Phenols and polyphenols are widely distributed in plant tissues, where they are linked to diverse biological functions such as chemical defense, pigmentation, structural support, and prevention of radiation damage.[1] Members of this large family of compounds include epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC), and tannic acid (TA), to name a few (Figure 1 B). Plant polyphenols display a rich and complex spectrum of physical and chemical properties,[1a] giving rise to broad chemical versatility including absorption of UV radiation, radical scavenging, and metal ion complexation. In addition, significant attention has been given to the purported health benefits associated with consumption of foods and beverages rich in plant polyphenols.[2]
Figure 1.
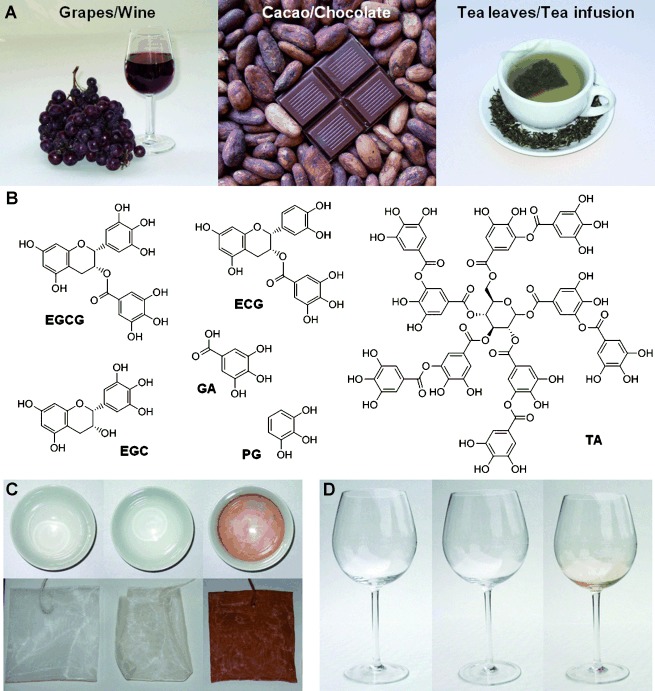
Polyphenol-rich foods and beverages deposit thin adherent coatings on surfaces. A) The high polyphenol content of grapes, red wine, raw cacao, chocolate, green tea leaves, and tea infusion provided inspiration for a novel coating strategy. B) Chemical structures of selected plant polyphenols as well as phenolic mimics and building blocks. C) Colorless, adherent polyphenol coatings deposit on surfaces exposed to tea infusion. The images on the left are of a clean tea cup and unused tea bag. These objects appear unchanged after exposure to tea infusion and rinsing with tap water (middle). The presence of a coating is revealed after treatment of the surfaces with aqueous AgNO3 (right). D) Colorless, adherent polyphenol coating formed on glass exposed to red wine. The images show a clean wine glass before (left), after exposure to red wine and rinsing with tap water (middle), and after treatment with aqueous AgNO3 (right).
Of interest to us in this report is the strong solid–liquid interfacial activity exhibited by plant polyphenols, a property that is reflected in their historical use as binding agents (lignin) and in leather manufacturing (vegetable tannins). The high dihydroxyphenyl (catechol) and trihydroxyphenyl (gallic acid, GA) content of plant polyphenols further attracted our interest in the context of surface modification, as catechols are known to strongly bind to surfaces through covalent and noncovalent interactions[3] and are prominent constituents of marine polyphenolic protein adhesives.[4] Inspired by the high catecholamine content of mussel adhesive proteins and by the involvement of catecholamines in melanin biosynthesis, in situ oxidative polymerization of dopamine at alkaline pH was recently discovered as a universal route for deposition of multifunctional coatings onto surfaces.[5] Although polydopamine (pDA) is simple to apply to substrates, deposits on a wide range of materials and offers many potential applied uses, the high costs of dopamine and the characteristically dark color of pDA coatings may be impediments for some practical applications. While a colorless pDA approach was recently reported,[6] the method employed a 2:1 mixture of 2-bromoisobutyryl-substituted dopamine to dopamine and was achieved at the expense of a roughly fourfold reduction in coating thickness.
Herein, we describe the use of low-cost plant polyphenols, their building blocks and trihydroxyphenyl-containing molecules as precursors for the formation of multifunctional coatings. In contrast to previous studies where plant polyphenols have been investigated as monolayer adsorbates or as ingredients in multicomponent coatings,[7] our strategy features either a plant polyphenol (TA) or a simple phenolic mimic (pyrogallol, PG) as the sole coating precursor. Plant polyphenol-inspired coatings retain many of the advantages of pDA and deposit under similar conditions, yet are colorless and derived in some cases from reagents hundredfold less costly than dopamine.
The coating potential of plant polyphenols was first illustrated with a simple experiment involving unadulterated tea and wine, yielding the surprising finding that thin polyphenol coatings form spontaneously on surfaces exposed to these polyphenol-rich beverages (Figure 1). A freshly prepared green tea infusion left undisturbed in a covered porcelain cup for several hours and then rinsed with tap water, appeared to leave no residue or produce any observable color changes to the cup. However, the presence of a thin polyphenol coating was revealed by immersing the tea cup in a AgNO3 solution, resulting in the deposition of a dark metallic silver film on the surface, presumably through a redox reaction between Ag+ ions and the polyphenolic coating (Figure 1 C). The tea bag used to prepare the infusion also appeared unchanged but darkened markedly upon treatment with AgNO3. The presence of surface-bound silver nanoparticles was evident upon inspection using scanning electron microscopy (SEM, Figure S2). Similarly, red wine also deposited an indiscernible polyphenol coating on glass that was visible only after treatment with AgNO3 (Figure 1 D).
Next we investigated crude extracts of red wine (RWE), cacao bean (CBE), and green tea (GTE) for their ability to form polyphenol films on polymeric, metallic, and native-oxide surfaces. Aqueous extracts were prepared and assayed for gallic acid equivalents (GAE),[8] revealing the high polyphenol content of the crude extracts (Figure S3). Immersion of polysulfone (PS) substrates into dilute solutions of RWE, GTE, and CBE (2 mg mL−1) left a uniform colorless polyphenol coating on the surface, attenuating the underlying substrate signal as measured by XPS (Figure 2 A). Coating deposition was most effective from buffered saline (0.6 m NaCl, pH 7.8) as compared to pure water, with coatings derived from CBE totally obscuring the S2p signal of the substrate, indicating thickness of about 10 nm or more (Figure S4).
Figure 2.
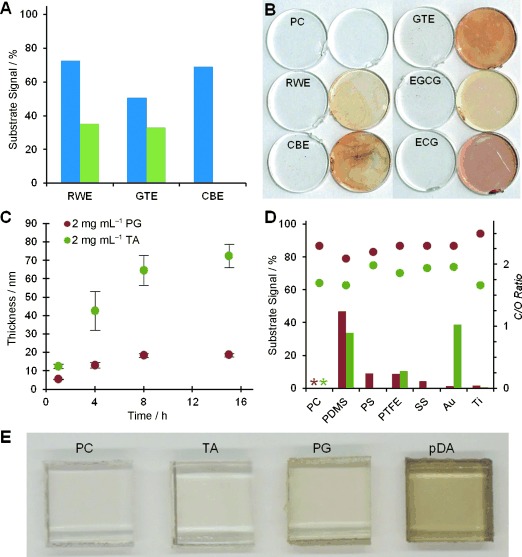
Deposition and physicochemical properties of coatings derived from polyphenol-rich food extracts, tannic acid (TA), and pyrogallol (PG). A) PS substrate signal (S2p) detected by XPS after modification with crude polyphenol-rich extracts in unbuffered water (blue) and in buffered saline (green). A value of 100 % indicates the absence of detectable coating. B) Coatings deposited onto clear polycarbonate (PC) disks from RWE, CBE, GTE, EGCG, and ECG. For each pair of disks, the disk on the right has also been treated with aqueous silver nitrate to visualize the coating. C) Time dependence of PG and TA film deposition from buffered saline on gold and TiO2, respectively, as determined by ellipsometry. D) Formation of PG (red) and TA (green) coatings occurs on a variety of substrates as indicated by the disappearance of the relevant substrate signal and by the corresponding carbon/oxygen ratio (C/O, secondary y-axis, circles), as assessed by XPS. Theoretical C/O ratios for molecular TA and PG are 1.65 and 2.00, respectively. *: the similarity of coating and substrate chemical signature prevented accurate analysis of coatings on PC. E) PC substrates modified with TA and PG reveal little or no discoloration when compared to pDA coatings of comparable thickness.
We subsequently investigated pure ECG and EGCG, two flavan-3-ols known to be present in high concentration in green tea, by immersing polycarbonate (PC, Figure 2 B) and titanium dioxide (TiO2, Figure S5) substrates in buffered saline containing 0.1–0.5 mg mL−1 of each compound. Chemical analysis of the resulting surfaces by XPS (Figure S5) and by silver visualization as described above showed that EGCG and ECG formed colorless coatings in a similar fashion (Figure 2 B), suggesting that the coatings derived from crude GTE may be a result of the presence of EGCG, ECG or related polyphenols.
Taking the results of these exploratory experiments into consideration, as well as the fact that the trihydroxyphenyl functional group is a common feature of EGCG, ECG, and other plant polyphenols, we selected PG and the gallate ester-rich TA as simple, readily available and low-cost precursors for deposition of multifunctional coatings on substrates. Thin coatings spontaneously deposited onto gold and titanium dioxide from PG and TA solutions, increasing in thickness with incubation time (Figure 2 C) and precursor concentration (Figure S6). Maximal thickness of approximately 20 nm occurred after 8 h of incubation in PG, and 65 nm in TA (2 mg mL−1 in buffered saline). Furthermore, the versatility of the coating method was illustrated by forming coatings on a variety of organic and inorganic substrates, including metals such as stainless steel (SS) and hydrophobic polymers like polytetrafluoroethylene (PTFE; Figure 2 D and Figure S7). Coatings derived from PG appeared to induce only minor changes in surface roughness relative to the bare substrate, as assessed by SEM (Figure S8), while being sufficiently thick as to completely mask the XPS signal of most underlying substrates (Figure 2 D).
TA-modified surfaces especially, and to some extent coatings derived from PG, appear colorless when compared to pDA coatings of comparable thickness (Figure 2 E and Figure S9). This was confirmed with UV/Vis measurements, which showed little change in the absorption spectrum of TA-coated PC (Figure S10). Exposure of coatings derived from TA and PG to aqueous AgNO3 (100 mm) afforded electroless silver metallization as indicated by the presence of silver nanoparticles bound to the surface of the coating (Figures S11–S13).
Oxidation of plant polyphenols is known to lead to the formation of higher molecular weight species[9] and these reactions are intimately involved in the browning of fruits and vegetables, in leather tanning, and in the fermentation of tea leaves.[10] We suspect that oxidation reactions are responsible for the formation of coatings from ECGC, ECG, PG, and TA. Similar to coatings derived from dopamine polymerization,[5] plant polyphenol-inspired coatings form spontaneously at mildly alkaline pH (7.8) in the presence of available dissolved oxygen (Figure S14), likely through phenolate ion intermediaries. Although further studies will be necessary to understand the exact mechanism of coating formation, we surmise that oxidation followed by oligomerization decreases the solubility of plant polyphenols and combined with their inherent affinity toward surfaces, ultimately leads to surface deposition. Mass spectrometry analysis of PG coatings and polymerization solutions indicated the presence of high-molecular-weight species, consistent with this general model (Figure S15).
Motivated by the many diverse functions of plant polyphenols in nature, we found that polyphenol coatings could display numerous useful functional properties. For example, emulating the natural role of plant polyphenols in biological defense,[11] we found that polyphenol coatings derived from TA (Figure 3 A) and PG (Figure S16) displayed strong contact-based antibacterial properties against both gram-negative and gram-positive strains. P. aeruginosa and S. aureus viability was reduced at least 30-fold upon exposure to PC modified with TA for 3 h (Figure 3 A). Importantly, the same coatings were nontoxic to mammalian cells, as exposure of NIH 3T3 fibroblast cells to TA- and PG-coated substrates revealed no observable cytotoxicity.
Figure 3.
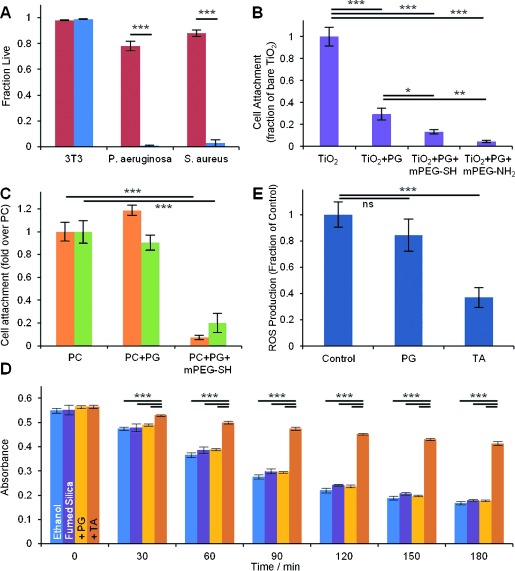
Biological applications of PG and TA coatings. A) Viability of 3T3 fibroblast cells upon contact with unmodified polystyrene (red) and TA-modified polystyrene (blue), and of P. aeruginosa and S. aureus cells in contact with unmodified PC (red) and TA-modified PC (blue). B) 24 h attachment of 3T3 fibroblasts on TiO2, PG-modified TiO2, and mPEG-SH or mPEG-NH2 grafted PG-modified TiO2. C) 24 h attachment of P. aeruginosa (orange) and S. aureus (green) on PC, PG-modified PC, and on mPEG-SH grafted PG-modified PC. D) Antioxidant performance of PG- and TA-coated fumed silica nanoparticles, as measured by β-carotene degradation assay. A decrease in absorbance is indicative of oxidation. E) Intracellular ROS production of 3T3 fibroblasts adhered to bare and polyphenol-modified polystyrene. *: p<0.05, **: p<0.01, ***: p<0.001.
Resistance to fouling by bacterial and mammalian cells was conferred by grafting antifouling polymers onto polyphenol coatings, exploiting the proclivity of polyphenols to covalently react with nucleophilic groups of polypeptides and other molecules.[1a, 12] Grafting of monofunctional thiol- or amine-terminated poly(ethylene glycol) (mPEG-SH and mPEG-NH2) was performed under the same deposition conditions that were used to deposit PG coatings (Figure S17), and the resultant surfaces resisted attachment of NIH 3T3 fibroblasts (Figure 3 B and Figure S18), P. aeruginosa, and S. aureus over a 24 h period (Figure 3 C).
Additionally, our coatings display one of the most celebrated features of plant polyphenols: the ability to scavenge radical and non-radical reactive oxygen species (ROS).[13] We examined the antioxidant properties of polyphenol-coated fumed silica (FS) nanoparticles and flat tissue culture polystyrene (TCPS) surfaces, using both chemical and cell-based assays. Radical scavenging properties were assessed using a stable radical assay based on 2,2-diphenyl-1-picrylhydrazyl (DPPH; Figure S19), and antioxidant behavior was probed through β-carotene degradation (Figure 3 D). A significant antioxidant effect was observed for polyphenol coatings derived from TA, although PG coatings were less effective (Figure 3 D). Intracellular ROS production in NIH 3T3 fibroblasts was notably attenuated in the presence of unmodified and TA-modified fumed silica nanoparticles (Figure S20). Most interestingly, measured intracellular ROS levels for 3T3 cells grown on TA-coated TCPS were substantially lower than for cells grown on bare TCPS (Figure 3 E). The reduction in ROS levels cannot be attributed to dissolved polyphenols, as culture media exposed to TA-coated TCPS failed to display measureable GAE content. This result implies an intracellular protective effect for cells cultivated on an extracellular polyphenol film, a finding that may have important implications for modulating the acute inflammatory response to implanted medical devices. We speculate that membrane-associated esterases could hydrolytically degrade portions of the coating, which then become internalized within the cell and provide protective effects similar to those observed by soluble polyphenols.
Finally, we demonstrated the use of plant polyphenol-inspired coatings for modulation of the optical properties of inorganic nanoparticles by depositing a PG coating onto cetyltrimethylammonium bromide (CTAB)-stabilized gold nanorods (Au NRs) displaying a native longitudinal surface plasmon resonance (SPR) at 895 nm. Suspending CTAB-stabilized Au NRs in 0.1 mg mL−1 PG resulted in displacement of the CTAB ligand and formation of a thin adherent PG coating on the Au NRs (Au-PG NRs). Secondary electron imaging revealed a less electron-dense 5 nm thick PG adlayer visible against the metallic core (Figure S21). Subsequent addition of AgNO3 to the suspension yielded bimetallic nanorods (Au-PG-Ag), with energy-dispersive X-ray spectroscopy (EDS) revealing the constructs to consist of a gold core and a silver shell (Figure 4 A). The thickness of the silver shell increased with concentration of AgNO3, facilitating fine-tuning of the longitudinal SPR wavelength through a blue shift of the SPR (Figure 4 B and C). The ability to tune optical properties of nanoparticles is desirable in a variety of contexts, including medical applications of metallic nanoparticles where the SPR is often employed for diagnostic and therapeutic purposes.[14]
Figure 4.
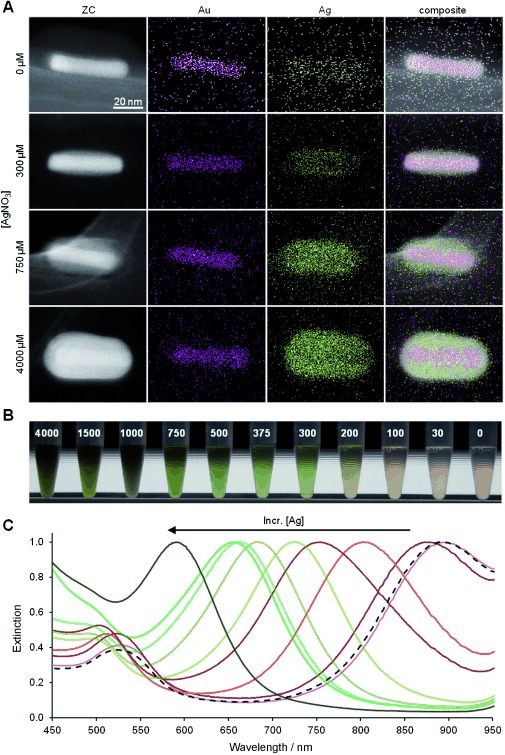
Plasmonic tuning of metal nanoparticles through templated synthesis of core–shell nanorods using plant polyphenol-inspired coatings. A) Z-contrast TEM and EDS elemental micrographs of Au-PG-Ag NRs prepared with increasing AgNO3 concentrations (top to bottom). B) A blue shift of the longitudinal surface plasmon resonance wavelength was indicated by a color change of the nanorod suspension. The magnitude of the blue shift was controlled by the concentration of silver nitrate (μm) used in nanoparticle modification. C) Normalized optical extinction spectra of Au-PG-Ag NRs illustrating plasmon tuning through control of the Ag shell thickness.
In summary, we described a novel bioinspired approach to the formation of colorless multifunctional coatings, exploiting the versatility and multifunctionality of plant polyphenols and their mimics. We believe that the simplicity and versatility of the strategy, combined with the low costs and wide availability of TA and PG, are attractive features that will lead to many additional applications beyond the ones we have described. Plant polyphenol-inspired coatings are not only possible through the use of low-cost precursors like PG and TA, but also through the direct use of extracts from polyphenol-rich foods, therefore representing a “green” family of surface modification strategies.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1a.Haslam E. Practical polyphenolics: from structure to molecular recognition and physiological action. Cambridge University Press; 1998. [Google Scholar]
- 1b.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Angew. Chem. 2011;123:610–646. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 2a.Pandey KB, Rizvi SI. Oxid. Med. Cell. Longevity. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b.Preedy VR. Tea in health and disease prevention. London: Elsevier; 2013. [Google Scholar]
- 2c.Ross JA, Kasum CM. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. USA. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Lee B, Messersmith PB, Israelachvili JN, Waite JH. Annu. Rev. Mater. Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b.Jensen R, Morse D. J. Comp. Physiol. B. 1988;158:317–324. [Google Scholar]
- 5.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohri M, Kohma H, Shinoda Y, Yamauchi M, Yagai S, Kojima T, Taniguchi T, Kishikawa K. Polym. Chem. 2013;4:2696–2702. doi: 10.1002/marc.201300395. [DOI] [PubMed] [Google Scholar]
- 7a.Erel-Unal I, Sukhishvili SA. Macromolecules. 2008;41:3962–3970. [Google Scholar]
- 7b.Kozlovskaya V, Harbaugh S, Drachuk I, Shchepelina O, Kelley-Loughnane N, Stone M, Tsukruk VV. Soft Matter. 2011;7:2364–2372. [Google Scholar]
- 7c.Lin D, Xing B. Environ. Sci. Technol. 2008;42:5917–5923. doi: 10.1021/es800329c. [DOI] [PubMed] [Google Scholar]
- 7d.Shutava T, Prouty M, Kommireddy D, Lvov Y. Macromolecules. 2005;38:2850–2858. [Google Scholar]
- 7e.Wu H, Wu C, He Q, Liao XP, Shi B. Mater. Sci. Eng. C. 2010;30:770–776. [Google Scholar]
- 7f.Huang J, Huang K, Liu S, Luo Q, Xu M. J. Colloid Interface Sci. 2007;315:407–414. doi: 10.1016/j.jcis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7g.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK, Cui J, Caruso F. Science. 2013;341:154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 8.Singleton VL, Orthofer R, Lamuela-Raventos RM. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 9.Drynan JW, Clifford MN, Obuchowicz J, Kuhnert N. Nat. Prod. Rep. 2010;27:417–462. doi: 10.1039/b912523j. [DOI] [PubMed] [Google Scholar]
- 10.Haslam E. Phytochemistry. 2003;64:61–73. doi: 10.1016/s0031-9422(03)00355-8. [DOI] [PubMed] [Google Scholar]
- 11.Cowen MM. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Haslam E. Plant polyphenols: vegetable tannins revisited. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- 12b.Spencer CM, Cai Y, Martin R, Gaffney SH, Goulding PN, Magnolato D, Lilley TH, Haslam E. Phytochemistry. 1988;27:2397–2409. [Google Scholar]
- 13.Bahorun T, Neergheen-Bhujun V, Toolsee NA, Somanah J, Luximon-Ramma A, Aruoma OI. Tea in Health and Disease Prevention. New York: Academic Press; 2013. pp. 361–374. [Google Scholar]
- 14a.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 14b.Homola J, Yee SS, Gauglitz G. Sens. Actuators B. 1999;54:3–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


