To the Editor
Last month, the US National Institutes of Health (NIH) issued its final guidelines defining the types of human embryonic cell (hESC) lines that would be eligible for public funding. The number of NIH-approved hESC lines available for federal funding was a bellwether issue for scientists during the Bush era prohibition. Originally, the agency announced 78 lines could be used. This was later revised downward to 64, then downward again to 21, the number most often cited in the literature1,2.
Among the criticisms of the remaining lines in the National Stem Cell Bank (NSCB) is that they have a limited range of genetic diversity3. Now that the NIH has announced its intention to set up a national registry of hESC lines, the biggest issue facing the agency will be how to rebuild the bank into a robust and valuable research resource.
Getting many different stem cell lines—the fundamental tool of regenerative medicine—into numerous laboratories with varied scientific and clinical foci is a worthy goal. We caution, however, that social and institutional factors such as intellectual property rights, access fees, use restrictions and competition shape the choices scientists make about research materials4,5. Federal policies and institutional guidelines, established and well-understood protocols, assays, reagents and the prominence of some lines in the published literature also have an impact. State-level policies can be even more restrictive than federal rules, and the possibility of legal challenges makes transfer of materials to some locales more difficult than others.
Indeed, initial social differences (in accessibility, ease of use or availability of technical complements such as known reagents and laboratory procedures) can lead to increasing returns for the selection of particular materials and eventually to lock-in on a dominant but often sub-par technological standard for a field6. In short, the diversity of lines actually in use at the bench has as much to do with past policies and the field's history as it does with the variety of lines available in the banks.
We enter this discussion with systematic data on the patterns of use of cell lines from the largest US repositories, the NSCB and the Harvard Stem Cell Institute (HSCI; Cambridge, MA, USA). In prior work, we examined the geographical patterns of distribution of hESC lines from these two sources. We showed that despite federal funding restrictions, hESC research is vigorous and growing in the United States. We see that states with high levels of research activity can blunt local laws and policies that would seek to restrict or chill hESC research7.
Using data on cell line shipments obtained from HSCI and WiCell—which curates the NSCB—we see that lines have been neither uniformly available nor uniformly used, indicating far less diversity of materials than most believe8. Our most recent data set tracks WiCell's distribution of five of its own lines (derived by James Thomson) beginning in 1999, those available from the NSCB after its inception in 2005 and those distributed by HSCI since April 2004. No more than 18 approved cell lines have ever been available through the NSCB and that number is only accurate for the first months of 2009. Importantly, we find that just two NSCB lines—H1 and H9—are commonly used. The story is slightly better for HSCI, where all 28 of the lines derived by Harvard's Doug Melton were shipped at least once within 16 months of the announcement that they would be made broadly available9. HSCI has distributed nearly the same number of lines as WiCell/NSCB in half the time, but a large proportion of those shipments have gone to investigators outside the United States.
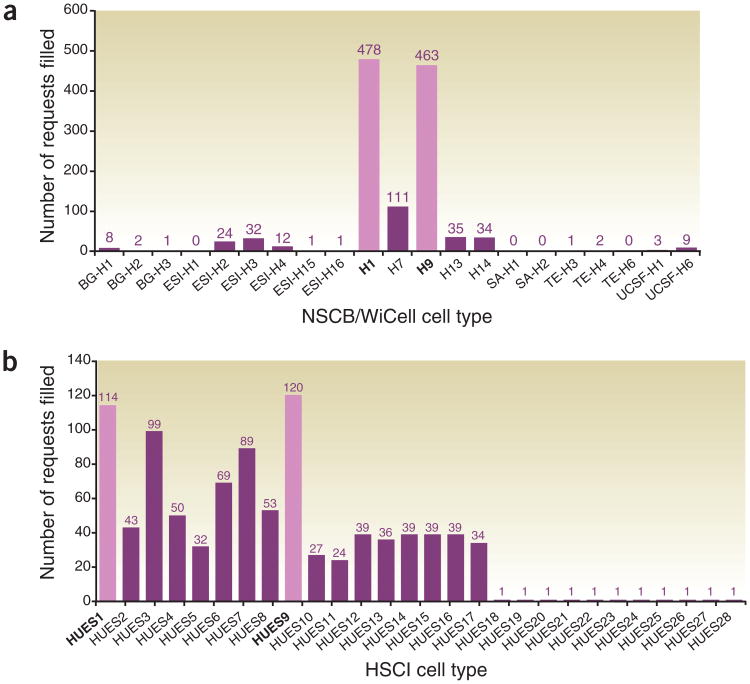
This pattern of diversity in banks is reflected somewhat differently at the bench. Figure 1 reports the number of unique requests that were filled, which we dub ‘vials’, for each available line. The patterns of use of materials are startling. Notice first that of the 1,217 unique requests for NSCB cell lines, fully 77% (941) asked for just two lines (H1, H9). Only one other line in the NSCB (H7) has been requested more than 100 times since 1999 and nine of the lines that have been available have been requested less than 10 times each. On a widely used measure of market concentration, 1-1/Herfindahl, a value of 1 represents a case of pure monopoly. For NSCB cell shipments, this measure is 0.968. For all intents and purposes, then, just two approved cell lines are widely used at the bench.
Figure 1.
Requests for hESC lines. (a) Twenty-one approved cell lines with number of unique shipments from WiCell and NSCB from March 1999 to December 2008. Lines that are unavailable are listed as zeros. Highlighted bars denote the most requested cell lines. (b) Twenty-eight cell lines available from HSCI with number of unique shipments from April 2004 to December 2008. HUES18 through HUES28 have shipped only once and appeared in the published literature in 2009. The most requested lines are highlighted.
Shipments of HSCI lines also manifest high levels of concentration. We see that eleven of the 28 available have shipped just one time each. We note, however, that although those lines were shipped in 2005, they appear in peer-review literature only very recently10. We thus focus our attention on the 17 cell lines available from HSCI starting in 2004. Here we see less concentration. We observe 946 unique shipments of these 17 HSCI cell lines, but only 24.7% (234) of those are for the two most commonly requested lines. Nevertheless, the more nuanced concentration measure we mobilize is 0.868, reflecting a moderately high level of reliance on a relatively small number of available HSCI cell lines. HSCI has dramatically broadened the diversity of cell lines available for use, but it is notable that WiCell's H1 and H9 lines have been requested nearly as many times as all HSCI lines combined. We suspect one reason the ‘diversity’ bottleneck between the banks and the bench is stronger for NSCB lines is because federal funding restrictions introduce constraints that might cause investigators to select materials conservatively with an eye toward those with known procedures for access from the banks. It is possible other factors could have led to greater concentration of the WiCell lines. These include the quality of the cells, their early characterization and proven record of productivity. There is also a first-mover advantage: laboratories requested the lines to repeat Thomson's methods and to establish local culture conditions. Understanding the scientific and social reasons behind investigator choice is an area for further study.
How does shipment data translate into on-the-ground research results? Do hESC lines used outside our data sets—including those approved lines before establishment of NSCB—find their way into the stem cell research community? We address these questions with a systematic search of 534 peer-reviewed publications using hESC lines during the period 1999–2008. Preliminary analysis shows that the high demand for three NSCB lines is reflected in the literature: 83.3% of publications used H9, 60.9% used H1 and 24.2% used H7. Approximately 68% of publications used one or more in combination with other NSCB lines. Fewer than 36% of the publications used NSCB lines other than H1, H7 and H9. By contrast, the HSCI lines, although nearly five years old, are unlikely to supplant the Thomson lines in the published literature any time soon. Our analysis suggests that the two most commonly requested Harvard lines each appear in just under 3% of stem cell publications to date. Even where federal restrictions on material use do not hold, scientists select an artificially limited range of lines and the early pattern of such selections has led a small number of lines to dominate research in the field11.
It seems unlikely that the role the four most used cell lines—H1, H9, HUES1 and HUES9—have played in the field's development will be quickly diminished by the creation of new materials. New policy prescriptions concerned with increasing the diversity of research materials must attend to both lines that can be used and those that actually are used.
The lasting legacy of Bush era policies is an hESC science that relies very heavily on a small number of well-used but less than ideal cell lines derived under fragmented and inconsistent regulatory regimes12. This complicated tangle of rules and restrictions may lead researchers to focus on well-used and documented materials regardless of their technical and scientific characteristics. A consequence of restrictive policy may indeed have produced a benefit: a reproducible yet small number of well-characterized lines are now used as references for the community of stem cell researchers. Yet the startling near monopoly of just two cell lines among those that have been approved for federal funding suggests that future policies must take care to preserve the continued use of these materials while developing incentives to create, benchmark and utilize new, more appropriate materials in pursuit of scientific and clinical goals.
In sum, attending to both the scientific and the social pressures on materials selection by focusing attention on the diversity of lines in the banks and at the bench is necessary to create an efficacious policy. The first step is that federal funding be extended to all lines under reasonable ethical standards of derivation. The second step is to determine which lines are best suited to help researchers reach their goals, thereby creating the greatest public benefit. We suggest that new NIH policies focus equally on rationalizing rules and standards for derivation and use of new lines and creating incentives to develop and disperse diverse materials that are necessary to realize this field's promise.
Acknowledgments
We gratefully acknowledge the assistance of Andrew Cohn and Susan Langbehn of Wisconsin Alumni Research Foundation and WiCell, Douglas Melton, Claire Fitzgerald and Brock Reeve of HSCI, and Mariana Craciun of the University of Michigan. C.T.S. was supported by funds from the Stanford Institute for Neuro-Innovation and Translational Neurosciences. J.O.-S. was supported by National Science Foundation grant no. 0545634. J.B.M.'s work on this publication was made possible by Grant Number 1 UL1 RR024150-01* from the National Center for Research Resources (NCRR), a component of NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Contributor Information
Christopher Thomas Scott, Email: cscott@stanford.edu.
Jason Owen-Smith, Email: jdos@umich.edu.
References
- 1.Abbot A, et al. Nature. 2006;442:336–337. [Google Scholar]
- 2.Anonymous. AAAS Center for Science, Technology and Congress. 2009 Mar 10; http://www.aaas.org/spp/cstc/briefs/stem-cells/
- 3.Daley G. N Engl J Med. 2004;351:627–628. doi: 10.1056/NEJMp048200. [DOI] [PubMed] [Google Scholar]
- 4.Murray F. Am J Sociol. in the press. [Google Scholar]
- 5.Walsh JP, Cho C, Cohen WM. Science. 2005;309:2002–2003. doi: 10.1126/science.1115813. [DOI] [PubMed] [Google Scholar]
- 6.David PA. Am Econ Rev. 1985;75:332–337. [Google Scholar]
- 7.McCormick JB, Owen-Smith J, Scott CT. Cell Stem Cell. 2009;4:107–110. doi: 10.1016/j.stem.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott CT, Owen-Smith J, McCormick JB. Nature. 2009;460:33. doi: 10.1038/460033b. [DOI] [PubMed] [Google Scholar]
- 9.Cromie WJ. Harvard University Gazette. 2004 Mar 4; http://www.news.harvard.edu/gazette/2004/03.04/01-stemcells.html.
- 10.Chen AE, et al. Cell Stem Cell. 2009;4:103–106. doi: 10.1016/j.stem.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen-Smith J, McCormick JB. Nat Biotechnol. 2006;24:391–392. doi: 10.1038/nbt0406-391. [DOI] [PubMed] [Google Scholar]
- 12.Caulfield T, et al. Stem Cell Rev. 2009;5:82–88. doi: 10.1007/s12015-009-9071-3. [DOI] [PubMed] [Google Scholar]