Abstract
Objective:
Colorectal cancer (CRC) is one of the most common and aggressive cancers worldwide. The majority of CRC cases are sporadic that caused by somatic mutations. The Adenomatous Polyposis Coli (APC; OMIM 611731) is a tumor suppressor gene of Wnt pathway and is frequently mutated in CRC cases. This study was designed to investigate the spectrum of APC gene mutations in Iranian patients with sporadic colorectal cancer.
Materials and Methods:
In this descriptive study, Tumor and normal tissue samples were obtained from thirty randomly selected and unrelated sporadic CRC patients. We examined the hotspot region of the APC gene in all patients. Our mutation detection method was direct DNA sequencing.
Results:
We found a total of 8 different APC mutations, including two nonsense mutations (c.4099C>T and c.4348C>T), two missense mutations (c.3236C>G and c.3527C>T) and four frame shift mutations (c.2804dupA, c.4317delT, c.4464_4471delATTACATT and c.4468_4469dupCA). The c.3236C>G and c.4468_4469dupCA are novel mutations. The overall frequency of APC mutation was 26.7% (8 of 30 patients).
Conclusion:
This mutation rate is lower in comparison with previous studies from other countries. The findings of present study demonstrate a different APC mutation spectrum in CRC patients of Iranian origin compared with other populations.
Keywords: Colorectal Cancer, APC, Iran
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second in women worldwide. The incidence rates of CRC in Iranian males and females are 8.7 and 6.4 in 100,000 respectively (1). Compared with Western countries, CRC incidence rates are low in south Asia, but recent studies in Iran have shown a significant increase in the rate of colorectal cancer (2-4). The majority (75-80%) of CRC cases is without a family history and arises by somatic mutations in colon and rectum (5, 6). Mutations in the APC gene occur in 34- 80% of sporadic CRC cases (7, 8). The APC tumor suppressor gene consists of 15 exons with exon 15 covering more than 75% of the coding sequence. About 60% of all mutations in APC occur in the mutation cluster region (MCR) between codons 1286 and 1513 in exon 15 (9, 10). The majority of APC mutations in the MCR introduce a stop codon, resulting in a truncated protein that lacks the binding site for two important interactants, β-catenin and axin, which act together in the Wnt signaling pathway (11).
The APC protein participates in many of the fundamental cellular processes such as proliferation, differentiation, migration and apoptosis (12). This protein is a negative regulator of the Wnt signaling pathway and is involved in cellular proliferation and differentiation. APC is also involved in the dynamics of cytoskeleton, and has an impact on apoptosis (13-15). This multifunctionality of APC protein may explain why disrupting APC is harmful to the epithelium of the intestine (12).
In 1990, Fearon and Vogelstein (16) first proposed a multistep genetic model for colorectal tumorigenesis. According to this hypothesis, activation of the Wnt signaling through disruption of APC gene is the earliest genetic event in colorectal tumorigenesis. Inactivation of APC gene occurs by mutation, loss of heterozygosity (LOH) or promoter hypermethylation (9). Due to the lack of a systematic investigation of APC gene mutations in Iran, we attempted to screen the MCR region for putative changes in a cohort of Iranian individuals that suffer from CRC.
Materials and Methods
Patients
In this descriptive study, fresh colon tissue from thirty sporadic CRC patients with no family history (18 men, 12 women) were collected that referred to Mehr and Emam hospitals in Ahvaz in the period from November 2009 to February 2011. Tumor and adjacent normal tissue specimens were obtained from all patients after obtaining formal consent and were stored at -80˚C until use. The specimens had the histopathologic characteristics of adenocarcinoma.
This project was approved by the Ethical Committee of Ahvaz Jundishapur University of Medical Sciences.
Genomic DNA extraction
The DNA from tumor and normal tissue was separately extracted using the AccuPrep® Genomic DNA Extraction Kit (Bioneer Corporation, Daejeon, Korea).
Primers and polymerase chain reaction
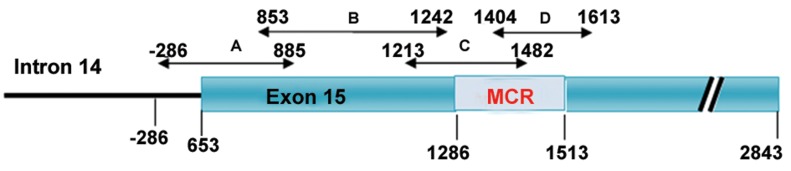
Four primer pairs were designed for four overlapping fragments (codons 653-885, 853- 1242, 1213-1482 and 1404-1613) of exon 15 (Table 1). The positions and the size of polymerase chain reaction (PCR) products are illustrated in figure 1. Amplification was performed in a total volume of 30 μl of reaction mixture containing 80-130 ng genomic DNA, 1X PCR buffer, 1.5 mmol/L of MgCl2, 0.2 mmol/L of dNTP, 0.2-0.4 μmol/L of each primer, and 3 U Super Taq DNA polymerase (Gen Fanavaran Ldt, Tehran, Iran). PCR conditions were as follows: initial denaturation at 94˚C for 5 minutes, 30 cycles of denaturation at 94˚C for 1 minute, annealing for 45 seconds and extension at 72˚C for 1 minute, and a final extension at 72˚C for 7 minutes.
Table 1.
Primers characteristics using for PCR amplification.
| Fragment | Primer | Ta (°C) | Fragment length (bp) |
|---|---|---|---|
| A | AF: 5´-AGTAAATGTATGTGCCCCACCCCC-3´ | 68 | 984 |
| AR: 5´-GGGCTGCAGTGGTGGAGATCTG-3´ | |||
| B | BF: 5´-TGGAGAGAGAACGCGGAATTGG-3´ | 66 | 1173 |
| BR: 5´-GCTGACCACTTCTACTCTGTGCAG-3´ | |||
| C | CF: 5´-CAAGCAGTGAGAATACGTCCACAC-3´ | 64 | 808 |
| CR: 5´-AGAACCTGGACCCTCTGAACTGCA-3´ | |||
| D | DF: 5´-TCCGTTCAGAGTGAACCATGCA-3´ | 65 | 628 |
| DR: 5´-GCAGCTGACTTGGTTTCCTTGCCA-3´ | |||
Fig 1.
The structure of the APC gene is partly demonstrating the intron 14 and exon 15. Horizontal arrows show the positions of the overlapped fragments covering the nucleotide -286 in intron 14 and codon 1613 within exon 15.
DNA sequencing
The purified PCR products were sequenced by Macrogen (Seoul, Korea) using an Applied Biosystems 3730 DNA Analyzer. Sequence chromatograms were analysed using the Chromas software and NCBI BLAST tool. When a mutation was found in a tumor DNA sample, the corresponding normal DNA was systematically checked for the absence of this mutation.
Results
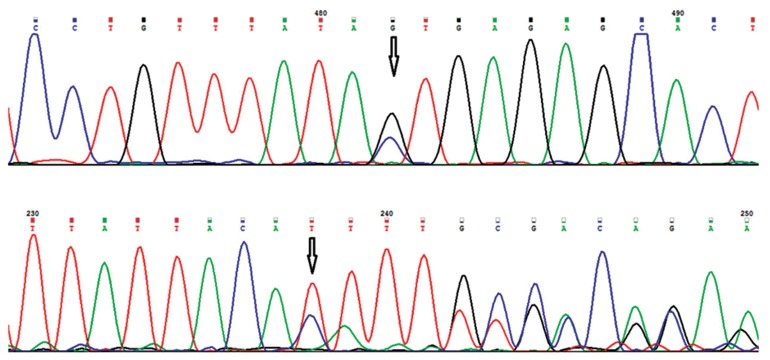
Genomic DNA sequencing of codon 653 to 1613 of APC gene and exon 15-intron 14 boundary enabled us to identify a total of 8 different mutations in 8 sporadic CRC patients (Table 2), including two nonsense mutations (25%), two missense mutations (25%) and four frameshift mutations (50%). Two of 8 mutations namely c.3236C>G (p.Thr1079Ser) and c.4468_4469dupCA (p.Phe1491IlefsX17) have not previously been reported in Human Gene Mutation Database (HGMD), Sanger-APC mutations database, the Leiden Open Variation Database (LOVD) and the literature, indicating that they are novel mutations (Table 3, Fig 2). The c.3236C>G mutation (Fig 2A) results in an amino acid substitution (p.Thr1079Ser) with unknown effect and the c.4468_4469dupCA mutation (Fig 2B) leads to a premature stop-codon at position 1507. The remaining 6 APC mutations had been reported previously. Of the 8 identified mutations, 5 (62.5%) occurred in the MCR. All mutations, with the exception of p.Thr1079Ser and p.Pro1176Leu, lead to truncated APC protein. The patients 7 and 18 showed two mutations; each had a truncating mutation and a missense change. The overall frequency of APC mutation was 26.7% (8 of 30 cases). Pathogenic mutation was found in 7 patients (23.3%) of which 6 (20% of total) showed mutation in the MCR. We detected three polymorphisms, including one non-synonymous (p.Glu1317Gln), one synonymous (p.Thr1493Thr) and one intronic (c.1959-143_1959-140dupAGAA) polymorphism. These polymorphisms were reported previously according to Human Variation database. Two patients had p.Glu1317Gln polymorphism. This non-synonymous variant is considered to play a role in the development of colorectal tumors (17). The p.Thr1493Thr polymorphism was observed in twenty three patients. Twenty two patients carrying the c.1959-143_1959-140dupAGAA polymorphism. We also found the novel variant c.3345G>T (p.Val1115Val) in 2 patients. Human Variation: http://www.ncbi.nlm.nih.gov/projects/SNP/ tranSNP/tranSNP.cgi.
Table 2.
APC mutation status in relation to clinicopathological variables.
| Characteristic | Frequency (%) | Yes (n=8) | No (n=22) |
|---|---|---|---|
| Age at diagnosis | |||
| <60 | 12 (40) | 4 (33.3%) | 8 (66.7%) |
| ≥60 | 18 (60) | 4 (22.2%) | 14 (77.8%) |
| Sex | |||
| Male | 18 (60) | 4 (22.2%) | 14 (77.8%) |
| Female | 12 (40) | 4 (33.3%) | 8 (66.7%) |
| Tumor location | |||
| Right colon | 12 (40) | 2 (16.7%) | 10 (83.3%) |
| Left colon | 9 (30) | 3 (33.3%) | 6 (66.7%) |
| Rectum | 9 (30) | 3 (33.3%) | 6 (66.7%) |
| Tumor histology | |||
| Poorly differentiated | 2 (6.67) | 0 | 2 (100%) |
| Moderately differentiated | 11 (36.67) | 4 (36.4%) | 7 (63.6%) |
| Well differentiated | 17 (56.67) | 4 (23.5%) | 13 (76.5%) |
Table 3.
APC mutations in Iranian sporadic CRC patients; novel mutations are in bold.
| Patient ID | DNA change | Protein change | Mutation type | Origin |
|---|---|---|---|---|
| 3 & 7 | c.3527C>T | p.Pro1176Leu | Missense | Somatic |
| 7 | c.2804dupA | p.Tyr935fsX1 | Frameshift | Somatic |
| 18 | c.3236C>G | p.Thr1079Ser | Missense | Germline |
| 18 | c.4099C>T | p.Gln1367X | Nonsense | Somatic |
| 20 | c.4317delT | p.Pro1440HisfsX33 | Frameshift | Somatic |
| 23 & 30 | c.4348C>T | p.Arg1450X | Nonsense | Somatic |
| 25 | c.4464_4471delATTACATT | p.Leu1488PhefsX23 | Frameshift | Somatic |
| 28 | c.4468_4469dupCA | p.Phe1491IlefsX17 | Frameshift | Somatic |
Fig 2.
DNA sequences of novel APC mutations. A. Chromatogram of c.3236C>G (p.Thr1079Ser) mutation. B. Chromatogram of c.4468_4469dupCA (p.Phe1491IlefsX17) mutation.
Discussion
The CRC has a high incidence of mortality in western countries that was also subjected for intensive investigations. Otherwise, its molecular characteristics remained widely unknown in Middle Eastern countries. Recent epidemiologic studies in Iran show rapid increase in the rate of CRC (2, 4) and with rising of the CRC cases in the developing countries, attention should be given to extend the underlying molecular knowledge of this mostly heterogeneous cancer (18). For instance, beside (loss of heterozygosity) LOH and hypermethylation, inactivation of the APC gene by mutation has been observed in different type of colon cancer, such as familial or sporadic and with or without polyposis (9). It is widely accepted that the APC gene inactivation is the first event in a multistep process of the CRC (16). Surprisingly and because of heterogeneous nature of the sporadic CRC, some tumors show no mutation in the APC gene and some others reveal high mutation rate (19). This attitude appears to be dependent on ethnicity, geographic region, dietary and genetic predisposition (20, 21).
In the present study, the APC gene was subjected for mutation survey in individuals suffering from sporadic type of CRC in southwest Iran.
We observed a mutation rate of 26.7% in a large part of exon 15 in the APC gene. Truncating mutations (nonsense and frameshift mutations) in the MCR were observed in 6 (20%) tumor samples, which is in concordance with the rate in Hungary and Tunisia (22, 23), but lower than in other European countries, the USA and Japan (Table 4, 24- 29). The mutation cluster region (codon 1286 to 1513) corresponds to the 20-amino acid sequence, exhibiting the β-catenin and the axin binding sites (9). A truncating mutation in the MCR abolishes this functional domain, which in turn leads to the cytoplasmic and nuclear accumulation of the β-catenin protein in the colorectal cells. The β-catenin accumulation is associated with activation of Wnt signaling pathway that promotes the generation of tumors (30).
Table 4.
Frequency of APC mutation in different populations.
| Country /Study | The studied area (codons) | Number of patients | Frequency of APC mutation (%) |
|---|---|---|---|
| Iran (present study) | 653-1613 | 30 | 23.3 |
| Hungary (22) | 1285-1465 | 70 | 21.4 |
| Tunisia (23) | 1240-1513 | 48 | 20.8 |
| Netherland (24) | 1286-1520 | 656 | 37.3 |
| Germany (25) | 1260-1547 | 99 | 49.5 |
| Norway(8) | 653-2843 | 218 | 66 |
| UK (11) | 1028-1712 | 106 | 56.6 |
| France(26) | 653-2843 | 85 | 57.6 |
| USA(27) | 1286-1585 | 90 | 34.4 |
| Japan(28) | 582-1580 | 61 | 47.5 |
| South Korea(29) | 1202-1674 | 78 | 33.3 |
Seven of our patients (23.3%) showed here somatic inactivation of one of the APC alleles. Besides mutation in other part of the APC gene, LOH and promoter hypermethylation may be the possible second hit mechanism in our cases. From two mutation hotspots at codons 1309 and 1450 (31), we only found 2 cases with nonsense mutation at codon 1450. The p.Phe1491IlefsX17 frameshift mutation and the p.Thr1079Ser missense mutation are novel that have not been published before. All detected nonsense and frameshift mutations in this study lead to the truncated form of the APC protein, underlining their pathogenic nature. The functional consequence of the p.Thr1079Ser and the p.Pro1176Leu changes is not clear. Furthermore, we propose these amino acid substitutions may cause damaging impact on the structure and function of APC protein. We also found a novel variant (p.Val1115Val) that its effect in the development of CRC is unknown and needs to be screened in a number of normal individuals.
Conclusion
Our findings demonstrate a unique APC mutation profile in Iranian CRC patients and support the idea that the spectrum of somatic APC mutations in CRCs are considerably variable and distinct among populations (32).
Although inactivating mutation of the APC gene was present in 23.3% of all the tumor cases studied, the actual percentage might be higher, because the present study focused only on the 5' half of APC exon 15, which includes the mutation cluster region. Hence, further mutation studies have to be conducted for the whole length of the APC gene for more evaluation. We also conclude that the MCR does not represent the hotspot region for mutation, at least in Iranian CRC patients.
To date, some hundred mutations have been reported in the APC gene. Some of these mutations occur across all ethnicity and populations and some others are specific to distinct geographic regions. The novel mutations presented in this study may be private to this region and therefore need to be screened country- wide in a large cohort of sporadic CRC patients in Iran.
Acknowledgments
This study was financially supported by The Toxicology Research Center of Ahvaz Jundishapur University of Medical Sciences. We are thankful to Mr. Mohammad Moghaddam for technical assistance. The authors declare that there is no conflict of interest in this article.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, cancer incidence and mortality worldwide: IARC cancerBase number 10. Lyon, France: International agency for research on cancer; 2010. Available from: http://www.globocan.iarc.fr. (19 Sep 2011) [Google Scholar]
- 2.Ansari R, Mahdavinia M, Sadjadi A, Nouraie M, Kamangar F, Bishehsari F, et al. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer Lett. 2006;240(1):143–147. doi: 10.1016/j.canlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular genetics of colorectal cancer in iran: a review. Arch Iran Med. 2009;12(2):161–169. [PubMed] [Google Scholar]
- 4.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20(3):556–563. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 5.Morán A, Ortega P, de Juan C, Fernández-Marcelo T, Frías C, Sánchez-Pernaute A, et al. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2(3):151–158. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4(10):769–780. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 7.Luchtenborg M, Weijenberg MP, Roemen GM, de Bruine AP, van den Brandt PA, Lentjes MH, et al. APC mutations in sporadic colorectal carcinomas from The Netherlands Cohort Study. Carcinogenesis. 2004;25(7):1219–1226. doi: 10.1093/carcin/bgh117. [DOI] [PubMed] [Google Scholar]
- 8.Løvig T, Meling GI, Diep CB, Thorstensen L, Norheim Andersen S, Lothe RA, et al. APC and CTNNB1 mutations in a large series of sporadic colorectal carcinomas stratified by the microsatellite instability status. Scand J Gastroenterol. 2002;37(10):1184–1193. doi: 10.1080/003655202760373407. [DOI] [PubMed] [Google Scholar]
- 9.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10(7):721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 10.van Es JH, Giles RH, Clevers HC. The many faces of the tumor suppressor gene APC. Exp Cell Res. 2001;264(1):126–134. doi: 10.1006/excr.2000.5142. [DOI] [PubMed] [Google Scholar]
- 11.Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, et al. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci USA. 2002;99(14):9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCartney BM, Nathke IS. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol. 2008;20(2):186–193. doi: 10.1016/j.ceb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senda T, Iizuka-Kogo A, Onouchi T, Shimomura A. Adenomatous polyposis coli (APC) plays multiple roles in the intestinal and colorectal epithelia. Med Mol Morphol. 2007;40(2):68–81. doi: 10.1007/s00795-006-0352-5. [DOI] [PubMed] [Google Scholar]
- 15.Qian J, Perchiniak EM, Sun K, Groden J. The mitochondrial protein hTID-1 partners with the caspase- cleaved adenomatous polyposis cell tumor suppressor to facilitate apoptosis. Gastroenterology. 2010;138(4):1418–1428. doi: 10.1053/j.gastro.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 17.Dallosso AR, Jones S, Azzopardi D, Moskvina V, Al-Tassan N, Williams GT, et al. The APC variant p.Glu1317Gln predisposes to colorectal adenomas by a novel mechanism of relaxing the target for tumorigenic somatic APC mutations. Hum Mutat. 2009;30(10):1412–1418. doi: 10.1002/humu.21089. [DOI] [PubMed] [Google Scholar]
- 18.Jass JR. Molecular heterogeneity of colorectal cancer: implications for cancer control. Surg Oncol. 2007;16(Suppl 1):S7–S9. doi: 10.1016/j.suronc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiencke JK. Impact of race/ethnicity on molecular pathways in human cancer. Nat Rev Cancer. 2004;4(1):79–84. doi: 10.1038/nrc1257. [DOI] [PubMed] [Google Scholar]
- 21.Koo JH, Kin S, Wong C, Jalaludin B, Kneebone A, Connor SJ, et al. Clinical and pathologic outcomes of colorectal cancer in a multi-ethnic population. Clin Gastroenterol Hepatol. 2008;6(9):1016–1021. doi: 10.1016/j.cgh.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Kámory E, Olasz J, Csuka O. Somatic APC inactivation mechanisms in sporadic colorectal cancer cases in hungary. Pathol Oncol Res. 2008;14(1):51–56. doi: 10.1007/s12253-008-9019-y. [DOI] [PubMed] [Google Scholar]
- 23.Bougatef K, Ouerhani S, Moussa A, Kourda N, Coulet F, Colas C, et al. Prevalence of mutations in APC, CTNNB1, and BRAF in Tunisian patients with sporadic colorectal cancer. Cancer Genet Cytogent. 2008;187(1):12–18. doi: 10.1016/j.cancergencyto.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Lüchtenborg M, Weijenberg MP, Wark PA, Saritas AM, Roemen GM, van Muijen GN, et al. Mutations in APC, CTNNB1 and K-ras genes and expression of hMLH1 in sporadic colorectal carcinomas from the Netherlands Cohort Study. BMC cancer. 2005;5:160–160. doi: 10.1186/1471-2407-5-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prall F, Weirich V, Ostwald C. Phenotypes of invasion in sporadic colorectal carcinomas related to aberrations of the adenomatous polyposis coli (APC ) gene. Histopathology. 2007;50(3):318–330. doi: 10.1111/j.1365-2559.2007.02609.x. [DOI] [PubMed] [Google Scholar]
- 26.Olschwang S, Hamelin R, Laurent-Puig P, Thuille B, De Rycke Y, Li YJ, et al. Alternative genetic pathways in colorectal carcinogenesis. Proc Natl Acad Sci USA. 1997;94(22):12122–12127. doi: 10.1073/pnas.94.22.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samowitz WS, Slattery ML, Sweeney C, Herrick J, Wolff RK, Albertsen H. APC mutations and other genetic and epigenetic changes in colon cancer. Mol Cancer Res. 2007;5(2):165–170. doi: 10.1158/1541-7786.MCR-06-0398. [DOI] [PubMed] [Google Scholar]
- 28.Miyaki M, Iijima T, Ishii R, Kita Y, Koike M, Kuroki T, et al. Increased frequency of p53 mutation in sporadic colorectal cancer from cigarette smokers. Jpn J Clin Oncol. 2002;32(6):196–201. doi: 10.1093/jjco/hyf047. [DOI] [PubMed] [Google Scholar]
- 29.Jeon CH, Lee HI, Shin IH, Park JW. Genetic alterations of APC, K-ras, p53, MSI, and MAGE in Korean colorectal cancer patients. Int J Colorectal Dis. 2008;23(1):29–35. doi: 10.1007/s00384-007-0373-0. [DOI] [PubMed] [Google Scholar]
- 30.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1(1):55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 31.Béroud C, Soussi T. APC gene: database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996;24(1):121–124. doi: 10.1093/nar/24.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JY, Hsieh JS, Lu CY, Yu FJ, Wu JY, Chen FM, et al. The differentially mutational spectra of the APC, K-ras, and p53 genes in sporadic colorectal cancers from Taiwanese patients. Hepatogastroenterology. 2007;54(80):2259–2265. [PubMed] [Google Scholar]