Abstract
A new technique for prospectively correcting head motion (called PROMO) during acquisition of high-resolution MRI scans has been developed to reduce motion artifacts. To evaluate the efficacy of PROMO, four T1-weighted image volumes (two with PROMO enabled, two uncorrected) were acquired for each of nine children. A radiologist, blind to whether PROMO was used, rated image quality and artifacts on all sagittal slices of every volume. These ratings were significantly better in scans collected with PROMO relative to those collected without PROMO (Mann-Whitney U test, P<0.0001). The use of PROMO, especially in motion-prone patients, should improve the accuracy of measurements made for clinical care and research, and potentially reduce the need for sedation in children.
Keywords: Motion correction, Head motion, MRI, Children
Introduction
Artifacts due to patient motion can be a significant impediment to acquiring clinically relevant MRI data. While all subject populations are susceptible to such artifacts, these problems are especially severe with young children, who frequently have difficulty remaining still during the time required to collect high-resolution data. Oftentimes, children must be sedated to obtain clinically useful data, a practice that is undesirable and adds a potential risk factor to the patient [1, 2]. Although methods to limit the severity of motion artifacts in MRI exist, they frequently have significant limitations attached, such as the inability to account for through-plane motion, additional required equipment or markedly increased scan time. In this paper, we present a new method of prospective motion correction, known as PROMO, that greatly reduces motion artifacts and significantly improves the clinical utility of structural MRI scans. PROMO provides the ability to account for both in-plane and through-plane motion and does so with only a minimal increase in scan time (depending on the degree of subject motion). To demonstrate the efficacy of PROMO, a small cohort of children (ages 9–12) was scanned with PROMO enabled or disabled. The resulting scans show a noticeable improvement in diagnostic image quality as rated by a neuroradiologist.
Description
A total of nine children (three girls; mean age: 10.8 years; standard deviation: 0.6 years; age range: 9.9 to 11.6 years) were recruited for this study, which was conducted with approval of the Institutional Review Board and in accordance with the rules and regulations of HIPAA.
A total of four T1-weighted scans were acquired for each subject, using a previously described sequence [3, 4]. All scans were performed on a 1.5-T Signa HDx Twin Speed system (GE Healthcare Technologies, Milwaukee, WI) with an 8-channel phased-array head coil. PROMO enabled and disabled scans were collected in an alternating pattern, and the acquisition order was counterbalanced across subjects. Children were instructed to remain as still as possible throughout the examination. Real-time motion tracking and correction with the navigator sequence was performed using the extended Kalman filter (EKF) algorithm [5] applied to MRI as reported by White and colleagues [6, 7] and as described previously for prospective motion correction in spiral-navigated 3-D pulse sequences [3, 8]. Five sets of three orthogonal low flip angle, single-shot spiral navigator scans (S-Navs) were interspersed within the dead time of the four 3-D IR-SPGR T1 scans acquired on each subject. These S-Navs were inserted in order to measure and adjust for head movement while scanning with PROMO enabled [7]. Online navigator-derived motion measures were used to adjust the image coordinate system with respect to brain position and to automatically rescan images that were acquired during intervals with particularly high motion, as determined by the position difference between the two navigator scans that bracket each acquisition partition.
Motion estimates for the PROMO-enabled volumes were computed from navigator scans, tracking position as a six-element vector. As an index of each individual’s magnitude of head motion, the L2 norm of the motion measures was computed across both PROMO-on scans for each child for both translation and rotation. This L2 norm is a normalized measure of the magnitude of variability in motion (in this case, the square root of the sum of squares of the range of motion) and has the advantage of being independent of relative position in the x, y, z coordinate space. Motion estimates were not calculated for the two PROMO-off scans since tracking and correction were disabled.
Neuroradiological rating system
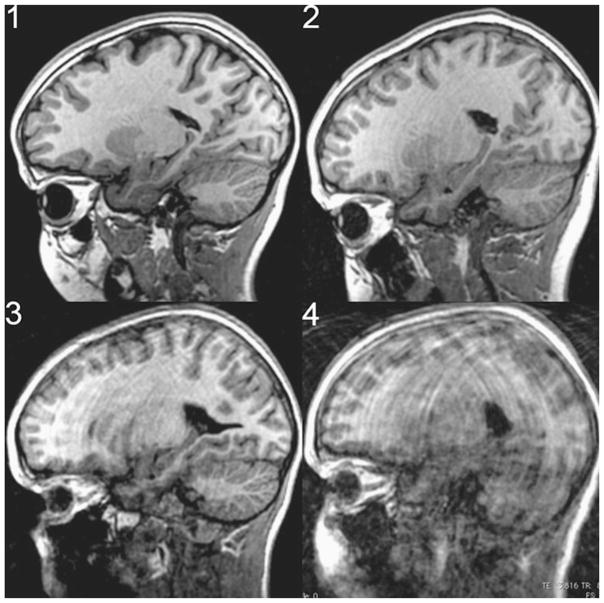
A numerical ratings system was developed by a clinical radiologist to reflect the degree of motion corruption and its effect on the clinical utility of the image. This rating system ranged from 0 to 4. A rating of zero indicates that a slice is free of motion. A rating of 1 would be characterized by curvilinear motion artifacts that are barely perceptible and occupy <50% of the surface area of the brain in the sagittal slice, or mild motion artifacts that are observed mostly in white matter. This motion is barely visible and could easily be overlooked without careful review. A slice given a rating of 2 would have motion artifacts that are perceptible in >50% of the brain in the given slice and extend to visibly involve the gray matter, or cause very minimal stepladder artifact at the gray/white matter interface. This motion can easily be seen on an image but would not compromise the evaluation of the scan for most medical indications. A rating of 3 would have motion artifacts that involved 100% of the image surface area, encompassing both brain and soft tissues, cause moderate motion artifact bands in white and gray matter, as well as moderate stepladder artifact at gray/white matter junction. This motion results in images that can still be useful, especially for life-threatening indications such as a large hemorrhage/neoplasm, but are barely interpretable. A rating of four indicates severe motion artifacts that render the images non-diagnostic for medical interpretation. Figure 1 shows typical examples of the image-quality rating scale used.
Fig. 1.
Examples of image quality ratings provided by a clinical radiologist. The slice rating is shown in the top left corner of each representative image
Analysis
To test the null hypothesis that there is no difference in the distribution of ratings between scans acquired with PROMO enabled versus those acquired without PROMO, we used the Mann-Whitney U test, which is appropriate for ordinal data and makes no assumptions about normality. Mean image-quality ratings by the radiologist blinded to scan type showed a robust overall difference among the four scans (Mann-Whitney U, F=18.77, P<0.0001, one-tailed). Post-hoc pair-wise comparisons revealed significantly inferior mean image quality ratings for the pair of PROMO-off (2.52) scans relative to the PROMO-on (1.1) scans (P<0.01).
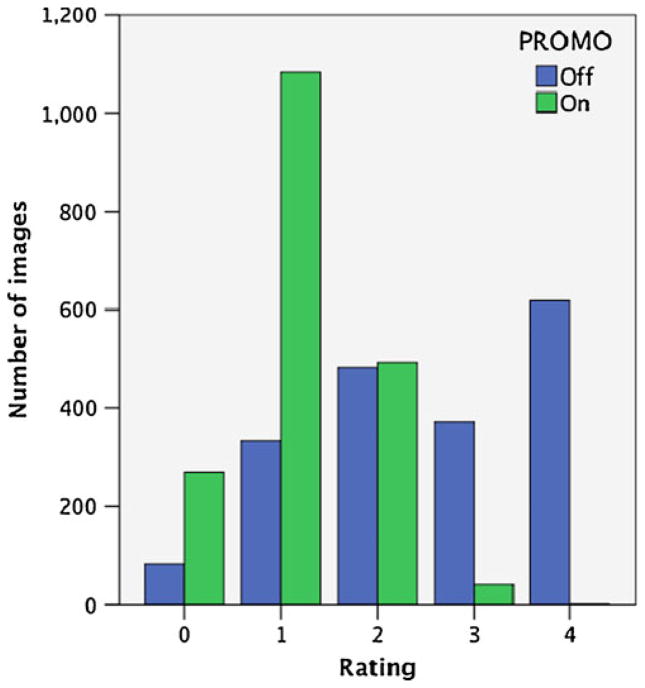
Figure 2 shows the total number of images rated at each image quality level for both methods. More than 600 images were rated as non-diagnostic with PROMO-off whereas PROMO-on produced only one non-diagnostic image. Table 1 shows the mean image quality rating for each of the four scans acquired collapsed across all subjects. The mean quality rating for PROMO-off scans was considerably higher than that acquired with PROMO enabled, indicating reduced diagnostic image quality when PROMO is not used. Table 2 shows the norm of the maximum translational and rotational displacement over the course of the two PROMO-on scans. These head motion measures showed relatively large-magnitude movements during the PROMO-on scans and varied considerably by individual, as expected of school-age children. Participant motion during scanning ranged from a norm of 2 mm translation and 2° of rotation to more than 1 cm of translation and 15° of rotation, depending on the individual.
Fig. 2.
Number of images rated at each quality level with PROMO on and PROMO off
Table 1.
Mean slice rating of each scan, averaged across all subjects. Slice ratings range from 0 (no motion artifacts) to 4 (severe motion artifacts, clinically uninterpretable)
| Scan | Mean slice rating | Standard deviation |
|---|---|---|
| PROMO off #1 | 2.3061 | 2.0172 |
| PROMO on #1 | 1.0044 | 0.9204 |
| PROMO off #2 | 2.7248 | 3.1284 |
| PROMO on #2 | 1.1933 | 1.2381 |
Table 2.
L2 norm of motion range for PROMO-on scans by subject
| Subject | Translation (mm) | Rotation (degree) |
|---|---|---|
| 1 | 10.7 | 12 |
| 2 | 10.5 | 9.2 |
| 3 | 7.5 | 15 |
| 4 | 6.5 | 5.8 |
| 5 | 3.9 | 5 |
| 6 | 3.3 | 5.5 |
| 7 | 2.6 | 2.4 |
| 8 | 2.1 | 2.9 |
| 9 | 2 | 5.1 |
Discussion
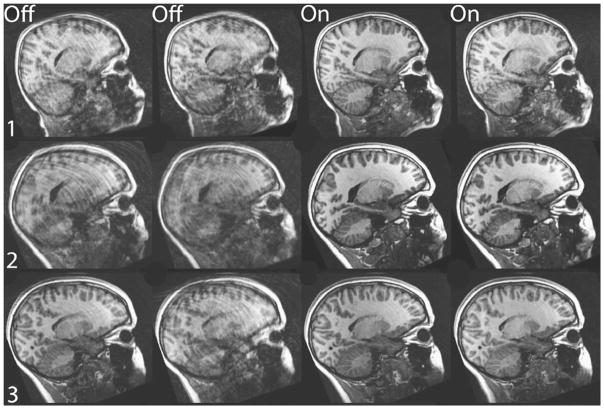
In this study, we tested a novel real-time prospective motion correction method, known as PROMO, for structural MRI of children. The advantages of PROMO can be seen clearly in Fig. 3. Scans obtained with PROMO enabled show visibly fewer motion artifacts, thereby increasing their diagnostic utility. This improvement is illustrated in Fig. 2, which shows far fewer non-diagnostic or minimally diagnostic images for scans acquired with PROMO. PROMO-on images achieved this high level of diagnostic utility in spite of large translational and rotational movements by the children during scanning, as indicated in Table 2. Mean image-quality ratings, shown in Table 1, are also universally superior for PROMO-on scans compared to those acquired without PROMO.
Fig. 3.
Single sagittal slice from each T1-W scan of the three children with the largest measured in-scan motion. Columns 1 and 2 were acquired with PROMO off and columns 3 and 4 were acquired with PROMO on
In addition to image quality, one of the key factors for successful clinical practice is effective cost management and efficient use of scanner time. If children require rescans or return visits, imaging time that could be used scanning additional children is instead spent obtaining diagnostic data on the same patient. Worse still, if the child is unable to hold still for the time required to obtain clinical data, sedation might be deemed necessary, which would involve considerable added personnel costs, in addition to the scanner time required for the reacquisition. In contrast, we showed naturalistically with children that PROMO resulted in diagnostically useful scans in all cases that we tested, even in this difficult subject population. Furthermore, PROMO does so with only a minimal increase in scan time (in most cases less than 10 s of additional scan time, depending on patient motion), allowing for more efficient use of scanner equipment. Obtaining clinically useful data without having to resort to rescanning or sedation allows for the maximum throughput in clinical practice and minimizes inconvenience and risk factors for patients.
Although the results of this study are highly encouraging, there are several limitations. We were unable to precisely measure subject motion during PROMO-off scans, so some differences in image quality could be due to increased subject motion during PROMO-off scans. However, any systematic bias should be minimized by the counterbalancing of the order in which scans were acquired with and without PROMO. Additionally, here we restricted our analysis to high-resolution, 3-D T1-weighted scans only. While PROMO has already been implemented in a 3-D FSE (CUBE) scanning sequence, these scans were not acquired in the current study, due to time limitations. However, preliminary results suggest comparable improvements in image quality of 3-D CUBE T2 and FLAIR acquisitions, using the PROMO technology.
Acknowledgments
The authors would like to thank the children and parents who volunteered to participate in this study, which was supported by grants from the National Institutes of Health Specialized Neuroscience Research Programs (U54 NS056883), the National Institute on Drug Abuse (RC2 DA029475), the National Institute of Neurological Disorders and Stroke (P50 NS022343), support from General Electric, and by a fellowship from the UCSD Institute for Neural Computation.
Contributor Information
Joshua M. Kuperman, Email: jkuperman@ucsd.edu, 8950 Villa La Jolla Drive, Suite C-101, La Jolla, CA 92093, USA. Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA
Timothy T. Brown, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Neurosciences, University of California, San Diego, La Jolla, CA, USA
Mazyar E. Ahmadi, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA
Matthew J. Erhart, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA
Nathan S. White, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA
J. Cooper Roddey, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA.
Ajit Shankaranarayanan, Applied Science Laboratory, GE Healthcare, Menlo Park, CA, USA.
Eric T. Han, Applied Science Laboratory, GE Healthcare, Menlo Park, CA, USA
Dan Rettmann, Applied Science Laboratory, GE Healthcare, Rochester, MN, USA.
Anders M. Dale, Multimodal Imaging Laboratory, University of California, San Diego, La Jolla, CA, USA. Department of Radiology, University of California, San Diego, La Jolla, CA, USA. Department of Neurosciences, University of California, San Diego, La Jolla, CA, USA
References
- 1.Hubbard A, Markowitz RI, Kimmel B, et al. Sedation for pediatric patients undergoing CT and MRI. J Comput Assist Tomogr. 1992;16:3–6. doi: 10.1097/00004728-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sanborn P, Michna E, Zurakowski D, et al. Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology. 2005;237:288–294. doi: 10.1148/radiol.2371041415. [DOI] [PubMed] [Google Scholar]
- 3.Shankaranarayanan A, Roddey C, White NS, et al. Motion insensitive 3D imaging using a novel real-time image-based 3D PROspective MOtion Correction Method (3D PROMO) ISMRM; Germany: 2007. [Google Scholar]
- 4.Brown TT, Kuperman JM, Erhart MJ, et al. Prospective motion correction of high-resolution magnetic resonance imaging data in children. Neuroimage. 2010;53:139–145. doi: 10.1016/j.neuroimage.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelb A. Applied optimal estimation. MIT; Cambridge: 1974. [Google Scholar]
- 6.White NS, Shankaranarayanan A, Han ET. Prospective motion correction using nonlinear predictive filtering. ISMRM; Germany: 2007. [Google Scholar]
- 7.White NS, Roddey C, Shankaranarayanan A. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 2010;63:91–105. doi: 10.1002/mrm.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roddey C, Shankaranarayanan A, Han ET. Motion insensitive imaging using 3D PROspective MOtion (PROMO) correction with region-of-interest tracking. ISMRM; Canada: 2008. [Google Scholar]