Abstract
Objective:
In vitro production of a definitive endoderm (DE) is an important issue in stem cell-related differentiation studies and it can assist with the production of more efficient endoderm derivatives for therapeutic applications. Despite tremendous progress in DE differentiation of human embryonic stem cells (hESCs), researchers have yet to discover universal, efficient and cost-effective protocols.
Materials and Methods:
In this experimental study, we have treated hESCs with 200 nM of Stauprimide (Spd) for one day followed by activin A (50 ng/ml; A50) for the next three days (Spd- A50). In the positive control group, hESCs were treated with Wnt3a (25 ng/ml) and activin A (100 ng/ml) for the first day followed by activin A for the next three days (100 ng/ml; W/A100-A100).
Results:
Gene expression analysis showed up regulation of DE-specific marker genes (SOX17, FOXA2 and CXCR4) comparable to that observed in the positive control group. Expression of the other lineage specific markers did not significantly change (p<0.05). We also obtained the same gene expression results using another hESC line. The use of higher concentrations of Spd (400 and 800 nM) in the Spd-A50 protocol caused an increase in the expression SOX17 as well as a dramatic increase in mortality rate of the hESCs. A lower concentration of activin A (25 ng/ml) was not able to up regulate the DE-specific marker genes. Then, A50 was replaced by inducers of definitive endoderm; IDE1/2 (IDE1 and IDE2), two previously reported small molecule (SM) inducers of DE, in our protocol (Spd-IDE1/2). This replacement resulted in the up regulation of visceral endoderm (VE) marker (SOX7) but not DE-specific markers. Therefore, while the Spd-A50 protocol led to DE production, we have shown that IDE1/2 could not fully replace activin A in DE induction of hESCs.
Conclusion:
These findings can assist with the design of more efficient chemically-defined protocols for DE induction of hESCs and lead to a better understanding of the different signaling networks that are involved in DE differentiation of hESCs.
Keywords: Definitive Endoderm, Embryonic Stem Cells, Differentiation, Activin A, Stauprimide
Introduction
Definitive endoderm (DE) is the origin of all embryonic endodermal organs such as the thyroid, lungs, liver, pancreas and intestines (1-5). Endodermal organs are subject to a number of illnesses, including type 1 diabetes mellitus and liver diseases, for which cell replacement therapies are a promising cure (6-8). Human embryonic stem cells (hESCs) are considered a valuable cell source for these therapeutic applications because of their ability to self-renew and potential to differentiate into different cell types (9). Since these stem cells mimic the in vivo developmental events during differentiation, the knowledge of embryology has been used to develop different stepwise protocols to produce endodermal tissues from hESCs (10- 12). The first step in these directed differentiation protocols is the induction of hESCs into DE.
Studies on amniote gastrulation show that the epiblast cells which pass through the anterior primitive streak encounter various concentrations of nodal, a member of the transforming growth factor-beta family (TGF-β) and form mesoderm, in addition to DE (13, 14). Other in vitro studies indicate that WNT, phosphatidylinositol 3-kinase (PI3K) and bone morphogenic proteins (BMPs) are important signaling pathways during the DE induction of embryonic stem cells (ESCs) (10, 15-17). The main growth factor inducer in DE differentiation protocols is activin A, which is also a member of the TGF-β family and a replacement for nodal. For example, it has been shown that the use of Wnt3a and activin A induces up to 80% of hESCs to express DE-specific markers such as SOX17 (15).
During recent years, as an alternative for growth factor inducers, cell-permeable bioactive small molecules (SMs) have been introduced as a means to manipulate stem cell signaling pathways (18-20). SMs can modulate DNA, RNA and protein functions. Their modulatory functions are specific, rapid and reversible. Additionally, they are less expensive (21). SMs are able to efficiently induce ESCs into different cell fates such as neural cells (22, 23), cardiomyocytes (24) and pancreatic cells (23). Inducers of definitive endoderm; IDE1/2 (IDE1 and IDE2), two SM inducers of DE formation, have the capability to efficiently produce DE cells from ESCs (25). SMs also can be used as suppressors of pluripotency in ESCs (21). For example, a 20000 SM screening study has shown that a SM named Stauprimide (Spd) can suppress pluripotency by inhibiting cellular myelocytomatosis oncogene (c-MYC) signaling. This suppression primes ESCs for lineage-specific differentiation (26).
During our previous study (27), we found that Rapamycin priming before activin A induction could efficiently differentiate hESCs into DE. We also observed high expression levels of SOX17 and FOXA2 in the hESCs which were primed with Spd before activin induction. Therefore, in this study we further tested the priming capability of Spd and its different concentrations toward activin-induced DE differentiation. We used Spd (200 nM) for the first day and activin A (50 ng/ml) for the following three days (Spd-A50) and after that, we attempted to replace activin A with IDE1/2. Our study showed that treatment of hESCs with Spd- A50 lead to endodermal differentiation. However activin A could not be replaced by SM IDE1/2.
Materials and Methods
Human embryonic stem cells culture
Royan H6 (passages 30-40) hESC (28) and Royan H5 (passages 25-30) hESC lines (from Royan Stem Cell Bank,Iran) were used in this experimental study. hESCs were maintained on Matrigel (Sigma-Aldrich, E1270, USA) in hESC medium that consisted of Dulbecco’s modified Eagle’s/Ham’s F12 medium (DMEM/F12, Invitrogen, USA, 21331-020); 20% (v/v) knockout serum replacement (KOSR, Invitrogen, USA, 10828-028); 1% (v/v) non-essential amino acids (Invitrogen, USA, 11140-050); penicillin/ streptomycin (Invitrogen, USA, 15140-122); ITS (insulin 1 mg/mL, transferrin 0.55 mg/mL, selenium 0.00067 mg/mL; Invitrogen, USA, 41400-045); 2 mM L-glutamine (Invitrogen, USA, 25030-032); 0.1 mM B-mercaptoethanol (2 ME, Sigma-Aldrich, USA, M7154); and 100 ng/mL basic fibroblast growth factor (bFGF, Royan Institute, Iran). Cells were grown in 5% CO2 at 95% humidity and passaged at a 1:4-1:6 split ratio every seven days with daily media changes.
Treating hESCs for endoderm formation
Before each differentiation step, cultured cells were given a brief wash in Dulbecco’s Phosphate-Buffered Saline with calcium and magnesium (DPBS, Gibco, 104040-182, USA). During differentiation (Fig 1A), 80% confluent hESCs were treated for one day with 200 nM Spd (Santa Cruz, USA, sc-202346) and for next three days with the 50 ng/ml activin A (R&D Systems, 338-AC) or 100/200 nM IDE1/IDE2 (Stemgent, USA, 04-0026 & 04-0027) in RPMI 1640 (Invitrogen, USA, 51800-035) supplemented with nonessential amino acids, L-glutamine, penicillin/ streptomycin, and 0.2% defined fetal bovine serum (FBS, HyClone, SH3007002, USA). For the positive control, as reported previously (29), hESCs were treated with 100 ng/ml activin A and 25 ng/ml Wnt3a (R&D Systems, USA, 5036-WN) in RPMI without FBS for one day followed by 100 ng/ml activin A in RPMI that contained 0.2% FBS for three days. As a negative control, cells were treated with 0.1% DMSO (Sigma-Aldrich, USA, D2650) for four days.
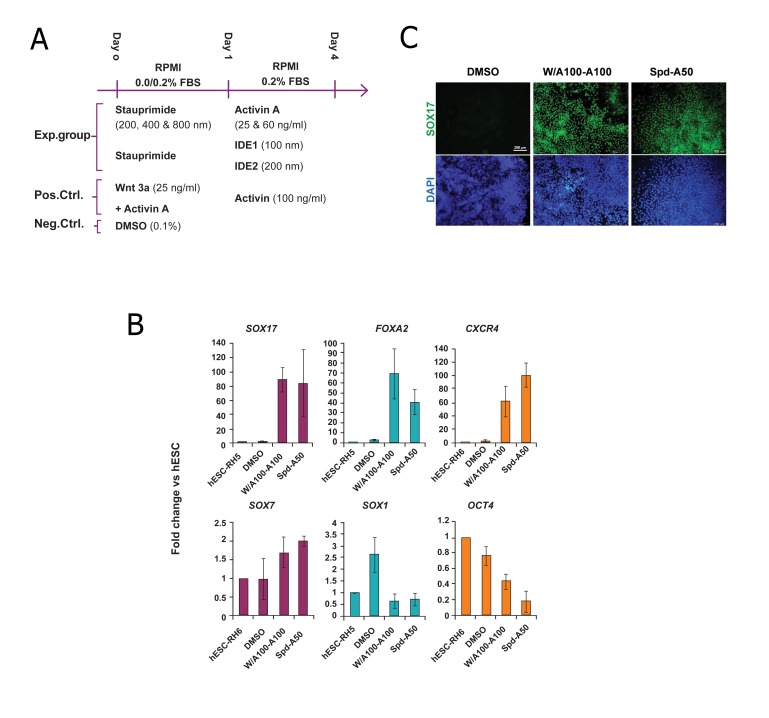
Fig 1.
Gene and protein expression analysis of definitive endoderm (DE) markers in experimental and control groups at day 4. A. Diagrammatic representation of the experimental groups (Spd-A50/A25 and Spd-IDE1/2), positive control (W/A100-A100) and negative control (DMSO) for endoderm induction of human embryonic stem cells (hESCs). B. Lineage-specific gene expression analysis of Spd-A50-treated Royan H6 human embryonic stem cells (hESC-RH6). In control groups, hESCs were treated with 0.1% dimethyl sulfoxide (DMSO) for 4 days and considered as the negative control. Cells were treated with Wnt3a and activin A for the first day, followed by treatment with activin A for the next three days (W/A100-A100) as the positive control. As determined by Q-PCR, the DE markers SOX17, FOXA2 and CXCR4 highly expressed in Spd-A50-treated Royan H6 hESCs while SOX7 [visceral endoderm (VE) marker], SOX1 (neuroectoderm marker) and OCT4 (pluripotency marker) had low levels of expression. The target gene expression level was normalized to GAPDH and presented relative to hESC. Data are presented as mean ± SD. C. Immunofluorescent staining of Spd-A50-treated human embryonic stem cells (hESCs) showed SOX17+ (green) populations comparable to that of W/A100-A100- (positive control) cells. DAPI; 4=,6-diamidino-2-phenylindole.
RNA isolation and real time RT-PCR
Total RNA from samples of the hES, DE, PP, PE cells and hepatocytes were extracted using an RNeasy mini kit (Qiagen, Germany, 74104) according to the manufacturer’s protocol. RNA samples were treated with the RNase-free DNase Set (Qiagen, Germany, 79254) to remove contaminating genomic DNA. The total RNAs were reverse transcribed by the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas, USA, K1632) using 0.2 μg of random hexamer primer and 1 μg of total RNA according to the manufacturer’s instructions.
Real time RT-PCR was performed in a Rotor gene 6000 instrument (Corbett Life Sciences) using the following program. Stage 1:95˚C for 10 minutes, stage 2:95˚C for 10 seconds, 60˚C for 30 seconds and 72˚C for 30 seconds, for a total of 40 cycles. The PCR mix in each well included 10 μl of SYBR®Premix Ex Taq™ II (RR081Q, Takara Bio. Inc.), 6 μl of dH2O, 1 μl each of the forward and reverse primers (5 pmol/μl), and 2 μl of single strand cDNA (16 ng/μl) in a final reaction volume of 20 μl. Relative gene expression was analyzed using the comparative Ct method, 2−ΔΔCt (30). The output data from Rotor-Gene 6000 analysis software (version 1.7, Corbett Life Science) were transferred to Microsoft Excel for further analysis. For each sample, the relative expression level was calculated by normalization of target genes to GAPDH as a reference gene and then calibrated against day 0 hESCs. Samples were gathered from 3 to 5 independent biological replicates and all reactions were performed in duplicate. The primers used were designed using Perl Primer software (31). Primer sequences, expected product size and Gene Bank accession numbers are listed in table 1.
Immunoflourescent staining
For immunofluorescent staining, cells were fixed in 4% w/v paraformaldehyde (Sigma-Aldrich, USA, P6148) for 15 minutes, at 25˚C, permeabilized in 0.1% Triton X-100 for 10 minutes, at 25˚C, and blocked in 10% secondary antibody host serum in 0.5% BSA for 1 hour, at 37˚C, after which they were incubated for 24 hours with goat anti-human SOX17 antibody (R&D Systems, USA, AF1924) that was diluted 1:100 in 0.5% BSA at 4˚C. Cells were subsequently washed with PBS-Tween 20 (PBST) and incubated with diluted (1:400) rabbit anti-goat IgG-FITC antibody (Sigma-Aldrich, USA, F7367) for 1 hour at 25˚C followed by DNA staining with 4’,6-diamidino- 2-phenylindole (DAPI) (Sigma-Aldrich, USA, D8417) for 3 minutes. Cells were observed under a fluorescence microscope (Olympus, BX51, Japan) and imaged with an Olympus DP72 digital camera mounted to the fluorescent microscope. For negative controls, primary antibodies were omitted and the same staining procedure was followed.
Table 1.
Primer sequences, annealing temperature and lengths of the amplified products.
| Genes | Gene bank code | Forward primer (5' – 3') | Reverse primer (5' – 3') | Annealingtemp (˚C) | Productsize (bp) |
|---|---|---|---|---|---|
| GAPDH | NM_002046.3 | CTCATTTCCTGGTATGACAACGA | CTTCCTCTTGTGCTCTTGCT | 60 | 121 |
| ACTB | NM_001101.3 | AGCACAGAGCCTCGCCTT | CACGATGGAGGGGAAGAC | 60 | 163 |
| OCT3\4 | NM_001159542.1 | GTTCTTCATTCACTAAGGAAGG | CAAGAGCATCATTGAACTTCA | 60 | 101 |
| SOX1 | NM_005986.2 | GTGTACCCTGGAGTTTCTG | TAGTCTGTGCCTCTAAAGTG | 60 | 88 |
| SOX7 | NM_031439.2 | ACGCCGAGCTCAGCAAGAT | TCCACGTACGGCCTCTTCTG | 60 | 73 |
| CXCR4 | NM_001008540.1 | CACCGCATCTGGAGAACCA | GCCCATTTCCTCGGTGTAGTT | 60 | 80 |
| FOXA2 | NM_021784.4 | ATGCACTCGGCTTCCAGTAT | TGTTGCTCACGGAGGAGTAG | 60 | 89 |
| SOX17 | NM_022454.3 | CTCTGCCTCCTCCACGAA | CAGAATCCAGACCTGCACAA | 60 | 102 |
Statistical analysis
All experiments were conducted in at least three independent biological replicates. Data from real time RT-PCR were expressed as mean ± SD and the differences of the mean were statistically evaluated by SPSS software (version 16) using one-way analysis of variance (ANOVA) followed by Tukey’s HSD and LSD posthoc tests. P values less than 0.05 were considered significant.
Results
Treating Royan H6 hESCs with Spd prior to activin induction
Suppression of regulators of a pluripotency state can lead to the priming of ESCs for differentiation into different lineages (26, 27, 32). Treating hESCs with Spd has been shown to lead to the down regulation of c-MYC which is known as a main factor in self-renewal of hESCs. As shown in figure 1A, we treated Royan H6 hESCs with 200 nM Spd for one day followed by 50 ng/ml activin A for the next three days (Spd-A50). We used a previously reported protocol (W/A100-A100) (11) as the positive control and DMSO treatment as the negative control.
To evaluate the efficiency of our experimental groups for endoderm induction, by the fourth day, we analyzed the cells for expressions of the following DE markers: SOX17, FOXA2 and CXCR4 (33, 34); and SOX7 [visceral endoderm (VE) marker], SOX1 (neuroectoderm marker) and OCT4 (pluripotency marker) by QPCR (Fig 1B).
SOX17, FOXA2 and CXCR4 expression levels of Spd-A50-treated hESCs compared with the positive control (W/A100-A100-treated cells) revealed that the Spd-A50 regimen lead to the upregulation of DE marker genes at levels comparable to W/A100-A100. However, the levels of SOX7 and SOX1 expression remained unchanged and OCT4 down regulated during hESCs treatment with both Spd-A50 and W/ A100-A100 (Fig 1B).
To corroborate the SOX17 gene expression results in Spd-A50-treated cells, we investigated SOX17 protein expression. SOX17+ populations were detected by immunofluorescent staining in the cultures treated with the Spd-A50 protocol (Fig 1C). Similar populations were also detected in the cultures treated with W/A100- A100. Therefore, Spd-A50 protocol was able to upregulate DE-specific markers in hESCs and produce DE cells.
Effects of Spd and activin concentrations on the efficiency of Spd-A50 protocol
The primary concentration of Spd (200 nM) was replaced by higher concentrations (400 and 800 nM) of Spd in the Spd-A50 protocol. Treated hESCs were investigated for the expressions of DE markers SOX17 and FOXA2 by Q-PCR. Although the levels of DE marker genes in Spd800-A50-treated hESCs were significantly (p<0.05) higher than Spd-A50-treated cells (Fig 2A), phase contrast images showed that treating hESCs with 800 nM Spd lead to a very high cell mortality (Fig 2B). As the SOX17 and FOXA2 expression levels were not significantly different in Spd 400 and Spd-A50-treated cells, the 200 nM concentration of Spd was selected for the remainder of the experiments. Additionally, the lower concentration of activin A (25 ng/ml) was tested (Spd-A25). According to Q-PCR results, this concentration of activin A caused a significant decrease in the expression levels of DE marker genes.
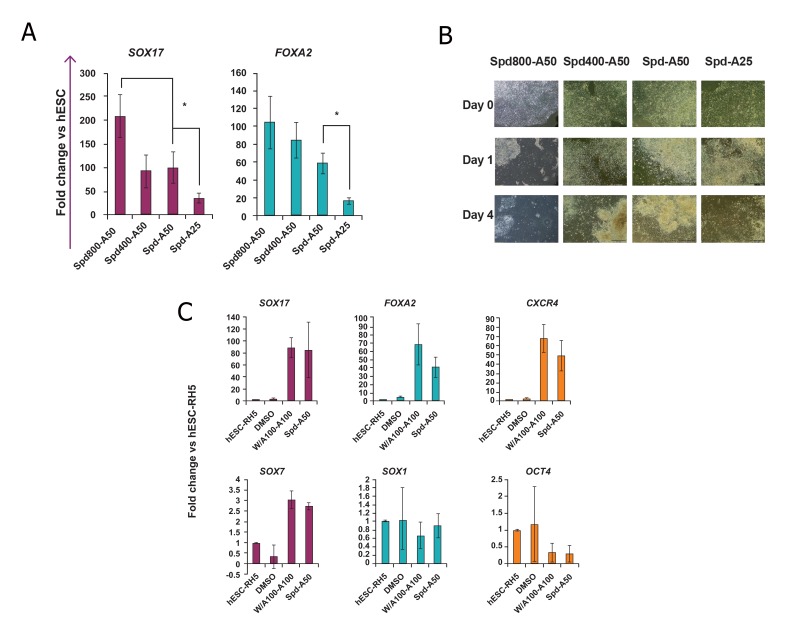
Fig 2.
The effects of stauprimide (Spd) and activin A (A) concentrations and human embryonic stem cell (hESC) line on the efficiency of the Spd-A50 protocol.
A. hESC-Rh6 were treated with Spd200/400/800-A50 and the Spd-A50 regimen. Samples were analyzed for expressions of SOX17 and FOXA2 by Q-PCR at the end of the fourth day. *; Significant difference from all other groups, at least p<0.05 as determined by ANOVA with Tukey’s LSD test. n=3-4.
B. Phase contrast images were taken at day 0 (hESC colonies at the beginning of the experiment), day 1 (the same colony, one day after treatment with Spd) and at day 4 (3 days after treatment with activin A). High mortality of the cells was observed after treatment with 800 nM Spd (Spd800-A50 group).
C. Lineage-specific gene expression analysis of Spd-A50-treated Royan H5 human embryonic stem cells (hESC-RH5). In control groups, hESCs were treated with 0.1% dimethyl sulfoxide (DMSO) for 4 days and considered as the negative control. Cells were treated with Wnt3a and activin A for the first day followed by activin A for the next three days (W/A100-A100) as the positive control. As determined by Q-PCR, the DE markers SOX17, FOXA2 and CXCR4 highly expressed in Spd-A50-treated Royan H5 hESCs while SOX7 [visceral endoderm (VE) marker], SOX1 (neuroectoderm marker) and OCT4 (pluripotency marker) exhibited low levels of expression. This expression pattern was similar to that obtained from the hESC-RH6 line. The target gene expression level was normalized to GAPDH and presented relative to hESC. Data are presented as mean ± SD.
The Spd-A50 protocol was tested on another hESC line (Royan H5) and the same gene expression results as hESC-RH6 were obtained (Fig 2C).
Effects of Spd and activin concentrations on the efficiency of Spd-A50 protocol
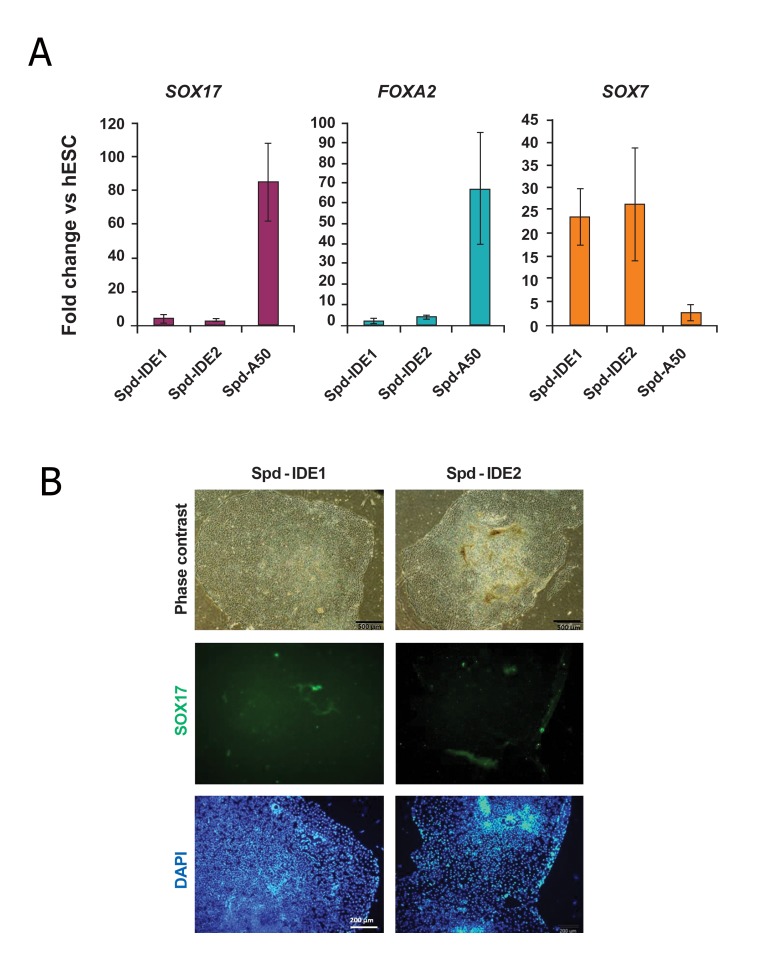
It has previously been shown that treatment of hESCs with IDE1/2 induced DE differentiation (25). Thus we replaced activin A with IDE1/2 in the Spd-A50 protocol and treated hESCs with this new regimen (Spd-IDE1/2). As shown in figure 3, this replacement caused a dramatic decrease in SOX17 (also at the protein level) and FOXA2 expression. However, the VE marker, SOX7 up regulated in cells treated with IDE1/2.
Fig 3.
Gene and protein expression analysis of definitive endoderm (DE) and visceral endoderm (VE) markers in human embryonic stem cell (hESCs) treated with Spd-IDEs.
A. After treating hESCs with Spd-IDE1/2, samples were analyzed for the expressions of SOX17, FOXA2 and SOX7 by Q-PCR at the end of the fourth day. The target gene expression level was normalized to GAPDH and presented relative to hESC. Data are presented as mean ± SD. Asterisk indicates significant difference between the shown groups (p<0.05 as determined by ANOVA with Tukey’s LSD test; n=3).
B. Immunofluorescent staining of Spd-IDE1/2-treated hESCs indicated no SOX17+ cells in the groups. DAPI; 4=,6-diamidino- 2-phenylindole.
Discussion
For DE differentiation, we used a twostep protocol. In the first step, hESCs were treated with Spd, a previously introduced SM suppressor of pluripotency (26, 27) and in the second step they were exposed to activin A, an inducer of endoderm formation. We showed that Spd-A50 regimen lead to upregulation of DE-specific markers (SOX17, FOXA2 and CXCR4) while no increase was observed in VE-specific gene (SOX7) expression. There was no difference in the DE-specific gene expression patterns between Spd- A50- and W/A100-A100-induced DE cells. As reported before, Spd has been shown to inhibit nuclear localization of nucleoside diphosphate kinase B (DNPK B), which led to the down regulation of c-MYC a known main factor in hESC self-renewal. Thus it could prime hESCs for differentiation (26). As we previously reported (27), pre-treatment of hESCs with SM suppressors of pluripotency could be a strategy to improve the efficiency of DE induction.
Activin/Smad2/3 is an important signaling pathway during endoderm formation (15, 35) and widely used in DE differentiation protocols. We reduced the concentration of activin A to 25 ng/ml in the Spd-A50 protocol, which led to a dramatic reduction in the expression of DE-specific genes. This agreed with previous reports that different concentrations of growth factor activin A have different inducing effects on hESCs (3).
IDE1 and IDE2 have been previously reported to be inducers of DE. While the exact mechanisms of their action are not known, the activation of the TGF-β signaling pathway has been shown in hESCs treated with these SMs(25). In contrast, we did not observe any up regulation of DE markers in Spd-IDE1/2-treated hESCs while the VE marker gene (SOX7) showed a significant up regulation in these cells. This was possibly due to the different abilities of hESC lines for differentiation under identical conditions as reported in numerous studies (36).
Conclusion
This study showed that while the Spd-A50 protocol could lead to efficient DE differentiation, replacement of activin A with two previously reported inducers of DE (IDE1/2) dramatically reduced the efficiency of DE differentiation in the tested hESC lines.
Acknowledgments
This study was funded by grants provided by Royan Institute. The authors declare they have no competing financial interests.
References
- 1.Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235(9):2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- 2.Grapin-Botton A. Endoderm specification. Cambridge (MA): Harvard Stem Cell Institute; 2008. Avalible fromhttp://www.ncbi.nlm.nih.gov/books/NBK27052/ (12 Mar 2013) [PubMed] [Google Scholar]
- 3.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay KD. Formation of the murine endoderm: lessons from the mouse, frog, fish, and chick. Prog Mol Biol Transl Sci. 2010;96:1–34. doi: 10.1016/B978-0-12-381280-3.00001-4. [DOI] [PubMed] [Google Scholar]
- 5.Benitez CM, Goodyer WR, Kim SK. Deconstructing pancreas developmental biology. Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a012401. doi: 101101/cshperspecta012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lysy PA, Weir GC, Bonner-Weir S. Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med. 2012;1(2):150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khodadadi L, Jafari H, Farrokhi A, Pirouz M, Baharvand H. From pancreatic development to mellitus diabetes treatment. Yakhteh. 2006;8(2):124–152. [Google Scholar]
- 8.Pournasr B, Farzaneh Z, Shahsavani M, Baharvand H. Liver development and in vitro differentiation of embryonic stem cells to hepatocytes. Cell J. 2010;11(4):348–373. [Google Scholar]
- 9.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26(5):1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 11.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 12.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15(4):509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17(13):1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 16.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo AK, Ali Y, Wong KY, Chipperfield H, Sadasivam A, Poobalan Y, et al. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30(4):631–642. doi: 10.1002/stem.1022. [DOI] [PubMed] [Google Scholar]
- 18.Efe JA, Ding S. The evolving biology of small molecules: controlling cell fate and identity. Philos Trans R Soc Lond B Biol Sci. 2011;366(1575):2208–2221. doi: 10.1098/rstb.2011.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl. 2011;50(1):200–242. doi: 10.1002/anie.201004284. [DOI] [PubMed] [Google Scholar]
- 20.Firestone AJ, Chen JK. Controlling destiny through chemistry: small-molecule regulators of cell fate. ACS Chem Biol. 2010;5(1):15–34. doi: 10.1021/cb900249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber SL. Small molecules: the missing link in the central dogma. Nat Chem Biol. 2005;1(2):64–66. doi: 10.1038/nchembio0705-64. [DOI] [PubMed] [Google Scholar]
- 22.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5(4):258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Hao J, Hong CC. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/beta-catenin signaling. ACS Chem Biol. 2011;6(2):192–197. doi: 10.1021/cb100323z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4(4):348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, et al. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4(5):416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Tahamtani Y, Azarnia M, Farrokhi A, Sharifi- Zarchi A, Aghdami N, Baharvand H. Treatment of human embryonic stem cells with different combinations of priming and inducing factors toward definitive endoderm. Stem Cells Dev. 2013;22(9):1419–1432. doi: 10.1089/scd.2012.0453. [DOI] [PubMed] [Google Scholar]
- 28.Baharvand H, Ashtiani SK, Taee A, Massumi M, Valojerdi MR, Yazdi PE, et al. Generation of new human embryonic stem cell lines with diploid and triploid karyotypes. Dev Growth Differ. 2006;48(2):117–128. doi: 10.1111/j.1440-169X.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 29.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen TD. Real-time quantitative PCR. Methods. 2001;25(4):383–385. doi: 10.1006/meth.2001.1260. [DOI] [PubMed] [Google Scholar]
- 31.Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics. 2004;20(15):2471–2472. doi: 10.1093/bioinformatics/bth254. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106(19):7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 34.Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119(3):567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 35.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 36.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26(3):313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]