Abstract
More nations are joining the human embryonic stem cell (hESC) “race” by aggressively publishing in the peer-reviewed journals. Here we present data on the international use and distribution of hESC using a dataset taken from the primary research literature. We extracted these papers from a comprehensive dataset of articles using hESC and human induced pluripotent cells (hiPSC). We find that the rate of publication by US-based authors is slowing in comparison to international labs, and then declines over the final year of the period 2008–2010. Non-US authors published more frequently and at a significantly higher rate, significantly increasing the number of their papers. In addition, international labs use a more diverse set of hESC lines and Obama-era additions are used more in non-US locations. Even considering the flood of new lines in the US and abroad, we see that researchers continue to rely on a few lines derived before the turn of the century. These data suggest the “embargo” effects of restrictive policy on the US stem cell field. Over time, non-US labs have freely used lines on the US registries, while federally funded US scientists have been limited to using those lines approved by the NIH.
Keywords: policy, ethics, law, human embryonic stem cell research, induced pluripotent stem cells, NIH, Obama, G.W. Bush
For human embryonic stem cell (hESC) researchers, the George W. Bush years were momentous. Bush severely limited the number of cell lines eligible for federal funding to those derived before August 9, 2001. Bush’s policy was controversial and provoked concerns for the future of US competitiveness in the biomedical sciences. There were fears that countries with less restrictive policies and ambitious funding initiatives would erode US dominance in stem cell research, hampering discovery and delaying the development of treatments and cures. In March 2009, President Barack Obama reversed the Bush policy with an executive order. Obama instructed the NIH to expand hESC funding and to issue new guidelines that would increase the number of lines approved for use with federal funds.
The effects expanded funding might have on the field were further obscured in August 2010 when a Washington, D.C. district judge, Royce Lamberth, issued a preliminary injunction to block implementation of the NIH guidelines and all federal funding for hESC research. The suit, brought by a pair of adult stem cell researchers, the Christian Medical Association, and an embryo adoption agency, alleged the NIH guidelines violated the 1997 Dickey-Wicker amendment, which prohibits the use of NIH funds for research that would destroy human embryos. The unsteady federal compromise that would fund a select list of approved lines while barring embryo destruction was now in jeopardy. In a 2-1 decision last April, a three-judge appeals panel reversed the district court ruling, and Lamberth released his stay. In January 2012, the two researchers appealed to the District of Columbia Circuit Court, raising the possibility the case will eventually reach the Supreme Court [1].
The reversal of the Bush policy was lauded for several reasons. Proponents argued it would decrease US researcher’s reliance on a small set of legacy lines, many of which were made with outdated culture methods. It would also increase the genetic diversity of registered lines, and enable the deposit of new, disease-specific lines. Coupled with expanded funding from the NIH, the policy would counter the ambitious efforts of countries such as China and Singapore, maintain and extend the productivity of US-based scientists, and advantage US corporations in the development of treatments. However, the effects of hESC policies on individual nations are just beginning to be explored. We previously reported a growing international gap in hESC research. Our search of the primary literature between November 1998 and December 2004 examined articles authored by investigators in 97 institutions around the globe. Though 46% of the authors’ institutions were US-based and US researchers out-published any other individual country, we found a growing gulf between US and total non-US publication rates. US labs lagged after 2002, with the deficit increasing through 2004. Also apparent were the appearance of newly derived hESC lines not on the NIH registry, which outnumbered approved lines over two to one (44/18). We argued in 2006 that if these trends continue unchecked, US research competitiveness would suffer [2]. In a bellwether of the results presented here, we surveyed research presented at the 2010 International Society for Stem Cell Research (ISSCR) meeting. Most of the lines (75%) reported at the meeting were not on the NIH registry and the appearance of many new lines resulted from research conducted outside of the United States [3].
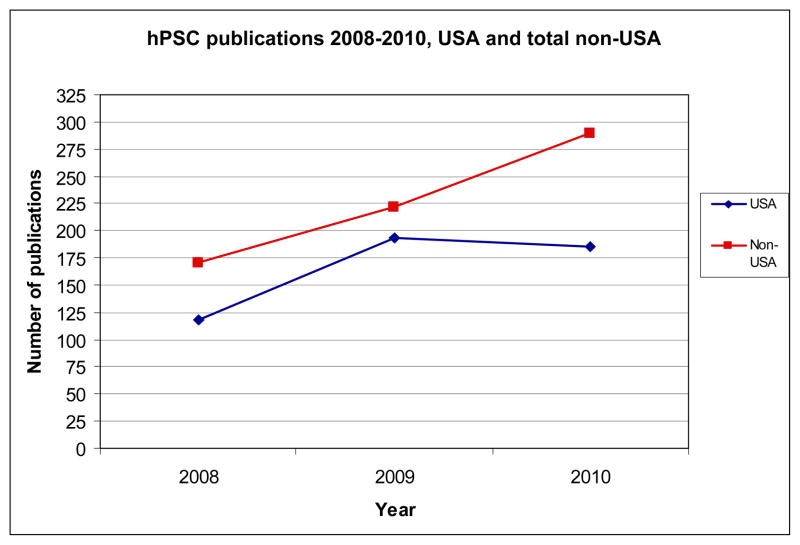
In recent years it appears that more nations are joining the hESC “race” by aggressively publishing in the peer-reviewed literature. Here we present data on primary research articles published between 2008 and 2010. We extracted these papers from a larger dataset of articles using hESC and human induced pluripotent cells (hiPSC). We used this strategy in prior work to report on the use and distribution of human pluripotent stem cell lines [4]. For each hESC article published between 2008 and 2010, we identified the location of the senior (last) author on the publication and used this as a proxy for the primary location for the work. With this data we determined the frequency of hESC used in publications (hESC alone or in combination with hiPSC) from the US and the top ten most productive foreign countries. Figure 1 shows the rate of publication by US-based authors slowing in comparison to international labs, and then declining over the final year of the period. By contrast, the publication rates of non-US authors were not significantly different than US authors in 2008–2009 (chi sq; p=0.165) but were significantly different between 2009 and 2010 (chi sq; p=0.024). In addition, non-US authors increased the number of their papers by a startling 70% between 2008 and 2010. The future ramifications of a relative decline in US productivity are yet to be determined. On the one hand, a greater number of productive nations in hESC research may result in therapeutic developments that are more numerous and more immediate. On the other, with more competitors in the hESC race, the US may cede its position at the forefront of discovery and innovation.
Figure 1.
Worldwide frequency of the appearance of pluripotent human stem cell (hPSC) publications in the primary literature for the period 2008–2110.
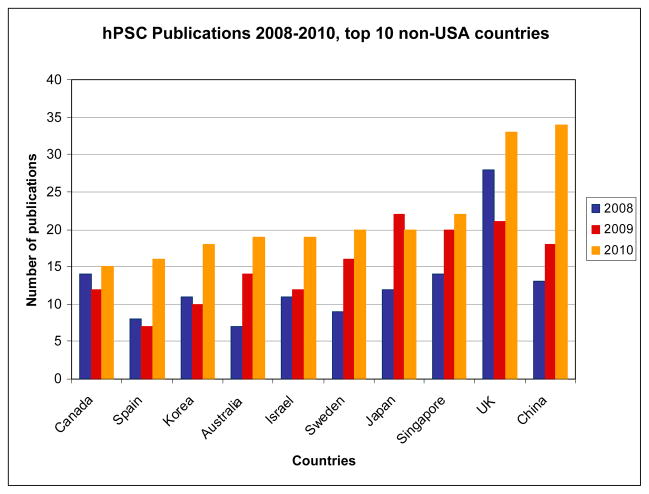
Figure 2 compares publication rates of the ten most productive foreign countries between 2008 and 2010. With the exception of Canada, these countries dramatically increased their 2008 publication totals. The UK (82) and China (65) led the way, followed by Singapore (56) and Japan (55). Four countries (China, Sweden, Australia and Spain) doubled the number of their hESC publications during the period. Even with a leveling trend in 2010, the US (496) leads productivity for the three-year period. Yet, all the non-US countries depicted in Figure 2 show overall positive trajectories, with especially strong growth in 2010, adding to the publication gap observed in the starting year. We believe the leveling and decline of US productivity in the latter years may be due to lingering effects of Bush era prohibitions and an uncertain policy environment the first half of the Obama administration. This lasting hangover might be due to any number of mechanisms, including loss of US scientists from the stem cell field as junior researchers opted not to pursue academic careers with human embryonic stem cell lines, laboratory and research group skills and infrastructure that were optimized for early, dominant hESC cells lines, or time spent identifying, developing, and maintaining alternatives to federal funding sources. The challenge raised to US productivity may also be exacerbated by the robust response of hESC laboratories in other nations, especially in the UK and China. We note that all of the listed countries have generally permissive or flexible hESC policy. Along the spectrum of nations on our list, Canada’s policy is considered to be the most restrictive: though spare IVF embryos may be used under certain conditions, it is not ethically permissible to create embryos for research purposes [5]. We further add that the use of hESC and hiPSC lines are tightly linked, with experienced hESC laboratories also experimenting with hiPSCs in comparative studies. For US-based researchers, then, policies that would restrict or prohibit hESC research may negatively impact the nascent iPSC field.
Figure 2.
Frequency of the appearance of pluripotent human stem cell (hPSC) publications in the primary literature for the period 2008–2110, by the top ten non-USA countries.
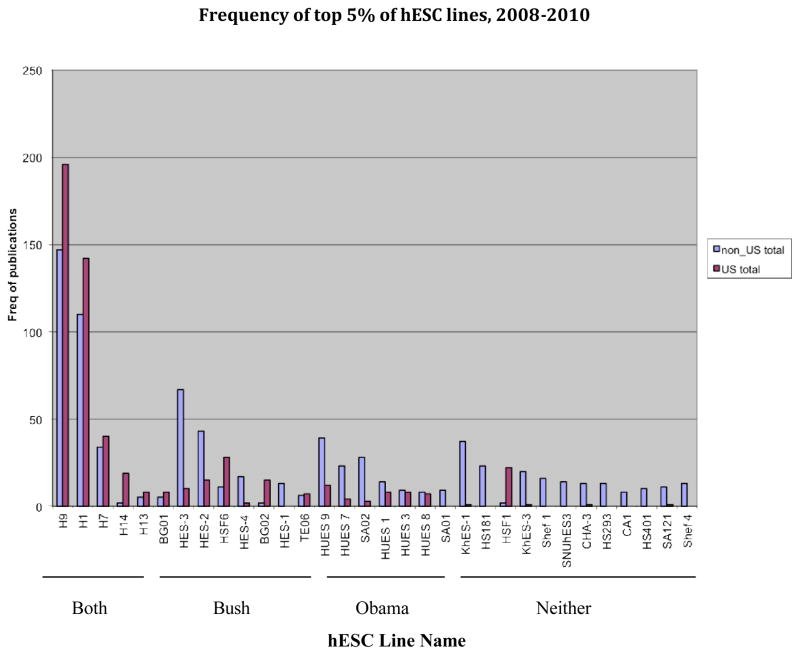
We further our analysis by examining the diversity of hESC lines used by researchers in our dataset. The scientific community has long called for a genetically diverse set of lines derived under new culture methods [6]. Overall, the number of different lines used in the literature has been startling, growing nearly tenfold from 44 in 1998–2004 to 414 during 2008–2010. Increases in the diversity of new lines can also be found on the NIH registry. While only 21 lines were available during the Bush era, 142 lines are now listed (NIH Registry 2012). Even considering the flood of new lines, researchers continue to rely on a few lines derived before the turn of the century. We previously showed that just two of these—H1 and H9—were used with any frequency, with H9 appearing in over 80% of peer-reviewed publications [7]. Three years later this trend continues. Figure 3 shows the absolute frequencies of the 32 lines appearing most often in the published literature between 2008 and 2010. Here, H1 and H9 continue to predominate. We note whether the lines appeared on the Bush registry, the Obama registry, both registries, or are unregistered. While the increase in registered lines is encouraging, most of the lines added since July 2009—when the new accession policy began—have yet to appear in the literature.
Figure 3.
World wide frequency of the top 5% of human embryonic stem cell lines appearing in the primary literature, by line type, for the period 2008–2110. Lines are categorized by whether they were approved during the Bush administration, the Obama administration, both administrations, or neither.
Several things stand out in Figure 3. The “H” lines, derived in 1998 by James Thomson, dominate the literature and are globally wide spread. These lines are used more in the US than abroad, but non-US use is robust and growing. The BG (Bresagen) series of lines, while derived in Australia, appear on more US-authored publications. This may be because Bresagen distributed the lines through a US affiliate, and because the lines were originally approved under the Bush rules and thus available for federal funding. There were questions about whether the BG lines were ethically derived, and later they were not re-registered under the stricter Obama guidelines [8]. Though the BG lines are used in relatively low numbers, we note their continued presence in the US-authored literature as an instance of the legacy of established research materials that persist irrespective of changing regulatory and funding policy.
The reverse seems to hold true for older lines approved under Obama but not under Bush. In response to the Bush restrictions, in 2004 Harvard University’s Doug Melton derived a suite of hESC lines with private funds. Though they were not NIH-eligible at first, Harvard distributed the lines widely, offering a less expensive alternative to the high fees charged by WiCell, the curator of the government-approved H lines. In a 2009 analysis, we tracked legal instruments called materials transfer agreements that enable the exchange of hESC lines. We found that compared to the distributions from the US stem cell bank, four times as many Harvard lines were shipped overseas during the period 2003–2007 [9]. In the primary literature, we pick up at 2008 and move through 2010. Here, we see that a suite of Harvard lines (HUES 1, 7, 8, and 9) continues to have an overseas presence. We believe this may be due to three factors: 1) between 2004 and July 2009, HUES lines could not be used in NIH projects because they were made with private funds; 2) during that period the lines were offered at low cost to those who requested them; and 3) though the lines were eventually approved under the new Obama guidelines, their current use in the US is restricted to federally-funded diabetes research. The Harvard lines, derived with private funds, were not eligible for NIH grants during the eight years of the Bush administration, and may not be used for federal projects that study neural and cardiac cells and their associated diseases. We note that the last point may have had a small effect in the present analysis, which tracks only 18 months of usage under the Obama policy.
With the exception of the robust use of the H lines, location effects may play a role in whether a line crosses the border to the US. Of the top lines appearing in the literature, many stay near to their points of origin. This fact holds even for foreign-made lines registered in the US over ten years ago. For example, Bush-approved lines derived in Singapore are used mostly or exclusively in non-US labs. In addition, lines derived in Sweden (HS-181, 293, 401), Singapore (HES-2, 3), and Japan (KhES-1, 3) dominate non-US publications, and some lines derived outside of the US – HS181, SA01 (Sweden), SNUhES-3 (South Korea), Shef 1, 4 (UK), HES 1 (Singapore), and CA-1 (Canada), are not affiliated with US last authors at all. Surprisingly, only 8 newly accessioned, NIH approved lines appear in our top 32: HUES1, 3, 7, 8, 9, SA01, 02 and KhES-1; but these Obama-era additions are used more in non-US locations. Nearly 60% (19/32) of the most frequently used lines are not on the US registry. Finally, less than 10% of the 142 lines on the current NIH registry appear in the top 5% of our census.
In conclusion, while the Obama policy has been generally positive for US scientists, legal wrangling, the effects of the Dickey-Wicker amendment, and persistence of social and political controversy have dampened the impacts of a more permissive hESC policy. The rate of US-authored publications appears to have slowed in contrast to other nations, which exhibit surprising increases in publication frequency. In terms of the diversity of lines, these data suggest lingering “embargo” effects on the US stem cell field. Over time, non-US labs have freely used lines on the US registries, and this is reflected in increasingly competitive publication frequencies. By contrast, during 2008–2010, US labs—which were mostly federally funded or anticipated federal grants—were limited to using NIH approved lines. A limitation of this study is the link we draw between last authors and the locations of lines used in their research. It is possible that cross-border collaborations among several authors create a mix of lines of national origin and registry status. In addition, it is not clear how state funds have contributed to US publication activity. Further interrogation of our database will help settle these questions.
Acknowledgments
CTS is supported by the Stanford Center for Biomedical Ethics and the Stanford Institute for Stem Cell Biology and Regenerative Medicine. The authors thank Molly Havard for her valuable assistance with the manuscript.
References
- 1.Sherley v. Sibelius No. 10- 5287 United States Court of Appeals dec April 29 2011
- 2.Owen-Smith J, McCormick J. An international gap in human ES cell research. Nat Biotechnol. 2006;24(4):391–392. doi: 10.1038/nbt0406-391. [DOI] [PubMed] [Google Scholar]
- 3.Scott CT, McCormick JB, Derouen MC, Owen-Smith J. Federal policy and the use of pluripotent stem cells. Nat Methods. 2010;7(11):866–867. doi: 10.1038/nmeth1110-866. [DOI] [PubMed] [Google Scholar]
- 4.Scott CT, McCormick JB, DeRouen MC, Owen-Smith J. Democracy derived? New trajectories in pluripotent stem cell research. Cell. 2011;145(6):820–826. doi: 10.1016/j.cell.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canada Tri-Council Statement: Ethical Conduct for Research Involving Humans (TCPS), the Assisted Human Reproduction Act (2004, c.2; AHRA)
- 6.Mosher JT, Pemberton TJ, Harter K, et al. N Engl J Med. 2010;362(2):183–185. doi: 10.1056/NEJMc0910371. [DOI] [PubMed] [Google Scholar]
- 7.Scott CT, McCormick JB, Owen-Smith J. And then there were two: use of hESC lines. Nat Biotechnol. 2009;27(8):696–697. doi: 10.1038/nbt0809-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streffer R. Informed Consent and Federal Funding for Stem Cell Research. Hastings Center Report. 2008;38(3):40–47. doi: 10.1353/hcr.0.0013. [DOI] [PubMed] [Google Scholar]
- 9.McCormick JB, Owen-Smith J, Scott CT. Distribution of human embryonic stem cell lines: who, when and where. Cell Stem Cell. 2009;4(2):107–110. doi: 10.1016/j.stem.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]