vitamin E and colon cancer: is there an association? Nutr Cancer. 1998;30(3):201–6. doi: 10.1080/01635589809514664. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 5.Hamidi MS, Corey PN, Cheung AM. Effects of vitamin E on bone turnover markers among US postmenopausal women. J Bone Miner Res. 2012 Jun;27(6):1368–80. doi: 10.1002/jbmr.1566. [Research Support, Non-U.S. Gov't]
- 6.Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008 Apr;16(4):515–21. doi: 10.1016/j.joca.2007.09.001. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 7.Sharma G, Saxena RK, Mishra P. Regeneration of static-load-degenerated articular cartilage extracellular matrix by vitamin C supplementation. Cell Tissue Res. 2008 Oct;334(1):111–20. doi: 10.1007/s00441-008-0666-9. [Research Support, Non-U.S. Gov't]
- 8.Clark AG, Rohrbaugh AL, Otterness I, Kraus VB. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol. 2002 Mar;21(2):175–84. doi: 10.1016/s0945-053x(01)00193-7. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]
- 9.Kraus VB, Huebner JL, Stabler T, Flahiff CM, Setton LA, Fink C, Vilim V, Clark AG. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004 Jun;50(6):1822–31. doi: 10.1002/art.20291. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 10.McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Levy D, Felson DT. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996 Apr;39(4):648–56. doi: 10.1002/art.1780390417. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]
- 11.Wang Y, Hodge AM, Wluka AE, English DR, Giles GG, O'Sullivan R, Forbes A, Cicuttini FM. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66. doi: 10.1186/ar2225. [Research Support, Non-U.S. Gov't]
- 12.Brand C, Snaddon J, Bailey M, Cicuttini F. Vitamin E is ineffective for symptomatic relief of knee osteoarthritis: a six month double blind, randomised, placebo controlled study. Ann Rheum Dis. 2001 Oct;60(10):946–9. doi: 10.1136/ard.60.10.946. [Clinical Trial Randomized Controlled Trial]
- 13.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004 Apr;134(4):927–34. doi: 10.1093/jn/134.4.927. [Comparative Study Research Support, U.S. Gov't, P.H.S.]
- 14.White E, Kristal AR, Shikany JM, Wilson AC, Chen C, Mares-Perlman JA, Masaki KH, Caan BJ. Correlates of serum alpha- and gamma-tocopherol in the Women's Health Initiative. Ann Epidemiol. 2001 Feb;11(2):136–44. doi: 10.1016/s1047-2797(00)00189-7. [Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov't, P.H.S.]
- 15.Jordan JM, De Roos AJ, Renner JB, Luta G, Cohen A, Craft N, Helmick CG, Hochberg MC, Arab L. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004 May 15;159(10):968–77. doi: 10.1093/aje/kwh133. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 16.Seki T, Hasegawa Y, Yamaguchi J, Kanoh T, Ishiguro N, Tsuboi M, Ito Y, Hamajima N, Suzuki K. Association of serum carotenoids, retinol, and tocopherols with radiographic knee osteoarthritis: possible risk factors in rural Japanese inhabitants. J Orthop Sci. 2010 Jul;15(4):477–84. doi: 10.1007/s00776-010-1491-z. [Research Support, Non-U.S. Gov't]
- 17.Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. The Journal of rheumatology. 2002 Dec;29(12):2585–91. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't]
- 18.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, Torner J, Lewis CE, Nevitt MC. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007 Sep;56(9):2986–92. doi: 10.1002/art.22851. [Multicenter Study Research Support, N.I.H., Extramural]
- 19.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, Liang MH. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995 Jul;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 20.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, Yu W, Genant HK. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003 Mar;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [Comparative Study Research Support, U.S. Gov't, P.H.S.]
- 21.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [Research Support, Non-U.S. Gov't]
- 22.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, Lewis CE, Aliabadi P, Sack B, McCulloch C, Zhang Y. A new approach yields high rates of radiographic progression in knee osteoarthritis. The Journal of rheumatology. 2008 Oct;35(10):2047–54. [Research Support, N.I.H., Extramural]
- 23.LaValley MP, McLaughlin S, Goggins J, Gale D, Nevitt MC, Felson DT. The lateral view radiograph for assessment of the tibiofemoral joint space in knee osteoarthritis: its reliability, sensitivity to change, and longitudinal validity. Arthritis Rheum. 2005 Nov;52(11):3542–7. doi: 10.1002/art.21374. [Evaluation Studies Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]
- 24.Behrens WA, Madere R. A highly sensitive high-performance liquid chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological fluids, and foods. Anal Biochem. 1987 Aug 15;165(1):102–7. doi: 10.1016/0003-2697(87)90206-5. [DOI] [PubMed] [Google Scholar]
- 25.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979 Oct;32(10):2143–9. doi: 10.1093/ajcn/32.10.2143. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 26.Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002 Mar-Apr;5(2):66–74. doi: 10.1046/j.1523-5408.2002.00005.x. [Research Support, U.S. Gov't, Non-P.H.S. Review]
- 27.Meydani M. Vitamin E. Lancet. 1995 Jan 21;345(8943):170–5. doi: 10.1016/s0140-6736(95)90172-8. [Review] [DOI] [PubMed] [Google Scholar]
- 28.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994 Nov;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 29.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, Lewis CE, Torner J, Nevitt MC, Zhang YQ, Felson DT. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007 Dec;56(12):4048–54. doi: 10.1002/art.23071. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]
- 30.Ness AR, Cappuccio FP, Atkinson RW, Khaw KT, Cook DG. Plasma vitamin C levels in men and women from different ethnic backgrounds living in England. Int J Epidemiol. 1999 Jun;28(3):450–5. doi: 10.1093/ije/28.3.450. [Comparative Study Research Support, Non-U.S. Gov't]
- 31.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Annals of internal medicine. 1996 Sep 1;125(5):353–9. doi: 10.7326/0003-4819-125-5-199609010-00001. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]
- 32.Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J. 2007;6:41. doi: 10.1186/1475-2891-6-41. [Meta-Analysis Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henriquez-Sanchez P, Sanchez-Villegas A, Doreste-Alonso J, Ortiz-Andrellucchi A, Pfrimer K, Serra-Majem L. Dietary assessment methods for micronutrient intake: a systematic review on vitamins. Br J Nutr. 2009 Dec;102(Suppl 1):S10–37. doi: 10.1017/S0007114509993126. [Research Support, Non-U.S. Gov't Review]
- 34.Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011 Apr;14(4):709–15. doi: 10.1017/S1368980010001783. [Research Support, Non-U.S. Gov't]
- 35.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010 Nov;62(11):1527–32. doi: 10.1002/acr.20287. [Research Support, N.I.H., Extramural Review]
- 36.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA : the journal of the American Medical Association. 2011 Feb 23;305(8):822–3. doi: 10.1001/jama.2011.163. [Research Support, N.I.H., Extramural]
- 37.Javaid MK, Lynch JA, Tolstykh I, Guermazi A, Roemer F, Aliabadi P, McCulloch C, Curtis J, Felson D, Lane NE, Torner J, Nevitt M. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage. 2010 Mar;18(3):323–8. doi: 10.1016/j.joca.2009.11.002. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]
- 38.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001 Dec;74(6):714–22. doi: 10.1093/ajcn/74.6.714. [Review] [DOI] [PubMed] [Google Scholar]
- 39.Kimmons JE, Blanck HM, Tohill BC, Zhang J, Khan LK. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed. 2006;8(4):59. [Comparative Study] [PMC free article] [PubMed] [Google Scholar]